Telomere Length and COVID-19 Severity: A Comparative Cross-Sectional Study Across the Clinical Spectrum
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Parameters
2.3. Measurement of Telomeres
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Study Participants
3.2. Medical History and Respiratory Support of the Study Participants
3.3. Biochemical Characteristics of the Study Participants
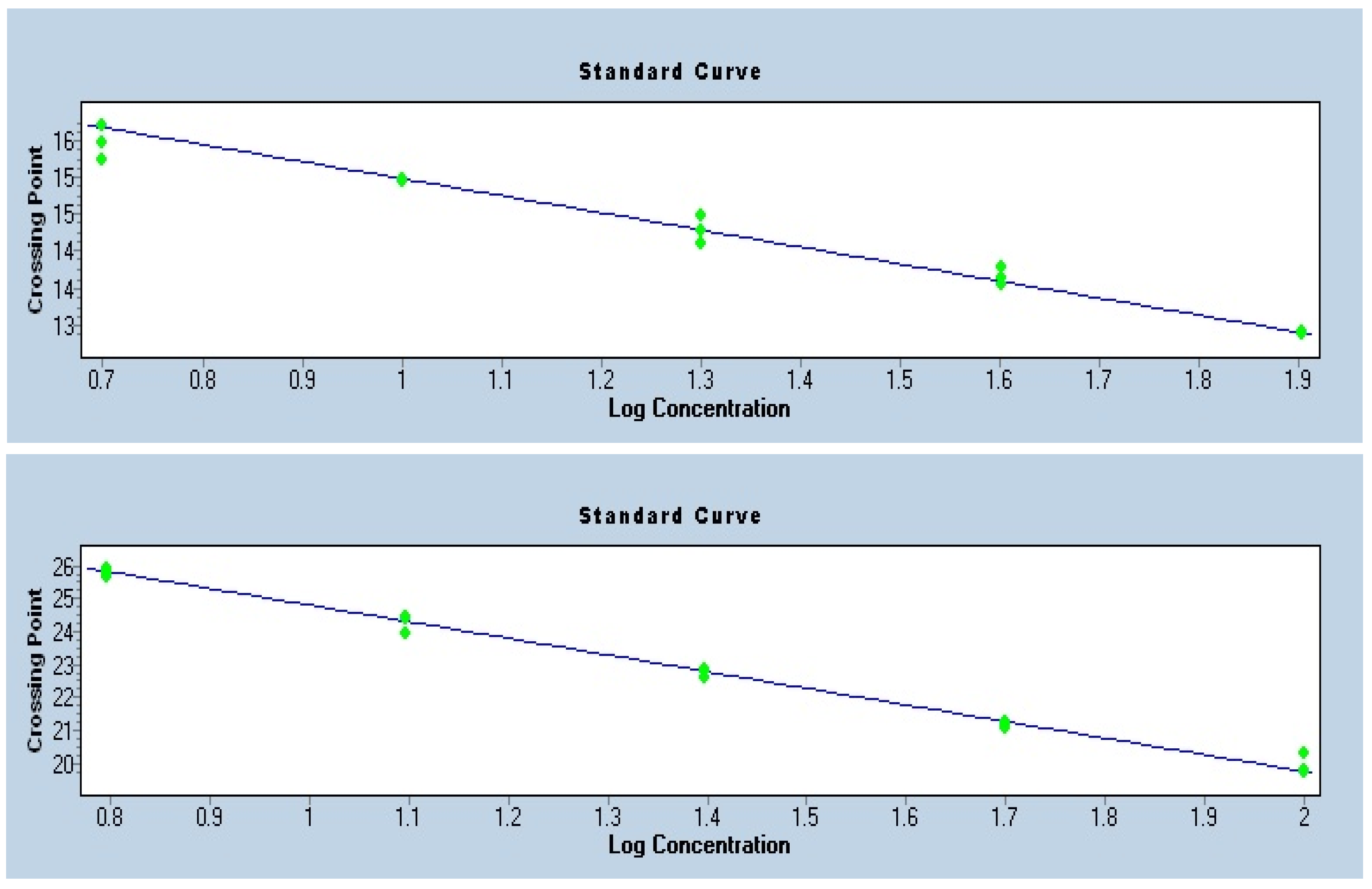
3.4. Quality Control
4. Discussion
4.1. Telomere Length in Severe vs. Non-Severe COVID-19
4.2. Mechanisms Linking COVID-19 Infection and Telomere Length
4.3. Association of Telomere Length with Measured Parameters
4.4. Telomere Length in COVID-19 Patients vs. Controls
4.5. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Tunnicliffe, L.; Muzambi, R.; Bartlett, J.W.; Howe, L.; Abdul Basit, K.; Warren-Gash, C. Infection and telomere length: A systematic review protocol. BMJ Open 2024, 14, e081881. [Google Scholar] [CrossRef]
- Dowd, J.B.; Bosch, J.A.; Steptoe, A.; Jayabalasingham, B.; Lin, J.; Yolken, R.; Aiello, A.E. Persistent Herpesvirus Infections and Telomere Attrition Over 3 Years in the Whitehall II Cohort. J. Infect. Dis. 2017, 216, 565–572. [Google Scholar] [CrossRef]
- Noppert, G.A.; Feinstein, L.; Dowd, J.B.; Stebbins, R.C.; Zang, E.; Needham, B.L.; Meier, H.C.S.; Simanek, A.; Aiello, A.E. Pathogen burden and leukocyte telomere length in the United States. Immun. Ageing 2020, 17, 36. [Google Scholar] [CrossRef]
- Benetos, A.; Lai, T.-P.; Toupance, S.; Labat, C.; Verhulst, S.; Perret-Guillaume, C.; Gautier, S.; Ungeheuer, M.-N.; Levy, D.; Susser, E.; et al. The Nexus Between Telomere Length and Lymphocyte Count in Seniors Hospitalized With COVID-19. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e97–e101. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.A.; Pimenta, R.; Viana, N.I.; Guimarães, V.R.; Romão, P.; Candido, P.; de Camargo, J.A.; Hatanaka, D.M.; Queiroz, P.G.; Teruya, A.; et al. Shorter leukocyte telomere length is associated with severity of COVID-19 infection. Biochem. Biophys. Rep. 2021, 27, 101056. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Barbi, V.; Gottardi Zamperla, M.; Atlante, S.; Forleo, L.; Nesta, M.; Massetti, M.; Pontecorvi, A.; Nanni, S.; Farsetti, A.; et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. Int. J. Mol. Sci. 2021, 22, 6151. [Google Scholar] [CrossRef]
- Cao, X.; Li, W.; Wang, T.; Ran, D.; Davalos, V.; Planas-Serra, L.; Pujol, A.; Esteller, M.; Wang, X.; Yu, H. Accelerated Biological Aging in COVID-19 Patients. Nat. Commun. 2022, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Krasnienkov, D.S.; Gorodna, O.V.; Kaminska, T.M.; Podolskiy, V.V.; Podolskiy, V.l.V.; Nechyporenko, M.V.; Antypkin, Y.G.; Livshits, L.A. Analysis of Relative Average Length of Telomeres in Leukocytes of Women with COVID-19. Cytol. Genet. 2022, 56, 526–529. [Google Scholar] [CrossRef]
- Retuerto, M.; Lledó, A.; Fernandez-Varas, B.; Guerrero-López, R.; Usategui, A.; Lalueza, A.; García-García, R.; Mancebo, E.; Paz-Artal, E.; Sastre, L.; et al. Shorter telomere length is associated with COVID-19 hospitalization and with persistence of radiographic lung abnormalities. Immun. Ageing 2022, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Dhabhar, F.S.; Su, Y.; Reus, V.I.; Rosser, R.; Burke, H.M.; Kupferman, E.; et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress—Preliminary findings. PLoS ONE 2011, 6, e17837. [Google Scholar] [CrossRef]
- Froidure, A.; Mahieu, M.; Hoton, D.; Laterre, P.-F.; Yombi, J.C.; Koenig, S.; Ghaye, B.; Defour, J.-P.; Decottignies, A. Short telomeres increase the risk of severe COVID-19. Aging 2020, 12, 19911–19922. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, R.; Guio-Carrion, A.; Zapatero-Gaviria, A.; Martinez, P.; Blasco, M.A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Wang, Q.; Codd, V.; Raisi-Estabragh, Z.; Musicha, C.; Bountziouka, V.; Kaptoge, S.; Allarae, E.; Di Angelantonioe, E.; Butterworthe, A.S.; Wood, A.M.; et al. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: A cohort study in UK Biobank. EBioMedicine 2021, 70, 103485. [Google Scholar] [CrossRef]
- Huang, D.; Lin, S.; He, J.; Wang, Q.; Zhan, Y. Association between COVID-19 and telomere length: A bidirectional Mendelian randomization study. J. Med. Virol. 2022, 94, 5345–5353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, B.S.; Guo, J.F.; Li, J.C. Telomere length and COVID-19 outcomes: A two-sample bidirectional Mendelian randomization study. Front. Genet. 2022, 13, 805903. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, F.; Shi, Y.; Chen, Y.; Shi, B.; Yu, G. Causal association of epigenetic aging and COVID-19 severity and susceptibility: A bidirectional Mendelian randomization study. Front. Med. 2022, 9, 989950. [Google Scholar] [CrossRef]
- Franzen, J.; Nuchtern, S.; Tharmapalan, V.; Vieri, M.; Nikolic, M.; Han, Y.; Balfanz, P.; Balfanz, P.; Dreher, M.; Brümmendorf, T.H.; et al. Epigenetic clocks are not accelerated in COVID-19 patients. Int. J. Mol. Sci. 2021, 22, 9306. [Google Scholar] [CrossRef]
- McGroder, C.F.; Zhang, D.; Choudhury, M.A.; Salvatore, M.M.; D’Souza, B.M.; Hoffman, E.A.; Wei, Y.; Baldwin, M.R.; Garcia, C.K. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021, 76, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Helby, J.; Nordestgaard, B.G.; Benfield, T.; Bojesen, S.E. Shorter leukocyte telomere length is associated with higher risk of infections: A prospective study of 75,309 individuals from the general population. Haematologica 2017, 102, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- MACHEREY-NAGEL. NucleoSpin 96 Blood—96-Well Kit for DNA from Blood. Available online: https://www.mn-net.com/nucleospin-96-blood-96-well-kit-for-dna-from-blood-740665.4 (accessed on 10 October 2025).
- Entringer, S.; Epel, E.S.; Lin, J.; Buss, C.; Shahbaba, B.; Blackburn, E.H.; Simhan, H.N.; Wadhwa, P.D. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obs. Gynecol. 2013, 208, 134.e1–134.e7. [Google Scholar] [CrossRef]
- Lin, J.; Epel, E.; Cheon, J.; Kroenke, C.; Sinclair, E.; Bigos, M.; Wolkowitz, O.; Mellon, S.; Blackburn, E. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J. Immunol. Methods 2010, 352, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Panelli, D.M.; Diwan, M.; Cruz, G.I.; Leonard, S.A.; Chueh, J.; Gotlib, I.H.; Bianco, K. An exploratory analysis of leukocyte telomere length among pregnant and non-pregnant people. Brain Behav. Immun. Health 2022, 25, 100506. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Tentolouris, A.; Eleftheriadou, I.; Tentolouris, N. Telomere length, epidemiology and pathogenesis of severe COVID-19. Eur. J. Clin. Inv. 2020, 50, e13376. [Google Scholar] [CrossRef]
- Haridoss, M.; Ayyasamy, L.; Bagepally, B.S. Is COVID-19 severity associated with telomere length? A systematic review and meta-analysis. Virus Genes 2023, 59, 489–498. [Google Scholar] [CrossRef]
- Jose, S.S.; Bendickova, K.; Kepak, T.; Krenova, Z.; Fric, J. Chronic inflammation in immune aging: Role of pattern recognition receptor crosstalk with the telomere complex? Front. Immunol. 2017, 8, 1078. [Google Scholar] [CrossRef]
- Liu, S.; Nong, W.; Ji, L.; Zhuge, X.; Wei, H.; Luo, M.; Zhou, L.; Chen, S.; Zhang, S.; Lei, X.; et al. The regulatory feedback of inflammatory signaling and telomere/telomerase complex dysfunction in chronic inflammatory diseases. Exp. Gerontol. 2023, 174, 112132. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Al Mutair, A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M.; et al. Role of inflammatory cytokines in COVID-19 patients: A review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef]
- Barrett, E.L.; Richardson, D.S. Sex differences in telomeres and lifespan. Aging Cell 2011, 10, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and telomere length: Systematic review and meta-analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Kyo, S.; Takakura, M.; Kanaya, T.; Zhuo, W.; Fujimoto, K.; Nishio, Y.; Orimo, A.; Inoue, M. Estrogen activates telomerase. Cancer Res. 1999, 59, 5917–5921. [Google Scholar]
- Simoncini, T.; Hafezi-Moghadam, A.; Brazil, D.P.; Ley, K.; Chin, W.W.; Liao, J.K. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000, 407, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Bramanti, B.; Serino, M.L.; Secchiero, P.; Zauli, G.; Tisato, V. COVID-19 and individual genetic susceptibility/receptivity: Role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int. J. Mol. Sci. 2020, 21, 3474. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lopez, F.R.; Fernandez-Alonso, A.M.; Ulloque-Badaracco, J.R.; Benites-Zapata, V.A.; Varikasuvu, S.R. Telomere length in subjects with and without SARS-CoV-2 infection: A systematic review and meta-analysis. Rev. Assoc. Med. Bras. 2024, 70, e20240387. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 139) | Control (n = 34) Group 1 | Non-Severe COVID-19 (n = 50) Group 2 | Severe COVID-19 (n = 55) Group 3 | p for All Groups | p 2 vs. 3 | p 1 vs. 2 | p 1 vs. 3 | |
|---|---|---|---|---|---|---|---|---|
| Female gender (n, %) | 67 (48.2%) | 22 (64.7%) | 27 (54.0%) | 18 (32.7%) | 0.008 † | 0.028 † | 0.329 † | 0.003 † |
| Age (years) | 53.52 ± 10.13 | 50.71 ± 9.57 | 53.66 ± 10.20 | 55.13 ± 10.21 | 0.134 ‡ | - | - | - |
| BMI (kg/m2) | 28.00 ± 4.83 | 28.38 ± 5.60 | 27.26 ± 4.29 | 28.43 ± 4.78 | 0.425 ‡ | - | - | - |
| Smoking status (n, %) | ||||||||
| No | 65 (53.7%) | 13 (40.6%) | 23 (56.1%) | 29 (60.4%) | 0.381 † | - | - | |
| Ex smoker | 31 (25.6%) | 12 (37.5%) | 10 (24.4%) | 9 (18.8%) | ||||
| Current | 25 (20.7%) | 7 (21.9%) | 8 (19.5%) | 10 (20.8%) | ||||
| Total (n = 139) | Control (n = 34) Group 1 | Non-Severe COVID-19 (n = 50) Group 2 | Severe COVID-19 (n = 55) Group 3 | p for All Groups | p 2 vs. 3 | p 1 vs. 2 | p 1 vs. 3 | |
|---|---|---|---|---|---|---|---|---|
| Hypertension (n, %) | 31 (22.3%) | 7 (20.6%) | 12 (24.0%) | 12 (21.8%) | 0.929 † | - | - | - |
| Disabled (n, %) | 21 (15.1%) | 3 (8.8%) | 9 (18.0%) | 9 (16.4%) | 0.487 † | - | - | - |
| Coronary Heart Disease (n, %) | 9 (6.5%) | 2 (5.9%) | 4 (8.0%) | 3 (5.5%) | 0.520 ‡ | - | - | - |
| Cerebrovascular disease (n, %) | 2 (1.4%) | 0 (0.0%) | 0 (0.0%) | 2 (3.6%) | 0.154 ‡ | - | - | - |
| Reperfusion (n, %) | 7 (5.0%) | 3 (8.8%) | 2 (4.0%) | 2 (3.6%) | 0.215 ‡ | - | - | - |
| Statin (n, %) | 20 (14.4%) | 1 (2.9%) | 11 (22.0%) | 8 (14.5%) | 0.051 † | 0.923 † | 0.014 † | 0.078 † |
| Chronic Obstructive Pulmonary Disease (n, %) | 5 (3.6%) | 0 (0.0%) | 1 (2.0%) | 4 (7.3%) | 0.050 ‡ | 0.205 † | 0.407 † | 0.108 † |
| Fever (n, %) | 88 (63.3%) | 26 (76.4%) | 21 (42%) | 41 (74.5%) | 0.457 ‡ | - | - | - |
| Cough (n, %) | 42 (30.2%) | 15 (44.1%) | 6 (12%) | 21 (38.2%) | 0.181 † | - | - | - |
| Dyspnea (n, %) | 26 (18.7%) | 8 (23.5%) | 2 (4%) | 16 (29.1%) | 0.003 | <0.001 † | 0.007 † | 0.566 † |
| Anosmia (n, %) | 3 (2.2%) | 1 (2.9%) | 2 (4%) | 0 (0.0%) | 0.267 ‡ | - | - | - |
| Hardship (n, %) | 28 (20.1%) | 6 (17.6%) | 8 (16%) | 14 (25.5%) | 0.443 † | - | - | - |
| Diarrhea (n, %) | 7 (5.0%) | 4 (11.8%) | 1 (2%) | 2 (3.6%) | 0.120 ‡ | - | - | - |
| Oxygen use (n, %) | 48 (34.5) | 17 (50.0%) | 0 | 31 (56.3%) | <0.001 † | <0.001 † | <0.001 † | 0.558 † |
| Nasal Oxygen (n, %) | 23 (16.5) | 9 (26.5%) | 0 | 14 (25.5%) | <0.001 † | <0.001 † | <0.001 † | 0.915 † |
| Venturi Mask (n, %) | 32 (23.0) | 11 (32.4%) | 0 | 21 (38.2%) | <0.001 † | <0.001 † | <0.001 † | 0.578 † |
| BiPAP/CPAP (n, %) | 1 (0.7) | 1 (2.9%) | 0 | 0 (0.0%) | 0.211 † | - | - | - |
| High flow nasal cannula (n, %) | 5 (3.6) | 3 (8.8%) | 0 | 2 (3.6%) | 0.103 † | - | - | - |
| Intubation /ICU (n, %) | 2 (1.4) | 0 (0.0%) | 0 | 2 (3.6%) | 0.212 † | - | - | - |
| Total (n = 139) | Control (n = 34) Group 1 | Non-Severe COVID-19 (n = 50) Group 2 | Severe COVID-19 (n = 55) Group 3 | p for All Groups | p 2 vs. 3 | p 1 vs. 2 | p 1 vs. 3 | |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin (mg/dL) | 13.7 ± 1.6 | 13.8 ± 1.9 | 13.7 ± 1.6 | 13.7 ± 1.5 | 0.939 | - | - | - |
| Platelets/μL | 212,000 (158,000, 272,000) | 212,000 (150,250, 268,250) | 253,000 (185,000, 282,000) | 194,000 (146,000, 259,000) | 0.014 ‡ | 0.014 † | 0.132 † | 0.685 † |
| White blood cell/μL | 6040 (4520, 7705) | 5200 (4345, 6865) | 6510 (5045, 8005) | 5600 (4200, 8210) | 0.259 ‡ | - | - | - |
| Neutrophils/μL | 3700 (2350, 5390) | 3200 (2275, 4525) | 3900 (3000, 5200) | 3850 (2175, 6215) | 0.971 ‡ | - | - | - |
| Lymphocytes /μL | 1346.0 ± 865.6 | 1213.7 ± 760.5 | 1627.7 ± 926.9 | 1173.8 ± 818.3 | 0.016 | 0.007 | 0.030 | 0.830 |
| Vitamin D (ng/mL) | 21.0 (14.0, 29.0) | 21.0 (9.8, 25.5) | 20.0 (16.3, 28.8) | 23.0 (13.0, 29.0) | 0.883 ‡ | - | - | - |
| Glucose (mg/dL) | 105.0 (94, 123) | 109.5 (95.8, 126.5) | 97.5 (88.8, 106.3) | 110.0 (97.0, 128.0) | 0.001 ‡ | 0.001 † | 0.004 † | 0.953 † |
| Sodium (mmol/L) | 138.8 ± 3.1 | 138.3 ± 3.9 | 139.6 ± 2.2 | 138.3 ± 3.1 | 0.040 | 0.024 † | 0.039 † | 0.949 † |
| Potassium‡ (mmol/L) | 4.3 (3.9, 4.6) | 4.2 (3.8, 4.7) | 4.4 (4.1, 4.6) | 4.3 (3.9, 4.6) | 0.309 | - | - | - |
| Hs Trop (ng/L) | 5.0 (3.0, 9.0) | 8.0 (4.3, 11.5) | 5.0 (3.0, 6.0) | 6.0 (3.0, 8.3) | 0.298 ‡ | - | - | - |
| Total bilirubin (mg/dL) | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.7) | 0.4 (0.3, 0.6) | 0.337 ‡ | - | - | - |
| Albumin (g/L) | 41.0 (30.7, 45.0) | 40.00 (29.5, 43.0) | 44.5 (41.4, 46.0) | 37.5 (33.6, 42.8) | <0.001 ‡ | <0.001 † | 0.006 † | 0.342 † |
| CK (mcg/L) | 105.0 (61.5, 191.5) | 119.0 (70.0, 219.5) | 105.0 (60.0, 202.0) | 91.0 (54.50, 172.5) | 0.442 ‡ | - | - | - |
| Urea ‡ (mg/dL) | 33.0 (25.0, 39.3) | 33.5 (27.3, 39.5) | 33.0 (25.8, 39.3) | 33.0 (24.8, 40.8) | 0.770 ‡ | - | - | - |
| Creatinine ‡ (mg/dL) | 0.8 (0.7, 1.0) | 0.9 (0.7, 1.1) | 0.8 (0.6, 0.9) | 0.8 (0.7, 1.1) | 0.049 ‡ | 0.049 † | 0.015 † | 0.557 † |
| CRP ‡ (mg/dL) | 6.1 (1.8, 24.5) | 10.6 (1.9, 33.4) | 3.0 (1.4, 10.0) | 8.3 (2.4, 45.4) | 0.025 ‡ | 0.025 † | 0.029 † | 0.913 † |
| Ferritin (ng/mL) | 300.0 (106.0, 546.0) | 365.0 (243.0, 715.0) | 150.5 (62.3, 252.8) | 359.5 (139.3, 809.5) | <0.001 ‡ | <0.001 † | <0.001 † | 0.978 † |
| LDH (U/L) | 242.5 (193.3, 332.8) | 262.0 (207.0, 368.5) | 205.0 (180.0, 244.5) | 291.0 (203.0, 343.5) | 0.001 ‡ | 0.001 † | 0.002 † | 0.885 † |
| AST (U/L) | 28.0 (20.0, 42.5) | 32.5 (22.5, 45.0) | 23.0 (17.0, 30.3) | 37.0 (22.5, 58.5) | <0.001 ‡ | <0.001 † | <0.001 † | 0.309 † |
| ALT (U/L) | 29.0 (18.5, 43.0) | 30.0 (23.0, 36.3) | 23.5 (16.0, 31.0) | 38.0 (22.5, 59.0) | <0.001 ‡ | 0.001 † | 0.043 † | 0.071 † |
| GGT (U/L) | 31.0 (19.5, 51.5) | 38.0 (28.5, 55.3) | 23.0 (14.5, 37.0) | 36.5 (21.8, 60.3) | 0.005 ‡ | 0.005 † | 0.004 † | 0.741 † |
| ALP (IU/L) | 64.5 (52.0, 85.0) | 60.50 (51.0, 73.3) | 65.0 (52.0, 85.0) | 68.0 (52.5, 86.5) | 0.673 ‡ | - | - | - |
| Fibrinogen (mg/dL) | 506.8 ± 159.6 | 446.2 ± 75.5 | 445.4 ± 196.1 | 579.4 ± 150.2 | 0.015 | 0.016 | 0.990 | 0.014 |
| INR | 0.99 (0.95, 1.03) | 0.98 (0.94, 1.07) | 0.99 (0.96, 1.03) | 0.99 (0.94, 1.02) | 0.521 ‡ | - | - | - |
| APTT | 35.4 (33.5, 37.3) | 35.2 (34.0, 38.6) | 34.6 (31.6, 36.7) | 35.6 (33.9, 37.8) | 0.403 ‡ | - | - | - |
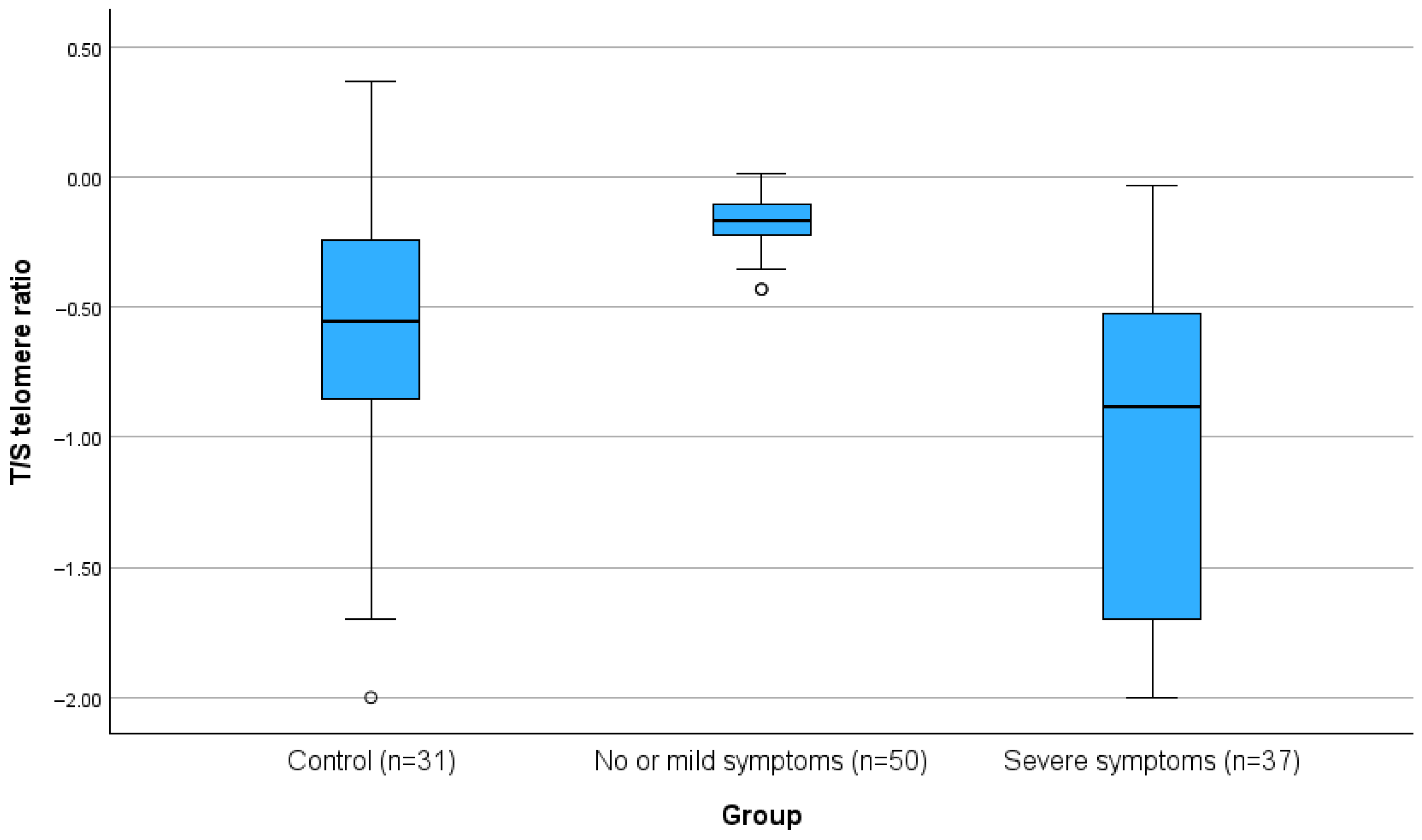
| RATIO T/S TELOMERES | 0.37 (0.02, 0.69) | 0.25 (0.10, 0.57) | 0.68 (0.59, 0.78) | 0.02 (0, 0.23) | <0.001 ‡ | <0.001 † | <0.001 † | <0.001 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacopoulou, F.; Tentolouris, A.; Koniari, E.; Kalogirou, D.; Basoulis, D.; Eleftheriadou, I.; Grigoropoulou, P.; Efthymiou, V.; Georgoulia, K.K.; Anastasiou, I.A.; et al. Telomere Length and COVID-19 Severity: A Comparative Cross-Sectional Study Across the Clinical Spectrum. Healthcare 2025, 13, 2656. https://doi.org/10.3390/healthcare13202656
Bacopoulou F, Tentolouris A, Koniari E, Kalogirou D, Basoulis D, Eleftheriadou I, Grigoropoulou P, Efthymiou V, Georgoulia KK, Anastasiou IA, et al. Telomere Length and COVID-19 Severity: A Comparative Cross-Sectional Study Across the Clinical Spectrum. Healthcare. 2025; 13(20):2656. https://doi.org/10.3390/healthcare13202656
Chicago/Turabian StyleBacopoulou, Flora, Anastasios Tentolouris, Eleni Koniari, Dimitrios Kalogirou, Dimitrios Basoulis, Ioanna Eleftheriadou, Pinelopi Grigoropoulou, Vasiliki Efthymiou, Konstantina K. Georgoulia, Ioanna A. Anastasiou, and et al. 2025. "Telomere Length and COVID-19 Severity: A Comparative Cross-Sectional Study Across the Clinical Spectrum" Healthcare 13, no. 20: 2656. https://doi.org/10.3390/healthcare13202656
APA StyleBacopoulou, F., Tentolouris, A., Koniari, E., Kalogirou, D., Basoulis, D., Eleftheriadou, I., Grigoropoulou, P., Efthymiou, V., Georgoulia, K. K., Anastasiou, I. A., Papadodima, S., Chrousos, G., & Tentolouris, N. (2025). Telomere Length and COVID-19 Severity: A Comparative Cross-Sectional Study Across the Clinical Spectrum. Healthcare, 13(20), 2656. https://doi.org/10.3390/healthcare13202656








