Abstract
Background/Objectives: The objective of this study is to investigate the patterns and characteristics of medication errors (MEs) associated with antipsychotic medication use in hospitals affiliated with the Ministry of Health (MOH) in Saudi Arabia and to identify areas for improvement. Methods: A retrospective descriptive analysis of MEs associated with antipsychotic use was conducted using data collected from MOH-affiliated hospitals between April 2020 and September 2022. The data were analyzed descriptively to identify the factors underpinning unsafe antipsychotic use. Results: The sample period produced 35,077 reported MEs. Reports from the Western region contributed the highest error percentage, and MEs were reported more frequently in male (76.1%, n = 26,705) and adult (97.7%, n = 34,275) patients. Pharmacists reported MEs more often than other healthcare professionals (66.5%, n = 23,312). Most MEs (89.9%, n = 31,524) originated in the prescribing stage, with missing prescription information being the most frequently reported ME type (40.5%, n = 14,206). Atypical antipsychotics accounted for the greatest proportion of reports (79.3%, n = 27,811) compared to typical antipsychotics (20.7%, n = 7262). Most ME outcomes fell into Category B: The error occurred but did not reach the patient (56.4%, n = 19,794). Factors related to staffing or workflow accounted for 21.3% (n = 7467) of the reported errors, followed by a lack of policies in relation to antipsychotics prescribing and monitoring (20.5%; n = 7195). Conclusions: MEs in hospitals in Saudi Arabia frequently involve antipsychotic medications. This study identified important targets that may help reduce such risks in the future.
1. Introduction
Medication errors (MEs) contribute significantly to preventable harm and impact 3.3% of the population, sometimes causing life-threatening complications [1,2]. The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) defines a medication error as “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is controlled by the healthcare professional, patient, or consumer. Such events may relate to professional practice, healthcare products, procedures, and systems, including prescribing, order communication, product labelling, packaging, nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use” [3].
The risk of MEs may be higher in psychiatric hospitals due to the complexities associated with patient care and factors such as disorganized cognitive function, emotion, and behavior [4,5]. Antipsychotic medications are one of three major classes that act on the central nervous system reported to be involved with MEs [6,7]. Antipsychotic medications are prescribed for schizophrenia, bipolar disorders, and schizoaffective disorders, but their use has expanded to include the treatment of depression, insomnia, autism spectrum disorders, and behavioral symptoms of dementia [8,9,10,11,12]. The wider application and off-label use of these medications have led to a significant increase in users, which raises concerns about the risk of MEs [13].
Several factors increase the likelihood of MEs associated with antipsychotic drugs, including prolonged action durations and extended treatment periods related to the chronicity of the health conditions they are treating [14]. Additionally, other factors, such as parenteral administration routes [15], polypharmacy [16,17,18], the use of high-risk medications (e.g., clozapine) [19,20], and high doses intended to tranquilize the patient rapidly, [21], also contribute to the risk of ME occurrence.
MEs in psychiatric hospitals are common and account for 5.7 to 88.8 MEs per 100 admissions [22] in which they are reported to be contributed by a variety of potential factors, including patient-, medication-, and hospital-related factors [23]. Historically, the ME reporting system has been the primary method of collecting MEs within healthcare organizations [24,25]. Many countries have established national reporting systems for this purpose, such as the National Reporting and Learning System (NRLS) in England and Wales [26], the British Columbia Patient Safety and Learning System in Canada [27], and the Ministry of Health (MOH)-ME reporting system in Saudi Arabia [28]. Such reporting systems are considered an important source of information that helps identify patterns and contributory factors of MEs, which are essential prerequisites for developing interventions that can be applied in practice [29]. In the United States, data from the National Poison Data System revealed a sharp rise in MEs between 2000 and 2012 due to the use of antipsychotics [30]. A study analyzing ME data from the NRLS used in England and Wales identified that a significant proportion of ME reports (10.4%) involved antipsychotic medications [31]. Similarly, in Thailand, a 10-year retrospective analysis of the national ME database found that 8.9% of all MEs involved antipsychotic medication [32]. In Saudi Arabia, an analysis of 23,355 ME reports retrieved from 21 psychiatric hospitals revealed antipsychotic medications to be involved in 7769 cases (59.4%), which indicates that antipsychotics are the drug class most frequently implicated in this setting [7]. In general hospitals, the factors related to patient harm from MEs are complex, including those related to healthcare providers [33,34], the healthcare system [35,36], and patient characteristics [16,23,37,38]. Several studies have highlighted that the risk of harm arising from MEs is common in psychiatric settings [6,22,31]. These findings reveal an urgent need to prioritize and detect MEs in these settings [23].
In Saudi Arabia, the MOH is the primary provider of mental health services, which it delivers through the regionally structured General Administration for Mental Health and Social Services [39,40]. Each region has hospitals that provide inpatient, outpatient, and emergency services alongside specialized facilities for children and adolescents [41] and additional support from private providers, community clinics, and primary healthcare centers. In the interest of improving medication safety in psychiatric treatment, the MOH has launched several initiatives to ensure equal, accessible, and integrated healthcare services for patients with psychiatric disorders [42]. More specifically, the MOH’s General Department of Pharmaceutical Care has supported efforts to detect MEs using ME reporting system to better understand the nature of MEs reported in MOH-affiliated hospitals, identify learning opportunities, and generate recommendations to improve medication safety [28]. This goal aligns with the World Health Organization’s third global initiative—Medication Without Harm [43]—as well as Saudi Arabia’s Vision 2030, which lists ‘enhancing the quality and safety of the healthcare system’ among the initiative’s priorities [44].
The reported prevalence of schizophrenia, schizotypal, and delusional disorders treated in MOH mental health facilities was 8624 inpatients and 141,775 outpatients [45]. Schizophrenia is also recognized as the third leading global cause of disability and reduced life expectancy [46,47], which points to its significance as a major public concern. Accordingly, it is necessary to conduct research across multiple hospitals in Saudi Arabia to assess the pattern and contributory factors of MEs associated with antipsychotic use. Therefore, this study aims to identify the ME patterns associated with antipsychotic medication use and to determine factors that might support the development of strategies to mitigate risk and improve patient outcomes in psychiatric settings.
2. Methods
2.1. Study Design
The study adopted a cross-sectional design to assess MEs associated with the use of antipsychotic medications in MOH-affiliated hospitals in Saudi Arabia from April 2020 to September 2022. Reports prior to April 2020 were excluded due to system updates that created inconsistencies with subsequent data, thereby undermining the reliability of the data coding and analysis. Data from private and non-MOH hospitals were not included because these institutions use separate internal reporting systems that do not communicate with the MOH-ME reporting system. The study was conducted and reported in accordance with ‘The REporting of studies Conducted using Observational Routinely collected health Data Statement’ and the completed RECORD checklist is included as Supplementary Material, Table S1 [48].
2.2. Data Source
Data were extracted from the National ME Reporting System, a standardized platform developed by the MOH’s General Administration of Pharmaceutical Care. The system was established to detect reports of MEs from MOH-affiliated hospitals across Saudi Arabia and is the largest national database of its kind. Reporting MEs is mandatory for all MOH hospitals and follows a structured format to ensure the capture of all information related to MEs. Over the years, the system has been continuously refined to improve data quality and standardization, with reporting arrangements coordinated and overseen by the MOH [28]. Healthcare providers in MOH hospitals submit ME reports through their local reporting systems for investigation and analysis. These reports are then transmitted to the MOH’s General Administration of Pharmaceutical Care for central review. Each ME report includes patient demographics (i.e., age and gender), the region in which the MEs occurred in Saudi Arabia (i.e., Central, Western, Eastern, Northern, and Southern Regions), reporter profession, error type (e.g., wrong drug, wrong dose), error stage (i.e., prescribing, transcribing, dispensing, administration, or monitoring), factors identified by the reporter to increase the likelihood of ME occurrence, and ME outcome according to NCC MERP ME index (see Table 1) [3]. Although several frameworks have been developed for ME classification [49,50], the MOH uses the NCC MERP framework, which was adopted for use in this study as it provides a standardized and validated internationally recognized method for classifying MEs [3].

Table 1.
The NCC MERP Index for Categorizing ME Outcomes.
2.3. Data Screening and Extraction
ME reports that listed “antipsychotic medications” under the ME reporting system’s “medication name/category” field were retrieved and included in this study. Three of the study’s authors independently classified antipsychotic medications as “typical” or “atypical” in accordance with the MOH’s Drug Formulary categorizations, ensuring full adherence to the official MOH classification system to maintain consistency with the national reporting framework. They also independently coded existing MOH variables, such as patient demographics, ME type and stage, reporter profession, contributory factor, and ME outcome. Supplementary Table S2 presents the list of antipsychotic drugs included in the analysis.
Any missing values in the database variables were retained and categorized as “Not reported.” The action plan reported for each ME was not included in the analysis, as these are beyond the scope of this study. All ME reports included in this study were fully anonymized to maintain patient and reporter confidentiality.
2.4. Data Analysis
Descriptive analyses were conducted to summarize the main characteristics of antipsychotic-related MEs, including the frequency of MEs over time, patient age, patient gender, reporter profession, ME type, ME stage, ME outcome, and contributing factors, all of which were presented as numbers and percentages. Cross-tabulations were used to explore the association between these variables, including ME stages, error types, drug class (typical or atypical), and their associated outcomes. This association was tested using the chi-square test, with statistical significance set at p < 0.05. The analyses were conducted using SPSS Version 30 (2024).
3. Results
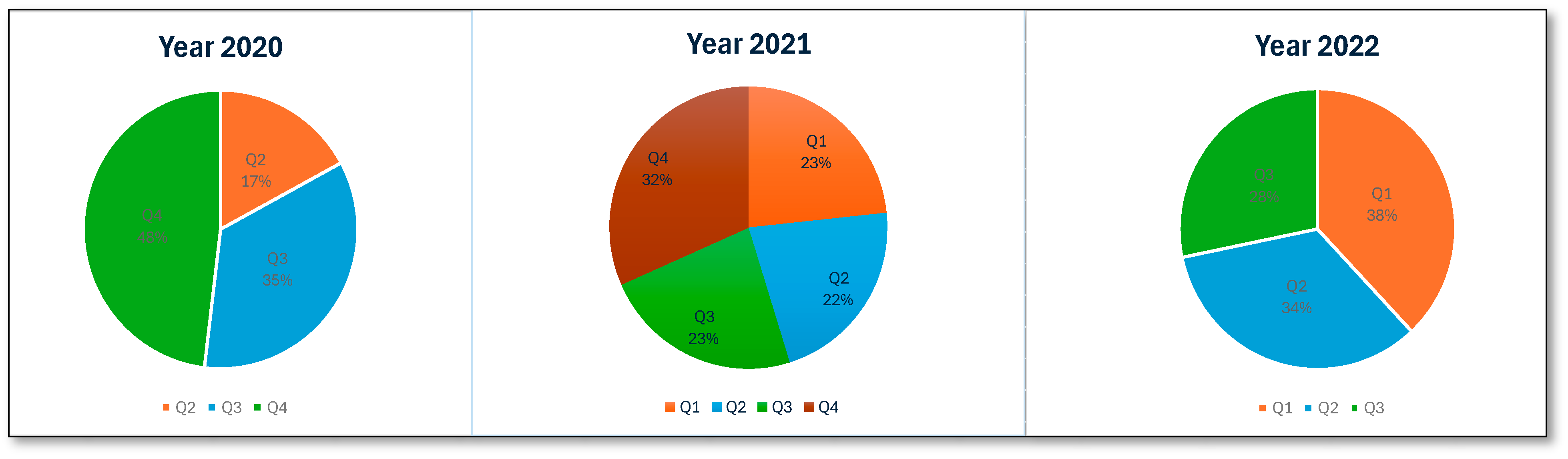
An analysis of ME reports related to patients receiving antipsychotic medications in Saudi Arabia between 2020 and 2022 identified 35,077 errors. The total number of MEs revealed a higher number in 2021 (45.1%, n = 15,819), while a significantly lower number was observed in 2020 (22%, n = 7707). Figure 1 presents the number of MEs per quarter. MEs were reported more frequently in male patients (76.1%, 26,705) than in female patients. The highest proportion of MEs was reported in adult patients (97.7%, n = 34,275). An examination of the reporting pattern among healthcare professionals showed that pharmacists and pharmacy assistants were most frequently involved in ME reporting: 66.5% (n = 23,312) and 21.8% (n = 7651), respectively. Physicians and patients less frequently report MEs, accounting for 3.6% (n = 1234) of the total reports, while nurses accounted for 5.2% (n = 1807) of the reported errors. An examination of the number of MEs reported in the five regions of Saudi Arabia revealed that the Western region accounted for the highest percentage of reports (32.8%, n = 11,496). The proportion of reports from other regions was much lower, with the Central region accounting for 18.5% (n = 6477) and the Northern, Southern, and Eastern regions reporting figures similar to or lower than the Central region. Regarding the stages at which MEs occurred, the prescribing stage accounted for the highest percentage (89.9%, n = 31,524), the dispensing stage accounted for 5.8% (n = 2047), and the transcribing and monitoring stages contributed minimally. The most negligible errors were reported at the administration stage (0.8%, n = 285). Table 2 presents the summary statistics for antipsychotic-related MEs.
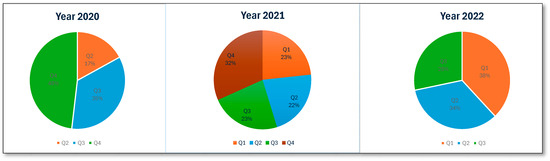
Figure 1.
Number of antipsychotic-related MEs per quarter.

Table 2.
ME Dataset Descriptive Statistics.
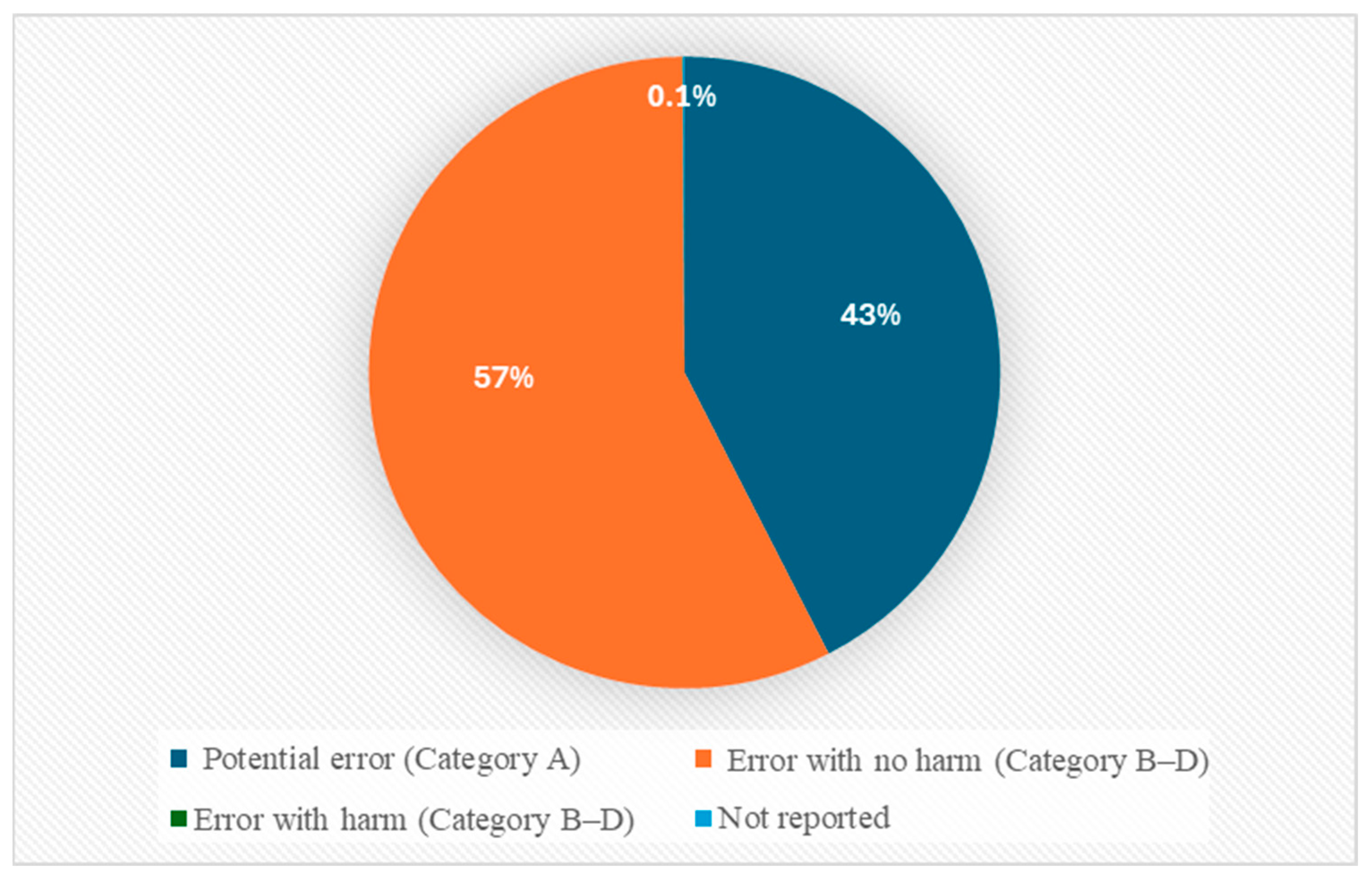
Most of the reported antipsychotic-related MEs were recorded in Categories A and B, representing potential error and MEs that did not reach patients (42.5%, n = 14,891 and 56.4%, n = 19,794, respectively). A smaller number were reported in Category C (MEs reaching the patient without harm: 0.9%, n = 326) and Category D (MEs requiring monitoring: 0.1%, n = 26). Few MEs were reported as involving actual harm (Categories E–I), which accounted for 0.04% (n = 13) of the reports. Overall, 98.9% of all reports represented potential harm (Categories A–D), while just 0.04% (n = 13) were linked to actual harm (Categories E and F). The remaining reports (0.1%, n = 27) did not provide any data on ME-related outcomes. No ME reports were classified as Category G or H (see Figure 2).
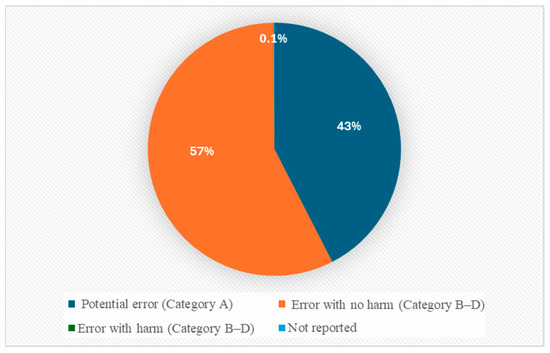
Figure 2.
ME categorization based on NCC MERP index.
A cross-tabulation analysis between ME stage and outcome was also conducted. Most antipsychotic-related MEs across all stages were reported to be associated with nonharmful outcomes. Prescribing MEs accounted for 31,487 reports (99.9%) that did not result in patient harm, with 12 MEs resulting in harmful outcomes. MEs in the dispensing (n = 2046; 100%), transcribing (n = 839; 100%), administration (n = 285; 100%), and monitoring stages (n = 354; 99.7%) were reported as having nonharmful outcomes. No significant association between ME stage and outcome was found using the chi-square test (χ2 = 5.236, p = 0.875) (see Table 3).

Table 3.
Antipsychotic-Related MEs by Stage and Outcome.
The number and percentage of MEs by type are summarized in Table 4. Most MEs were reported to be related to a lack of vital details, such as the patient’s age, allergies, and diagnostic information, collectively referred to as prescription information. Oversights in this category contributed to 40.5% (n = 14,206) of the identified MEs. Other MEs were reported as being related to the absence or incorrect documentation of dosage information (14.3%, n = 5033), with the remaining ME types contributing to 13.9% (n = 4874). A little over 15% (n = 5550) of the MEs were reported to be linked to incorrect dosing and frequency of administration or incorrect/unclear/missing medication names.

Table 4.
Descriptive analysis of ME dataset by ME type.
Of the total number of antipsychotic medications involved with MEs, around 80% (n = 27,811) of MEs were atypical. Olanzapine (33.1%; n = 11,607) and quetiapine (19.4%; 6804) were the most frequently reported drugs. Of the 20.7% (n = 7262) of reported MEs involving typical antipsychotics, the most frequently reported drugs were haloperidol (14.5%; 5098) and chlorpromazine (2.9%; 1025). The remining report was reported to involve both classes (n = 4). The top five reported typical and atypical antipsychotic drugs and their associated outcomes are presented in Table 5. No significant difference was observed between the antipsychotic drug type and ME outcome (χ2 = 16.241, p = 0.981).

Table 5.
Top Five Typical and Atypical Antipsychotics and Their Associated Outcomes.
Table 6 presents the factors that contributed to reported MEs. The three leading factors were staffing or workflow-related (21.3%, n = 7467), followed by the absence of policies related to prescribing and monitoring (20.5%, n = 7195) and environmental factors (16.7%, n = 5875). Poor handwriting (11%, n = 3876) and staff inexperience (9.2%, n = 3217) were also significant contributors. Issues with electronic systems, incorrect labeling, look-alike medications, and prohibited abbreviations contributed to a lesser extent. The other minor contributing factors were patient-related, such as those involving sound-alike medications and storage arrangements.

Table 6.
Frequency of potential contributory factors in reported MEs.
4. Discussion
This retrospective study assessed the pattern of MEs related to antipsychotic use in MOH-affiliated hospitals in Saudi Arabia. The total number of MEs related to antipsychotic use varied across the year; however, the higher number of ME reports from 2021 (n = 15,819) and 2022 (n = 11,551) compared to 2020 (n = 7707) likely reflects the inclusion of data over a full year (12 months) compared to the nine months (April to December) captured for 2020. Thus, the variation might be due to the timeframes included rather than a real temporal trend. Additionally, the COVID-19 pandemic might have influenced reporting rates [51,52,53]. While published studies showed inconsistent effects on reporting, a study based on Saudi Arabia specifically reported an increase from 1.5 to 19 MEs per 100 medication orders during the COVID-19 pandemic [54].
The data from this study revealed that MEs occurred most frequently in relation to male and adult patients. Antipsychotic medication use has been reported to increase with age [40], which may account, in part, for the higher rates of MEs observed in the adult population. The findings of our study are consistent with previous research reporting a higher percentage of MEs in males treated with clozapine [20]. Moreover, the fact that males are at a higher risk of developing schizophrenia than females [55] suggests that the former may be more susceptible to MEs due to the increased likelihood of being treated with medication. On the other hand, the higher proportion of adult males reported to be involved in MEs likely reflects the types of hospitals contributing to the MOH’s ME reporting system, as women and children may not be fully represented in this reporting system. This pattern may also be influenced by reporting bias related to errors involving these groups of patients [56]. A considerable regional disparity was also found in the reporting of antipsychotic-related MEs. Approximately one-third of the reported MEs originated in the Western region, while the remaining two-thirds were distributed across the other four. Future research could explore the potential factors for this regional disparity. Our findings suggest that pharmacists are the most likely to report MEs, while physicians and patients make far fewer reports. The fact that pharmacists are the most frequently involved in ME reporting has been substantiated in studies conducted in Saudi Arabia and other parts of the world [4,28,57].
Our study highlights the extent to which atypical antipsychotics are involved in MEs, specifically that they are reported more frequently than MEs associated with typical antipsychotics. These results are unsurprising, since atypical antipsychotic drugs are used more frequently than typical antipsychotics due to their efficacy in treating a wide range of negative schizophrenia symptoms and because they carry a lower risk of motor side effects, such as extrapyramidal symptoms and tardive dyskinesia, which negatively impact patients’ quality of life and may subsequently reduce their long-term treatment adherence [58,59,60]. Moreover, atypical antipsychotics, such as clozapine and risperidone, have been associated with potentially hazardous prescribing while treating psychiatric disorders [61,62]. Therefore, atypical antipsychotics should be prioritized to reduce the risk of MEs associated with their use.
The results of our study suggest that most MEs related to antipsychotic medication use throughout Saudi Arabia are caused by prescription errors. Approximately two-fifths of the prescriptions lacked complete details, which reveals a gap in the documentation process. Around 15% of the MEs were attributed to incomplete or missing dosage details, and a little over 20% omitted crucial medication information such as the name, frequency, and treatment duration. Other studies have shown that MEs are frequently reported in mental health settings and during prescription ordering or transcribing [6,63,64,65]. For instance, a 2007 study by Rothschild et al. reported an error rate of 68% related to prescription orders [66], and a study conducted in a French psychiatric hospital found that PEs accounted for 55.3% of the total number of MEs committed [4]. Further evidence from a UK that used Clinical Practice Research Datalink (CPRD) data has revealed a notable link between mental health prescribing patterns and patient safety outcomes, which supports our findings that antipsychotics, particularly in the prescribing stage, should take priority when implementing interventions to ensure safe medication use [67]. Other published studies conducted in Saudi Arabia showed that MEs are common even in nonpsychiatric settings, such as in primary care settings, which account for one-fifth of all reported MEs [68]. A systematic review examining MEs in the Middle East identified PEs as the most common ME type [5,55]. Another study conducted in secondary healthcare centers in Kuwait found that 62% of MEs are related to PEs [69]. However, it is important to note that while PEs represent a higher percentage of the reported errors, administration (0.8%) and monitoring (1.0%) accounted for only small proportions. This pattern should be interpreted cautiously, as this is likely to reflect the reporting pattern rather than genuinely low risk. Inconsistent capture of these errors across sites may introduce reporting bias and should be considered when interpreting these results [56].
Our findings demonstrate that the vast majority of antipsychotic-related MEs were classified as near misses or errors without patient impact (Categories A–D) and that only a very small proportion were associated with actual harm (Categories E–I). This distribution is consistent with the nature of reporting systems, which tend to capture near-miss and no-harm events rather than harmful outcomes. The predominance of near-miss reporting highlights opportunities for early intervention to prevent future patient harm [70].
Our study identified three primary factors that contribute to MEs, including staffing, workflow, and the absence of suitable policies related to antipsychotic medication use, a finding that aligns with the results of other studies conducted in psychiatric settings [7,16,20,31]. Increased patient turnover increases the workload, which may not be matched by the existing workforce and may, in turn, enhance the likelihood of errors. This particular issue has been identified in studies whose authors found that overburdened staff cannot work diligently, which compromises the quality of care [71]. Moreover, environmental factors, such as overly crowded workspaces, distracting noises, and inadequate illumination, also contributed to MEs. These findings are consistent with those reported by Keers et al., who highlighted the increased chance of slip-ups or lapses in workplaces where distractions are present [16,38].
Implications for Practice and Future Research
Several interventions have been recommended to reduce the risk of MEs in hospitals, such as staff education, the integration of technology in the medication use process (e.g., Pharmacist-Led Information Technology Intervention for Medication Errors [PINCER]) [72], and the involvement of ward-based clinical pharmacists [73,74,75]. A targeted implementation study could evaluate both the effectiveness and feasibility in preventing antipsychotic-related MEs. The predominance of MEs in the prescribing stage, as well as the high percentage of reported near-miss and no-harm MEs, suggest an opportunity to focus on strengthening early safeguards within the medication use process, specifically by implementing structured identification and monitoring of unsafe prescribing practices. The use of prescribing safety indicators (PISs) has been found to be effective in psychiatric settings and offers an evidence-based approach for enhancing antipsychotic prescribing in psychiatric hospitals in Saudi Arabia [61].
At the system level, this study highlights the importance of maintaining the MOH-ME reporting system to support ongoing improvements in the safe use of antipsychotic medications. Future developments might focus on expanding reporting system capacities to allow the documentation of multiple ME types and contributory factors in individual ME reports to better reflect the complexity of the reported MEs. Additionally, refining category definitions—for example, differentiating between “incorrect/unclear/missing dose” and “dose omission”—might facilitate the targeted prevention of specific ME types. In particular, the “Other” option, which comprised 13.9% of reports and represented MEs that could not be classified using the predefined MOH-ME type classifications, presents a valuable opportunity for future system improvement. Future research is warranted to investigate the root cause of antipsychotic-related MEs using qualitative approaches, such as Reason’s Model of Accident Causation [16,76,77], that might shed light on the contextual factors involved in MEs. Additionally, interventional studies are warranted such as pharmacist-led review, electronic prescribing and medicine administration, or educational initiatives aimed at reducing antipsychotic-related MEs [78,79].
This study has several strengths. First, it presents a large-scale investigation of antipsychotic-related MEs in the Middle East and provides novel insights into medication safety in this regional context. Second, a dataset of 35,077 reports was sufficient to explore subgroup patterns across demographics, regions, and antipsychotic drug classes. Finally, applying the NCC MERP classification framework facilitated comprehensive ME outcome categorization that facilitated meaningful comparisons with international studies. Nevertheless, this study has several limitations. First, due to the inherent limitations of all voluntary ME reporting systems, MEs are likely to be underreported, and the data reflects reporting patterns (i.e., differences in infrastructure, staffing, or reporting culture) rather than the actual occurrence of MEs. Second, the lack of denominator data, such as total prescriptions and number of patient-days, limited the ability to calculate ME rates. Future studies are encouraged to collect denominator data to support more meaningful comparisons with national and international studies. Third, the ME outcome assessment was classified using the NCC MERP framework, which reflects the reporter’s perception of harm rather than actual clinical consequences. Additionally, the study period coincided with the COVID-19 pandemic, which may have influenced ME reporting pattern due to changes in healthcare delivery processes, staffing, and workload during that period. Moreover, our study employed the MOH classification system, with no reclassification conducted by the research team, which makes interrater reliability checks unnecessary; however, while this approach supports consistency, the potential for misclassification cannot be ruled out. Finally, the database does not capture information related to patients’ underlying conditions and comorbidities, which limit interpretations based on the clinical context. Future system enhancements would allow reporters to capture the complexity of antipsychotic-related MEs and better support the development of targeted future safety interventions.
5. Conclusions
This is the first national study of patterns and contributory factors of MEs related to antipsychotic use in MOH-affiliated hospitals in Saudi Arabia. The study found that most MEs originated in the prescribing and dispensing stages, with MEs related to drug doses and prescription information being the most frequently observed. Regarding the potential contributory factors associated with antipsychotic use, we identified staff shortages and lack of policies among the most frequently reported. To improve safety, targeted training for high-risk antipsychotics, pharmacist-led intervention and computerized prescribing with electronic medication administration could be considered to ensure safe antipsychotic medication use.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare13212705/s1, Table S1: RECORD Checklist; Table S2: List of antipsychotic drugs included in the analysis. Reference [80] is cited in the supplementary materials.
Author Contributions
Conceptualization, G.H.A., L.I.A.A., S.M.A., W.S.A., R.A.A., A.S.A., R.F.H., S.F.A., A.M.A., N.T.A., L.S.A., N.A.A. and B.G.A.; Data curation, W.S.A., R.A.A., A.S.A., R.F.H., S.F.A., A.M.A., N.T.A. and B.G.A.; Formal analysis, O.J.F.B. and L.S.A.; Funding acquisition, G.H.A.; Investigation, G.H.A., L.I.A.A., S.M.A., L.S.A. and N.A.A.; Methodology, L.I.A.A., S.M.A., W.S.A., R.A.A., A.S.A., R.F.H., S.F.A., A.M.A., N.T.A., L.S.A. and B.G.A.; Project administration, G.H.A., L.I.A.A., S.M.A. and W.S.A.; Supervision, G.H.A.; Validation, G.H.A., W.S.A., R.A.A., R.F.H., S.F.A., A.M.A., N.T.A., O.J.F.B., N.A.A. and B.G.A.; Writing—original draft, G.H.A. and O.J.F.B.; Writing—review and editing, G.H.A., L.I.A.A., S.M.A., W.S.A., R.A.A., A.S.A., R.F.H., S.F.A., A.M.A., N.T.A., O.J.F.B., L.S.A., N.A.A. and B.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Princess Nourah bint Abdulrahman University Researchers Support Initiative (PNURSP2025R352), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
This study was granted ethical approval by the Central Institutional Review Board of the Ministry of Health (Reference no. 22-55 E, 16 November 2022).
Informed Consent Statement
The informed consent was waived because we used secondary data from MOH database.
Data Availability Statement
The data that support the findings of this study are available from the Ministry of Health. Restrictions apply to the availability of these data, which were used under the license for this study. Data are available from the author(s) with the permission of the Ministry of Health.
Acknowledgments
We would like to acknowledge Princess Nourah bint Abdulrahman University Researchers Support Project (PNURSP2025R352) for funding this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Mental Health Action Plan 2013–2020; World Health Organization: Geneva, Switzerland, 2013; Available online: https://iris.who.int/handle/10665/89966 (accessed on 25 October 2024).
- Hodkinson, A.; Tyler, N.; Ashcroft, D.M.; Keers, R.N.; Khan, K.; Phipps, D.; Abuzour, A.; Bower, P.; Avery, A.; Campbell, S. Preventable medication harm across health care settings: A systematic review and meta-analysis. BMC Med. 2020, 18, 313. [Google Scholar] [CrossRef]
- National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP). Medication Error Index. Available online: https://www.nccmerp.org/sites/default/files/index-color-2021-draft-change-10-2022.pdf (accessed on 25 October 2024).
- Lebas, R.; Calvet, B.; Schadler, L.; Preux, P.-M.; Laroche, M.-L. Relationships between medications used in a mental health hospital and types of medication errors: A cross-sectional study over an 8-year period. Res. Soc. Adm. Pharm. 2024, 20, 597–604. [Google Scholar] [CrossRef]
- Shehata, Z.H.A.; Sabri, N.A.; Elmelegy, A.A. Descriptive analysis of medication errors reported to the Egyptian national online reporting system during six months. J. Am. Med. Inform. Assoc. 2016, 23, 366–374. [Google Scholar] [CrossRef]
- Alshehri, G.H.; Ashcroft, D.M.; Nguyen, J.; Hann, M.; Jones, R.; Seaton, K.; Newton, G.; Keers, R.N. Prevalence, nature, severity and preventability of adverse drug events in mental health settings: Findings from the MedicAtion relateD harm in mEntal health hospitals (MADE) Study. Drug Saf. 2021, 44, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Alenzi, K.A.; Alsheikh, M.Y.; Alsuhaibani, D.S.; Alatawi, Y.; Alshammari, T.M. Medication Errors in Psychiatric Hospitals: A Nationwide Real-World Evidence Study in Saudi Arabia. Pharmaceuticals 2024, 17, 1514. [Google Scholar] [CrossRef] [PubMed]
- Højlund, M.; Pottegård, A.; Johnsen, E.; Kroken, R.A.; Reutfors, J.; Munk-Jørgensen, P.; Correll, C.U. Trends in utilization and dosing of antipsychotic drugs in Scandinavia: Comparison of 2006 and 2016. Br. J. Clin. Pharmacol. 2019, 85, 1598–1606. [Google Scholar] [CrossRef]
- Marston, L.; Nazareth, I.; Petersen, I.; Walters, K.; Osborn, D.P. Prescribing of antipsychotics in UK primary care: A cohort study. BMJ Open 2014, 4, e006135. [Google Scholar] [CrossRef]
- Carton, L.; Cottencin, O.; Lapeyre-Mestre, M.; A Geoffroy, P.; Favre, J.; Simon, N.; Bordet, R.; Rolland, B. Off-label prescribing of antipsychotics in adults, children and elderly individuals: A systematic review of recent prescription trends. Curr. Pharm. Des. 2015, 21, 3280–3297. [Google Scholar] [CrossRef]
- Jobski, K.; Höfer, J.; Hoffmann, F.; Bachmann, C. Use of psychotropic drugs in patients with autism spectrum disorders: A systematic review. Acta Psychiatr. Scand. 2017, 135, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Mok, P.L.; Carr, M.J.; Guthrie, B.; Morales, D.R.; Sheikh, A.; Elliott, R.A.; Camacho, E.M.; Van Staa, T.; Avery, A.J.; Ashcroft, D.M. Multiple adverse outcomes associated with antipsychotic use in people with dementia: Population based matched cohort study. BMJ 2024, 385, e076268. [Google Scholar] [CrossRef]
- Pirhonen, E.; Haapea, M.; Rautio, N.; Nordström, T.; Turpeinen, M.; Laatikainen, O.; Koponen, H.; Silvan, J.; Miettunen, J.; Jääskeläinen, E. Characteristics and predictors of off-label use of antipsychotics in general population sample. Acta Psychiatr. Scand. 2022, 146, 227–239. [Google Scholar] [CrossRef]
- Correll, C.U.; Rubio, J.M.; Kane, J.M. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 2018, 17, 149–160. [Google Scholar] [CrossRef]
- Wang, D.; Schneider-Thoma, J.; Siafis, S.; Qin, M.; Wu, H.; Zhu, Y.; Davis, J.M.; Priller, J.; Leucht, S. Efficacy, acceptability and side-effects of oral versus long-acting-injectables antipsychotics: Systematic review and network meta-analysis. Eur. Neuropsychopharmacol. 2024, 83, 11–18. [Google Scholar] [CrossRef]
- Keers, R.N.; Plácido, M.; Bennett, K.; Clayton, K.; Brown, P.; Ashcroft, D.M. What causes medication administration errors in a mental health hospital? A qualitative study with nursing staff. PLoS ONE 2018, 13, e0206233. [Google Scholar] [CrossRef] [PubMed]
- Stassen, H.; Bachmann, S.; Bridler, R.; Cattapan, K.; Herzig, D.; Schneeberger, A.; Seifritz, E. Detailing the effects of polypharmacy in psychiatry: Longitudinal study of 320 patients hospitalized for depression or schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Lelliott, P.; Paton, C.; Harrington, M.; Konsolaki, M.; Sensky, T.; Okocha, C. The influence of patient variables on polypharmacy and combined high dose of antipsychotic drugs prescribed for in-patients. Psychiatr. Bull. 2002, 26, 411–414. [Google Scholar] [CrossRef]
- Dabba, K.; Elswood, M.; Ameer, A.; Gerrett, D.; Maidment, I. A mixed methods analysis of clozapine errors reported to the National reporting and learning system. Pharmacoepidemiol. Drug Saf. 2019, 28, 657–664. [Google Scholar] [CrossRef] [PubMed]
- AlAmri, L.S.; Alluwaymi, W.S.; Alghamdi, B.G.; Alghanim, R.A.; Almordi, A.S.; Hettah, R.F.; Almushaikah, S.F.; AlShahrani, A.M.; Alshammri, N.T.; Aldossari, S.M. Characteristics and causes of reported clozapine-related medication errors: Analysis of the Ministry of Health database in Saudi Arabia. Int. J. Clin. Pharm. 2024, 46, 1410–1418. [Google Scholar] [CrossRef]
- Shahpesandy, H.; Tye, N.; Hegarty, A.; Czechovska, J.; Kwentoh, M.L.; Wood, A. Rapid tranquillisation of acutely disturbed and violent patients: A retrospective cohort examination of 24 patients on a psychiatric intensive care unit. J. Psychiatr. Intensive Care 2015, 11, e1. [Google Scholar] [CrossRef]
- Alshehri, G.H.; Keers, R.N.; Ashcroft, D.M. Frequency and nature of medication errors and adverse drug events in mental health hospitals: A systematic review. Drug Saf. 2017, 40, 871–886. [Google Scholar] [CrossRef]
- Alshaikhmubarak, F.Q.; Keers, R.N.; Lewis, P.J. Potential risk factors of drug-related problems in hospital-based mental health units: A systematic review. Drug Saf. 2023, 46, 19–37. [Google Scholar] [CrossRef]
- Mann, K.; Rothschild, J.M.; Keohane, C.A.; Chu, J.A.; Bates, D.W. Adverse drug events and medication errors in psychiatry: Methodological issues regarding identification and classification. World J. Biol. Psychiatry 2008, 9, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, T.K.; Seger, D.L.; Bates, D.W. Identifying drug safety issues: From research to practice. Int. J. Qual. Health Care 2000, 12, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Cousins, D.H.; Gerrett, D.; Warner, B. A review of medication incidents reported to the National Reporting and Learning System in England and Wales over 6 years (2005–2010). Br. J. Clin. Pharmacol. 2012, 74, 597–604. [Google Scholar] [CrossRef]
- British Columbia Patient Safety & Learning System. BC Patient Safety & Learning System. 2008. Available online: http://bcpslscentral.ca/wp-content/uploads/2014/04/PSLSEvaluationReport_FINAL_Jan2508_website1.pdf (accessed on 1 July 2025).
- Alshammari, T.M.; Alenzi, K.A.; Alatawi, Y.; Almordi, A.S.; Altebainawi, A.F. Current situation of medication errors in Saudi Arabia: A nationwide observational study. J. Patient Saf. 2022, 18, e448–e453. [Google Scholar] [CrossRef]
- Vincent, C. Incident reporting and patient safety. BMJ 2007, 334, 51. [Google Scholar] [CrossRef]
- Kamboj, A.; Spiller, H.A.; Casavant, M.J.; Chounthirath, T.; Hodges, N.L.; Smith, G.A. Antidepressant and antipsychotic medication errors reported to United States poison control centers. Pharmacoepidemiol. Drug Saf. 2018, 27, 902–911. [Google Scholar] [CrossRef]
- Alshehri, G.H.; Keers, R.N.; Carson-Stevens, A.; Ashcroft, D.M. Medication safety in mental health hospitals: A mixed-methods analysis of incidents reported to the national reporting and learning system. J. Patient Saf. 2021, 17, 341–351. [Google Scholar] [CrossRef]
- Tansuwannarat, P.; Vichiensanth, P.; Sivarak, O.; Tongpoo, A.; Promrungsri, P.; Sriapha, C.; Wananukul, W.; Trakulsrichai, S. A 10-Year Retrospective Analysis of Medication Errors among Adult Patients: Characteristics and Outcomes. Pharmacy 2023, 11, 138. [Google Scholar] [CrossRef]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Coping with medical error: A systematic review of papers to assess the effects of involvement in medical errors on healthcare professionals’ psychological well-being. Qual. Saf. Health Care 2010, 19, e43. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, T.L.; Houseman, B.; Vaqar, S.; Hipskind, J.E. Medical Error Reduction and Prevention. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Schwendimann, R.; Blatter, C.; Dhaini, S.; Simon, M.; Ausserhofer, D. The occurrence, types, consequences and preventability of in-hospital adverse events—A scoping review. BMC Health Serv. Res. 2018, 18, 521. [Google Scholar] [CrossRef]
- San Jose-Saras, D.; Valencia-Martín, J.L.; Vicente-Guijarro, J.; Moreno-Nunez, P.; Pardo-Hernández, A.; Aranaz-Andres, J.M. Adverse events: An expensive and avoidable hospital problem. Ann. Med. 2022, 54, 3156–3167. [Google Scholar] [CrossRef] [PubMed]
- Fernholm, R.; Holzmann, M.J.; Wachtler, C.; Szulkin, R.; Carlsson, A.C.; Pukk Härenstam, K. Patient-related factors associated with an increased risk of being a reported case of preventable harm in first-line health care: A case-control study. BMC Fam. Pract. 2020, 21, 20. [Google Scholar] [CrossRef]
- Keers, R.N.; Williams, S.D.; Cooke, J.; Ashcroft, D.M. Causes of medication administration errors in hospitals: A systematic review of quantitative and qualitative evidence. Drug Saf. 2013, 36, 1045–1067. [Google Scholar] [CrossRef]
- Koenig, H.G.; Al Zaben, F.; Sehlo, M.G.; Khalifa, D.A.; Al Ahwal, M.S. Current state of psychiatry in Saudi Arabia. Int. J. Psychiatry Med. 2013, 46, 223–242. [Google Scholar] [CrossRef]
- Qureshi, N.A.; Al-Habeeb, A.A.; Koenig, H.G. Mental health system in Saudi Arabia: An overview. Neuropsychiatr. Dis. Treat. 2013, 9, 1121–1135. [Google Scholar] [CrossRef]
- Al-Habeeb, A.A.; Qureshi, N.A.; Al-Maliki, T.A. Pattern of child and adolescent psychiatric disorders among patients consulting publicly-funded child psychiatric clinics in Saudi Arabia. East. Mediterr. Health J. 2012, 18, 112–119. [Google Scholar] [CrossRef]
- Koenig, H.G.; Al Zaben, F.; Sehlo, M.G.; Khalifa, D.A.; Al Ahwal, M.S.; Qureshi, N.A.; Al-Habeeb, A.A. Mental health care in Saudi Arabia: Past, present and future. Open J. Psychiatry 2014, 4, 113. [Google Scholar] [CrossRef]
- World Health Organization. Global Patient Safety Action Plan 2021–2030: Towards Eliminating Avoidable Harm in Health Care; World Health Organization: Geneva, Switzerland, 2021; Available online: https://iris.who.int/bitstream/handle/10665/343477/9789240032705-eng.pdf?sequence=1 (accessed on 25 October 2024).
- Chowdhury, S.; Mok, D.; Leenen, L. Transformation of health care and the new model of care in Saudi Arabia: Kingdom’s Vision 2030. J. Med. Life 2021, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Parentela, G.; Alharbi, H.; Alahmadi, A.; Alburkani, H.; Aljumayi, I. Socio-demography and psychosis symptom severity among male schizophrenia—Diagnosed patients of MOH Mental Health Facilities, Kingdom of Saudi Arabia; A correlational study. Arch. Psychiatr. Nurs. 2019, 33, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Noori, M.; Nejadghaderi, S.A.; Shamekh, A.; Sullman, M.J.; Collins, G.S.; Kolahi, A.-A. The burden of schizophrenia in the Middle East and North Africa region, 1990–2019. Sci. Rep. 2024, 14, 9720. [Google Scholar] [CrossRef] [PubMed]
- Semahegn, A.; Torpey, K.; Manu, A.; Assefa, N.; Tesfaye, G.; Ankomah, A. Psychotropic medication non-adherence and its associated factors among patients with major psychiatric disorders: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 17. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M.; Committee, R.W. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Carson-Stevens, A.; Hibbert, P.; Avery, A.; Butlin, A.; Carter, B.; Cooper, A.; Evans, H.P.; Gibson, R.; Luff, D.; Makeham, M.; et al. A cross-sectional mixed methods study protocol to generate learning from patient safety incidents reported from general practice. BMJ Open 2015, 5, e009079. [Google Scholar] [CrossRef]
- World Health Organization & WHO Patient Safety. Conceptual Framework for the International Classification for Patient Safety, Version 1.1: Final Technical Report January 2009 (WHO/IER/PSP/2010.2); World Health Organization: Geneva, Switzerland, 2010; Available online: https://iris.who.int/handle/10665/70882 (accessed on 1 July 2025).
- Bonheur, A.N.; Philips, K.; Hametz, P.; Choi, J.; Xie, X.; Soshnick, S.H.; Cabana, M.D.; Cassel-Choudhury, G. Incident reporting and harmful safety events during the COVID-19 pandemic in a children’s hospital. BMC Res. Notes 2025, 18, 266. [Google Scholar] [CrossRef]
- Al Meslamani, A.Z. Medication errors during a pandemic: What have we learnt? Expert Opin. Drug Saf. 2023, 22, 115–118. [Google Scholar] [CrossRef]
- Wysocki, V.; Grabe, D.; Meek, P. SA44 Trends in Medication Error Reporting during the COVID-19 Pandemic: An Analysis of Faers Data, 2016 to 2021. Value Health 2022, 25, S612. [Google Scholar] [CrossRef]
- Almazrou, D.; Egunsola, O.; Ali, S.; Bagalb, A. Medication misadventures among COVID-19 patients in Saudi Arabia. Cureus 2021, 13, e15513. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lawton, R.; Parker, D. Barriers to incident reporting in a healthcare system. BMJ Qual. Saf. 2002, 11, 15–18. [Google Scholar] [CrossRef]
- Sarvadikar, A.; Prescott, G.; Williams, D. Attitudes to reporting medication error among differing healthcare professionals. Eur. J. Clin. Pharmacol. 2010, 66, 843–853. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Gadaleta, E. Contrasting typical and atypical antipsychotic drugs. Focus 2021, 19, 3–13. [Google Scholar] [CrossRef]
- Wright, P.; O’Flaherty, L. Antipsychotic drugs: Atypical advantages and typical disadvantages. Ir. J. Psychol. Med. 2003, 20, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Alkhadhari, S.; Al Zain, N.; Darwish, T.; Khan, S.; Okasha, T.; Ramy, H.; Tadros, T.M. Use of second-generation antipsychotics in the acute inpatient management of schizophrenia in the Middle East. Neuropsychiatr. Dis. Treat. 2015, 11, 915–924. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khawagi, W.Y.; Steinke, D.T.; Nguyen, J.; Pontefract, S.; Keers, R.N. Development of prescribing safety indicators related to mental health disorders and medications: Modified e-Delphi study. Br. J. Clin. Pharmacol. 2021, 87, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Ascher-Svanum, H.; Zhu, B.; Faries, D.; Landbloom, R.; Swartz, M.; Swanson, J. Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia. Bmc Psychiatry 2006, 6, 8. [Google Scholar] [CrossRef]
- Stubbs, J.; Haw, C.; Taylor, D. Prescription errors in psychiatry–a multi-centre study. J. Psychopharmacol. 2006, 20, 553–561. [Google Scholar] [CrossRef]
- Stubbs, J.; Haw, C.; Cahill, C. Auditing prescribing errors in a psychiatric hospital. Are pharmacists’ interventions effective? Hosp. Pharm. 2004, 11, 203–207. [Google Scholar]
- Tabatabaee, S.S.; Ghavami, V.; Javan-Noughabi, J.; Kakemam, E. Occurrence and types of medication error and its associated factors in a reference teaching hospital in northeastern Iran: A retrospective study of medical records. BMC Health Serv. Res. 2022, 22, 1420. [Google Scholar] [CrossRef]
- Rothschild, J.M.; Mann, K.; Keohane, C.A.; Williams, D.H.; Foskett, C.; Rosen, S.L.; Flaherty, L.; Chu, J.A.; Bates, D.W. Medication safety in a psychiatric hospital. Gen. Hosp. Psychiatry 2007, 29, 156–162. [Google Scholar] [CrossRef]
- Khawagi, W.Y.; Steinke, D.; Carr, M.J.; Wright, A.K.; Ashcroft, D.M.; Avery, A.; Keers, R.N. Evaluating the safety of mental health-related prescribing in UK primary care: A cross-sectional study using the clinical practice research Datalink (CPRD). BMJ Qual. Saf. 2022, 31, 364–378. [Google Scholar] [CrossRef]
- Khoja, T.; Neyaz, Y.; Qureshi, N.A.; Magzoub, M.A.; Haycox, A.; Walley, T. Medication errors in primary care in Riyadh City, Saudi Arabia. East Mediterr Health J. 2011, 17, 156–159, Erratum in: East Mediterr Health J. 2011, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Alsaleh, F.M.; Alsaeed, S.; Alsairafi, Z.K.; Almandil, N.B.; Naser, A.Y.; Bayoud, T. Medication errors in secondary care hospitals in Kuwait: The perspectives of healthcare professionals. Front. Med. 2021, 8, 784315. [Google Scholar] [CrossRef] [PubMed]
- Caspi, H.; Perlman, Y.; Westreich, S. Managing near-miss reporting in hospitals: The dynamics between staff members’ willingness to report and management’s handling of near-miss events. Saf. Sci. 2023, 164, 106147. [Google Scholar] [CrossRef]
- Ayre, M.J.; Lewis, P.J.; Phipps, D.L.; Keers, R.N. unDerstandIng the cauSes of mediCation errOrs and adVerse drug evEnts for patients with mental illness in community caRe (DISCOVER): A qualitative study. Front. Psychiatry 2023, 14, 1241445. [Google Scholar] [CrossRef] [PubMed]
- Avery, A.J.; Rodgers, S.; Cantrill, J.A.; Armstrong, S.; Cresswell, K.; Eden, M.; Elliott, R.A.; Howard, R.; Kendrick, D.; Morris, C.J. A pharmacist-led information technology intervention for medication errors (PINCER): A multicentre, cluster randomised, controlled trial and cost-effectiveness analysis. Lancet 2012, 379, 1310–1319. [Google Scholar] [CrossRef]
- Onder, G.; Van Der Cammen, T.J.; Petrovic, M.; Somers, A.; Rajkumar, C. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing 2013, 42, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Krähenbühl-Melcher, A.; Schlienger, R.; Lampert, M.; Haschke, M.; Drewe, J.; Krähenbühl, S. Drug-related problems in hospitals: A review of the recent literature. Drug Saf. 2007, 30, 379–407. [Google Scholar] [CrossRef]
- Khalil, H.; Kynoch, K.; Hines, S. Interventions to ensure medication safety in acute care: An umbrella review. JBI Evid. Implement. 2020, 18, 188–211. [Google Scholar] [CrossRef]
- Sawamura, K.; Ito, H.; Yamazumi, S.; Kurita, H. Interception of potential adverse drug events in long-term psychiatric care units. Psychiatry Clin. Neurosci. 2005, 59, 379–384. [Google Scholar] [CrossRef]
- Ito, H.; Yamazumi, S. Common types of medication errors on long-term psychiatric care units. Int. J. Qual. Health Care 2003, 15, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, R.D.; Suess, T.M.; Lesko, M.G.; Feroli, A.A.; Kennel, J.A.; Mahler, J.M.; Sauders, T. Using bar-code technology and medication observation methodology for safer medication administration. Am. J. Health-Syst. Pharm. 2007, 64, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, C.; Roustit, M.; Bal, G.; Schwebel, C.; Pansu, P.; David-Tchouda, S.; Foroni, L.; Calop, J.; Timsit, J.-F.; Allenet, B. Automated drug dispensing system reduces medication errors in an intensive care setting. Crit. Care Med. 2010, 38, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Schmidt, S.; Wing, K.; Ehrenstein, V.; Nicholls, S.; Filion, K.; Klungel, O.; Petersen, I.; Sorensen, H.; Guttmann, A.; et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement for Pharmacoepidemiology (RECORD-PE). BMJ 2018, 363, k3532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).