The Digital Amplifier in Medical Insurance: How Chinese Provincial Pooling Policy Optimizes Chronic Disease Management
Abstract
1. Introduction
2. Policy Background and Hypotheses
2.1. Policy Background
2.2. Theoretical Analysis and Hypotheses
3. Data, Variables, and Model
3.1. Data
3.2. Variables
3.2.1. Explained Variables
3.2.2. Explanatory Variables
3.2.3. Control Variables
3.3. Model
4. Results
4.1. Baseline Results
4.2. Robustness Tests
4.2.1. Parallel Trend Assumption Assessment
4.2.2. PSM-DID
4.2.3. Alternative Variable Measurement
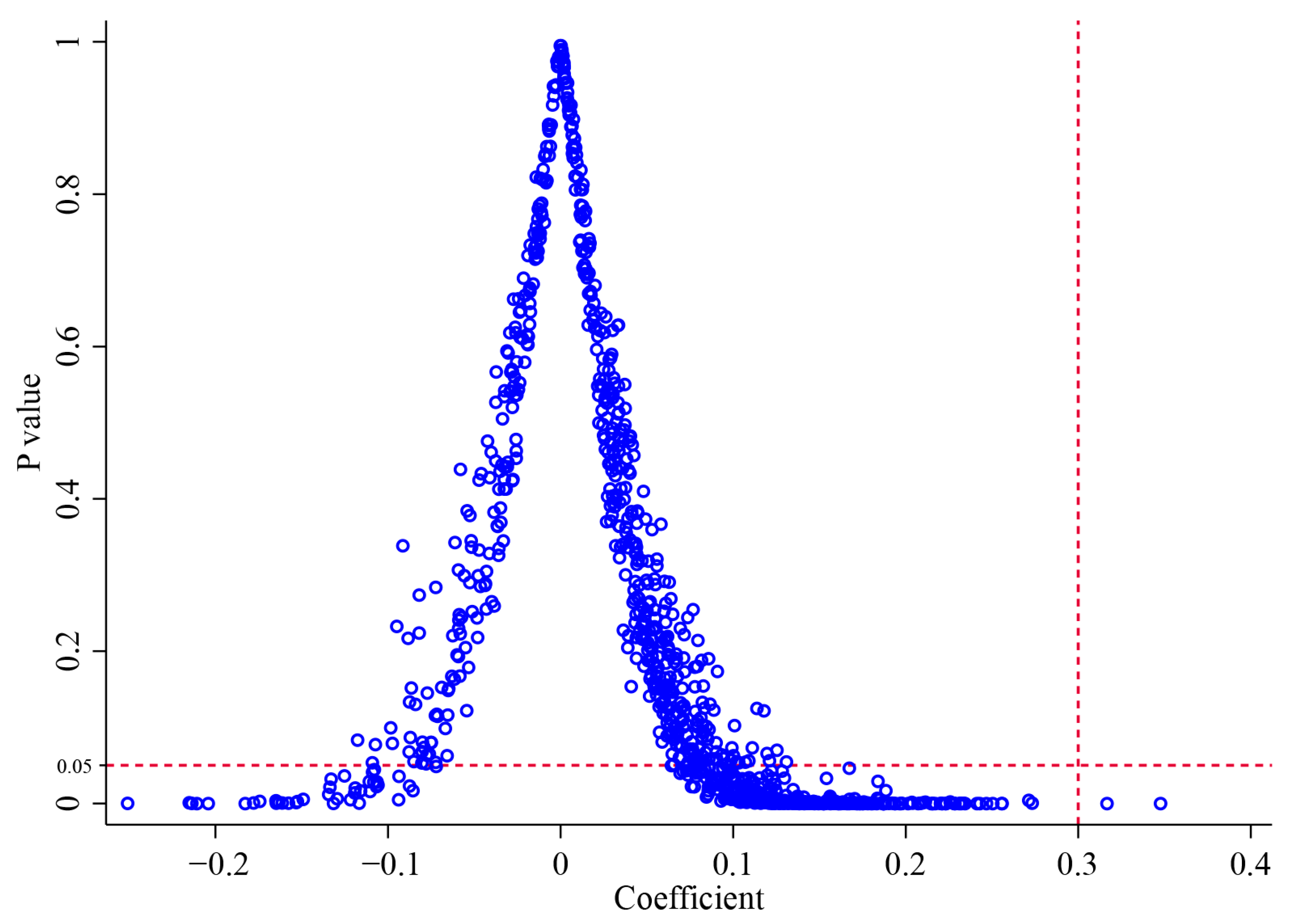
4.2.4. Placebo Tests
4.3. Heterogeneity Analysis
4.3.1. Region Heterogeneity
4.3.2. Income Heterogeneity
4.3.3. Education Heterogeneity
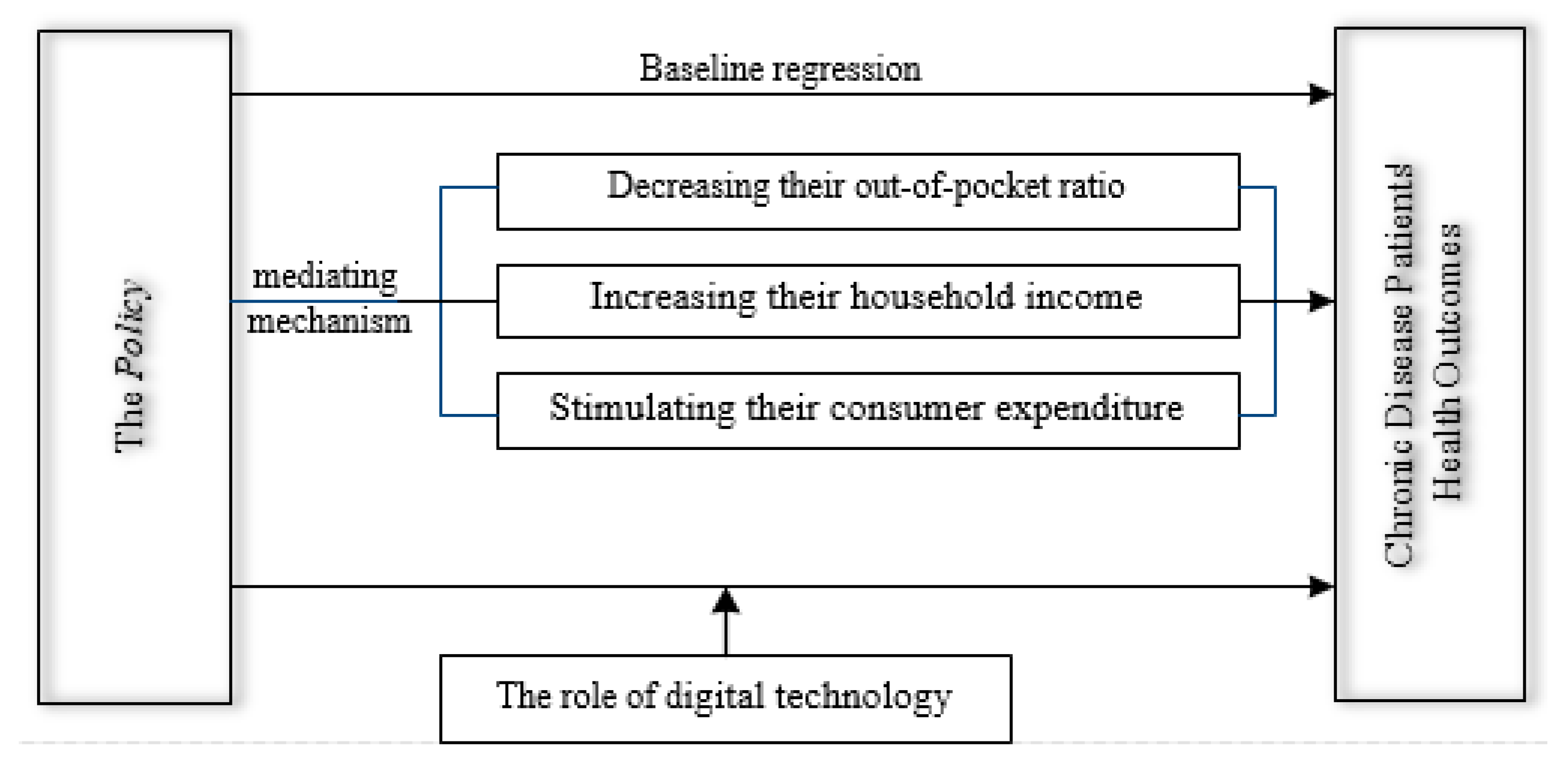
4.4. Underlying Mechanism Analysis
4.4.1. Out-of-Pocket Ratio
4.4.2. Household Income
4.4.3. Consumer Expenditure
4.5. Further Analysis: The Role of Digital Technology
5. Discussion
5.1. How the Policy Results in Better Health: Three Key Channels
5.2. Unlocking Policy Potential: How Digital Technology Acts as an Amplifier
5.3. Contributions
5.4. Limitations and Future Research Directions
5.5. Policy Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, L.L. Health promotion and chronic disease prevention at the workplace. Annu. Rev. Public Health 2024, 45, 337–357. [Google Scholar] [CrossRef]
- Allegrante, J.P.; Wells, M.T.; Peterson, J.C. Interventions to support behavioral self-management of chronic diseases. Annu. Rev. Public Health 2019, 40, 127–146. [Google Scholar] [CrossRef]
- Ma, H.F.; Mu, X.M.; Jin, Y.Z.; Luo, Y.N.; Wu, M.; Han, Z.Y. Multimorbidity, lifestyle, and cognitive function: A cross-cultural study on the role of diabetes, cardiovascular disease, cancer, and chronic respiratory diseases. J. Affect. Disord. 2024, 362, 560–568. [Google Scholar] [CrossRef]
- Syed, M.A.; Syed, M.A.; Lee, A.C. Integrated care for chronic diseases: An evolutionary step for emerging primary health care systems. Public Health 2024, 237, 456–457. [Google Scholar] [CrossRef]
- Dai, Q.Q.; Li, M.; Wang, Z.Y.; Xu, Q.Q.; Zhang, X.Y.; Tao, L.Y. The mediating effects of chronic diseases in the relationship between adverse childhood experiences and trajectories of depressive symptoms in later life: A nationwide longitudinal study. Healthcare 2024, 12, 2539. [Google Scholar] [CrossRef]
- Sun, S.C.; Li, T.; Zheng, A.Q.; Zhang, Z.X.; Wang, Q.Y.; Chen, C.; Zeng, Z.R. Doctor-patient-family collaboration in community-based chronic disease management to enhance multidimensional value. Patient Educ. Couns. 2025, 132, 108604. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.X.; Zou, S.Y.; Chen, Y.H.; Cai, Z.Q.; Zhu, Y.; Tang, K. The role of social integration in chronic disease prevalences among the internal migrant populations in China: Evidence from a national survey. Healthcare 2025, 13, 69. [Google Scholar] [CrossRef]
- Zhang, X.J.; Chen, W. Can provincial-pooling of urban and rural resident basic medical insurance improve the health performance of migrant population? Humanit. Soc. Sci. Commun. 2024, 11, 983. [Google Scholar] [CrossRef]
- Frank, F.; Bjerregaard, F.; Bengel, J.; Bitzer, E.M.; Heimbach, B.; Kaier, K.; Kiekert, J.; Krämer, L.; Kricheldorff, C.; Laubner, K.; et al. Local, collaborative, stepped and personalised care management for older people with chronic diseases (LoChro): Study protocol of a randomised comparative effectiveness trial. BMC Geriatr. 2019, 19, 64. [Google Scholar] [CrossRef]
- Pu, X.H.; Hou, R.Y.; He, S.C.; Zhang, W.K. How does the basic urban-rural medical insurance affect resident health inequality? Evidence from China. Healthcare 2025, 13, 1455. [Google Scholar] [CrossRef]
- Song, X.Y.; Hallensleben, C.; Li, B.; Zhang, W.H.; Jiang, Z.L.; Shen, H.X.; Gobbens, R.J.J.; Chavannes, N.H.; Versluis, A. Illness perceptions and self-management among people with chronic lung disease and healthcare professionals: A mixed-method study identifying the local context. Healthcare 2022, 10, 1657. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H.L.; Pan, X.Q. Forecasting health financing sustainability under the unified pool reform: Evidence from China’s Urban Employee Basic Medical Insurance. Health Econ. Rev. 2024, 14, 77. [Google Scholar] [CrossRef]
- Dong, B. The promotion of pooling level of basic medical insurance and participants’ health: Impact effects and mediating mechanisms. Int. J. Equity Health 2023, 22, 113. [Google Scholar] [CrossRef]
- Cockerham, W.C.; Hamby, B.W.; Oates, G.R. The social determinants of chronic disease. Am. J. Prev. Med. 2017, 52, S5–S12. [Google Scholar] [CrossRef]
- Allain, S.; Naouri, D.; Deroyon, T.; Costemalle, V.; Hazo, J.B. Income and professional inequalities in chronic diseases: Prevalence and incidence in France. Public Health 2024, 228, 55–64. [Google Scholar] [CrossRef]
- Du, Y.H.; de Bock, G.H.; Vonk, J.M.; Pham, A.T.; van der Ende, M.Y.; Snieder, H.; Smidt, N.; Krabbe, P.F.M.; Alizadeh, B.Z.; Lunter, G.; et al. Lifestyle factors and incident multimorbidity related to chronic disease: A population-based cohort study. Eur. J. Ageing 2024, 21, 37. [Google Scholar] [CrossRef]
- Shao, Y.J.; Yang, X.M.; Chen, Q.; Guo, H.Y.; Duan, X.C.; Xu, X.J.; Yue, J.X.; Zhang, Z.Y.; Zhao, S.; Zhang, S.Q. Determinants of digital health literacy among older adult patients with chronic diseases: A qualitative study. Front. Public Health 2025, 13, 1568043. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, W.K. Green finance: The catalyst for artificial intelligence and energy efficiency in Chinese urban sustainable development. Energy Econ. 2024, 139, 107883. [Google Scholar] [CrossRef]
- Shu, Z.; Han, Y.; Xiao, J.G.; Li, J. Effect of medical insurance and family financial risk on healthcare utilization by patients with chronic diseases in China: A cross-sectional study. BMJ Open 2019, 9, e030799. [Google Scholar] [CrossRef]
- Peng, Z.X.; Zhu, L. The impacts of health insurance on financial strain for people with chronic diseases. BMC Public Health 2021, 21, 1012. [Google Scholar] [CrossRef]
- Berete, F.; Demarest, S.; Charafeddine, R.; Bruyère, O.; van der Heyden, J. Comparing health insurance data and health interview survey data for ascertaining chronic disease prevalence in Belgium. Arch. Public Health 2020, 78, 120. [Google Scholar] [CrossRef]
- Peng, M.; Dai, B.Z.; Xin, L. Does the new rural cooperative medical scheme provincial pooling policy improve health equity among older adults? Evidence from China Longitudinal Healthy Longevity Survey Data. Int. J. Health Policy Manag. 2025, 14, 8671. [Google Scholar]
- Andersen, R.M. Revisiting the behavioral model and access to medical care: Does it matter? J. Health Soc. Behav. 1995, 36, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.W.; Yao, J. The dilemma of medical insurance under commissioning agency: The impact of provincial-level pooling on fund income and expenditure—Take Urban Employee Basic Medical Insurance as an example. Insur. Stud. 2023, 11, 104–118. [Google Scholar]
- Wang, Z.H.; Li, X.J.; Chen, M.S. Catastrophic health expenditures and its inequality in elderly households with chronic disease patients in China. Int. J. Equity Health 2015, 14, 8. [Google Scholar] [CrossRef]
- Li, X.H.; Yang, Z.Q.; Qian, X.W. Factoring in temporal variations of public transit-based healthcare accessibility and equity. Int. J. Transp. Sci. Technol. 2024, 13, 186–199. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, X.D.; Xu, Z.P.; Zhang, L.F. Social medical insurances, choices of medical institutions and the ‘siphon effect’ in the health service market: Evidence from 2021 Yangtze River Delta region of China. Risk Manag. Healthc. Policy 2024, 17, 1287–1299. [Google Scholar] [CrossRef]
- Tian, J.; Chen, Z.P.; Wang, Y.; Zhu, Y. Does the trans-provincial immediate reimbursement reduce health gap between urban and rural floating population? Evidence from China. BMC Public Health 2025, 25, 1826. [Google Scholar] [CrossRef]
- Shattnawi, K.K.; Ali, N.A.; Almanasreh, A.A.; Al-Motlaq, M.A. Caregiver burden among parents of children with chronic diseases: A cross-sectional study. J. Clin. Nurs. 2023, 32, 6485–6493. [Google Scholar] [CrossRef]
- Kushner, R.F.; Sorensen, K.W. Lifestyle medicine: The future of chronic disease management. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 389–395. [Google Scholar] [CrossRef]
- Han, L.L.; Nan, Y.Q.; Yang, F.; Zang, X.H.; Xue, X.L. Impact of chronic disease shocks on household consumption in China: The role of multi-level health insurance. Sage Open 2024, 14, 21582440241284422. [Google Scholar] [CrossRef]
- Wei, S.Q.; Kong, F.L.; Li, S.X. The effects of social support and morbidities on self-rated health among migrant elderly following children to Jinan, China. Healthcare 2021, 9, 686. [Google Scholar] [CrossRef]
- Idler, E.L.; Benyamini, Y. Self-rated health and mortality: A review of twenty-seven community studies. J. Health Soc. Behav. 1997, 38, 21–37. [Google Scholar] [CrossRef]
- Beck, T.; Levine, R.; Levkov, A. Big bad banks? The winners and losers from bank deregulation in the United States. J. Financ. 2010, 65, 1637–1667. [Google Scholar] [CrossRef]
- Li, C.J.; Wang, H.S.; Yuan, J.J.; Shi, L.L.; Chen, Y.H.; Gao, Z.F.; Zhao, L.B.; Oliveira, A. Current status of older people with chronic diseases adopting digital health technologies: A scoping review. Digit. Health 2025, 11, 20552076251348578. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Zhou, L.; Zhang, W.K. Help or hurt? The impact of digital finance on the physical health of the elderly in China. Healthcare 2024, 12, 1299. [Google Scholar] [CrossRef]
- Chien, S.Y.; Hu, H.C.; Cho, H.Y. Long-term monitoring of individuals with chronic obstructive pulmonary disease using digital health technology: Qualitative study. J. Med. Internet Res. 2025, 27, e63660. [Google Scholar] [CrossRef]
- Baek, S.J.; Choi, J.A.; Noh, J.W.; Jeong, H.S. A cost-minimization analysis of teleconsultation versus in-person care for chronic diseases and rehabilitation in medically underserved areas of South Korea. Healthcare 2025, 13, 445. [Google Scholar] [CrossRef]
- Wan, S.W.; Choe, L.; Wong, G.J.; Koh, W.L.; Ng, J.S.; Tan, W.H.; Ooi, J.L.X.; Melody, J.; Lau, J.; Tan, K.K. Telemedicine uptake behaviors and predictors of its acceptance among community-dwelling older adults with chronic diseases. Health Policy Technol. 2025, 14, 101007. [Google Scholar] [CrossRef]
- Ali, L.; Wallström, S.; Fors, A.; Barenfeld, E.; Fredholm, E.; Fu, M.; Goudarzi, M.; Gyllensten, H.; Kjellberg, I.L.; Swedberg, K.; et al. Effects of person-centered care using a digital platform and structured telephone support for people with chronic obstructive pulmonary disease and chronic heart failure: Randomized controlled trial. J. Med. Internet Res. 2021, 23, e26794. [Google Scholar] [CrossRef]
- Yao, R.; Zhang, W.L.; Evans, R.; Cao, G.; Rui, T.Q.; Shen, L.N. Inequities in health care services caused by the adoption of digital health technologies: Scoping review. J. Med. Internet Res. 2022, 24, e34144. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Zhou, L.; Zhang, W.K. Can e-commerce bring dividends to rural households? New evidence from the income inequality perspective. Econ. Anal. Policy 2025, 86, 363–379. [Google Scholar] [CrossRef]

| Variables | N | Mean | S.D. | Min | Max |
|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | |
| Core variables | |||||
| SRH (range from 1 to 7; higher self-assessment means better overall health) | 26,585 | 3.297 | 1.841 | 1 | 7 |
| Policy (have implemented the Policy = 1; not yet = 0) | 26,585 | 0.079 | 0.270 | 0 | 1 |
| Control variables | |||||
| Age | 26,585 | 55.718 | 14.073 | 16 | 95 |
| Gender (male = 1; female = 0) | 26,585 | 0.440 | 0.496 | 0 | 1 |
| Household registration (urban = 1; rural = 0) | 26,585 | 0.458 | 0.498 | 0 | 1 |
| Marital status (married = 1; others = 0) | 26,585 | 0.852 | 0.355 | 0 | 1 |
| Internet use (yes = 1; no = 0) | 26,585 | 0.206 | 0.404 | 0 | 1 |
| Number of family members | 26,585 | 4.067 | 1.955 | 1 | 17 |
| Household income per capita (in log, RMB) | 26,585 | 9.007 | 1.917 | 0 | 15.243 |
| (1) | (2) | |
|---|---|---|
| Policy | 0.459 *** | 0.300 *** |
| (0.082) | (0.076) | |
| Controls | √ | |
| Year FE | √ | √ |
| County × Year FE | √ | √ |
| N | 26,585 | 26,585 |
| R2 | 0.614 | 0.634 |
| PSM-DID (1) | Alternative Measurement of Policy (2) | |
|---|---|---|
| Policy | 0.300 *** | |
| (0.085) | ||
| Policy | 0.299 *** | |
| (0.074) | ||
| Controls | √ | √ |
| Year FE | √ | √ |
| County × Year FE | √ | √ |
| N | 6229 | 26,585 |
| R2 | 0.712 | 0.634 |
| Region Heterogeneity | Income Heterogeneity | Education Heterogeneity | ||||
|---|---|---|---|---|---|---|
| Urban | Rural | Low | High | Low | High | |
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Policy | 0.311 *** | 0.204 | 0.645 *** | 0.192 ** | 0.404 | 0.172 ** |
| (0.086) | (0.183) | (0.235) | (0.088) | (0.324) | (0.084) | |
| Controls | √ | √ | √ | √ | √ | √ |
| Year FE | √ | √ | √ | √ | √ | √ |
| County × Year FE | √ | √ | √ | √ | √ | √ |
| N | 12,179 | 14,406 | 12,279 | 14,306 | 9198 | 17,387 |
| R2 | 0.692 | 0.626 | 0.644 | 0.653 | 0.615 | 0.674 |
| Medical Burden (1) | Household Income (2) | Consumer Expenditure (3) | |
|---|---|---|---|
| Policy | −0.042 ** | 0.438 *** | 0.128 *** |
| (0.020) | (0.168) | (0.049) | |
| Controls | √ | √ | √ |
| Year FE | √ | √ | √ |
| County × Year FE | √ | √ | √ |
| N | 23,664 | 26,585 | 24,412 |
| R2 | 0.452 | 0.303 | 0.493 |
| Digital Technology Input | Digital Technology Output | |
|---|---|---|
| (1) | (2) | |
| Policy | −0.329 | 1.047 *** |
| (0.308) | (0.382) | |
| Input | −0.002 | |
| (0.031) | ||
| Policy × Input | 0.053 ** | |
| (0.026) | ||
| Output | 2.673 | |
| (2.632) | ||
| Policy × Output | 6.894 ** | |
| (3.475) | ||
| Controls | √ | √ |
| Year FE | √ | √ |
| County × Year FE | √ | √ |
| N | 26,585 | 26,585 |
| R2 | 0.634 | 0.634 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Cheng, H.; Zhang, W. The Digital Amplifier in Medical Insurance: How Chinese Provincial Pooling Policy Optimizes Chronic Disease Management. Healthcare 2025, 13, 2643. https://doi.org/10.3390/healthcare13202643
Zeng M, Cheng H, Zhang W. The Digital Amplifier in Medical Insurance: How Chinese Provincial Pooling Policy Optimizes Chronic Disease Management. Healthcare. 2025; 13(20):2643. https://doi.org/10.3390/healthcare13202643
Chicago/Turabian StyleZeng, Ming, Huan Cheng, and Weike Zhang. 2025. "The Digital Amplifier in Medical Insurance: How Chinese Provincial Pooling Policy Optimizes Chronic Disease Management" Healthcare 13, no. 20: 2643. https://doi.org/10.3390/healthcare13202643
APA StyleZeng, M., Cheng, H., & Zhang, W. (2025). The Digital Amplifier in Medical Insurance: How Chinese Provincial Pooling Policy Optimizes Chronic Disease Management. Healthcare, 13(20), 2643. https://doi.org/10.3390/healthcare13202643







