Chronic Implications of Bilateral Foot Pattern Variability in Schoolchildren
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Overview
2.2. Participants
2.3. Parameters and Measurements
2.4. Ethics Approval
2.5. Statistical Analysis
- Descriptive statistics: for continuous variables, measures of central tendency (mean, median) and variability (standard deviation, minimum, maximum, interquartile range) were calculated. For categorical variables, absolute frequencies and percentages were reported. Data distribution was examined using skewness and kurtosis coefficients, and potential outliers were identified through quartile analysis.
- Comparative analysis: differences between groups defined by foot morphology (flat, cavus, normal arches) were assessed with the Kruskal–Wallis H test, appropriate for non-normally distributed data and unequal group sizes. Post hoc pairwise comparisons were performed using Dunn’s test with Bonferroni correction. Sex-based differences in foot morphology were evaluated using the Chi-square test, with sex coded numerically in the dataset (boys = 1, girls = 2).
- BMI-based analysis: participants were also stratified according to BMI category, with coding as follows: 1 = normal weight, 2 = overweight, 3 = obese. For each subgroup, descriptive statistics (mean, SD, min, max, IQR) were calculated, and cross-tabulations were performed to explore potential relationships between BMI and functional parameters.
- Correlation analysis: relationships between anthropometric, gait, and functional parameters were assessed using Spearman’s rank correlation coefficient, suitable for ordinal or non-normally distributed continuous data.
- Effect size and significance: effect sizes were calculated to complement significance testing (η2 for Kruskal–Wallis, Cramer’s V for categorical data, and r for correlations). A significance threshold of α = 0.05 was applied for all tests.
3. Results
3.1. Baseline Characteristics
3.2. Descriptive Statistics of Continuous Variables
3.3. Gender-Based Comparisons
3.4. BMI-Based Comparisons
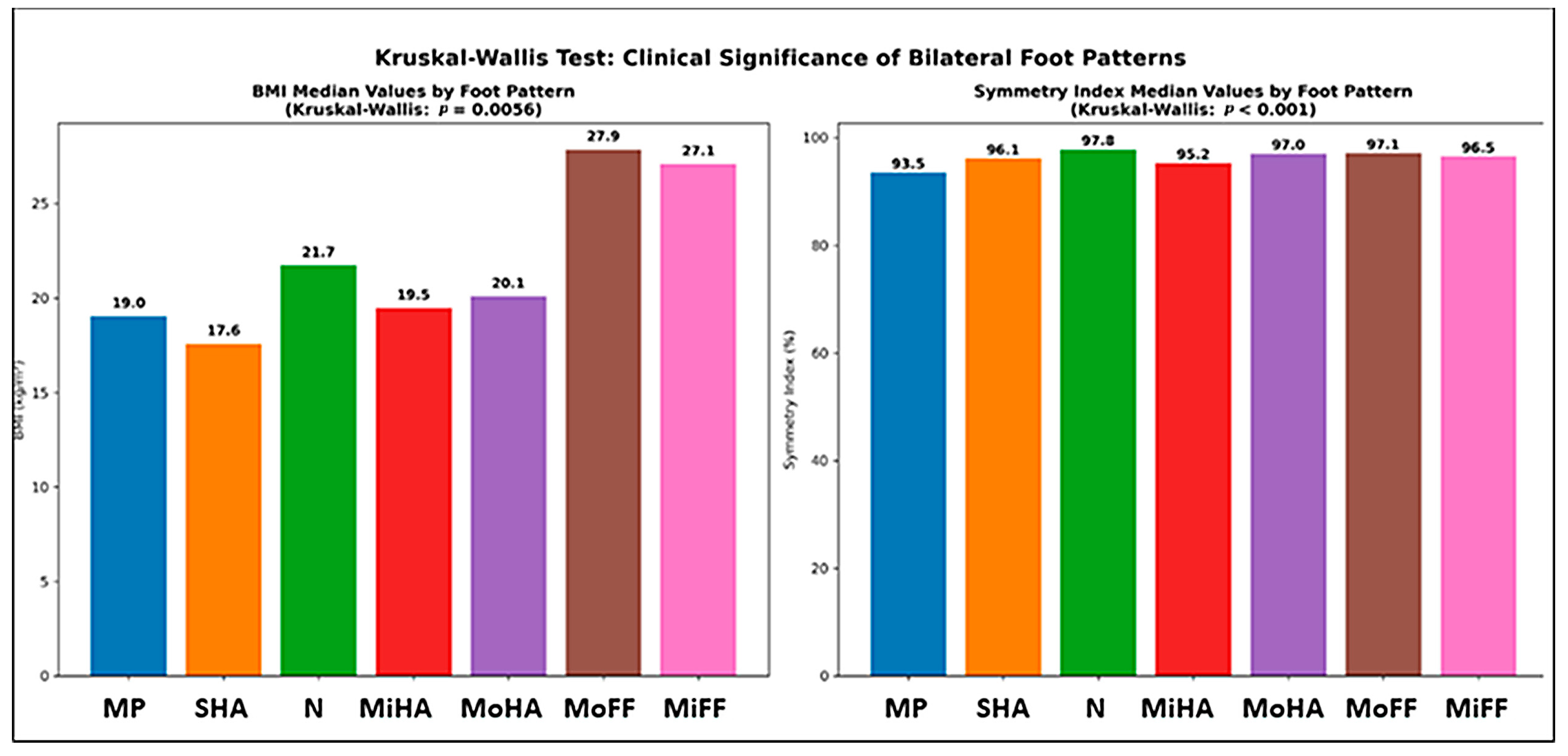
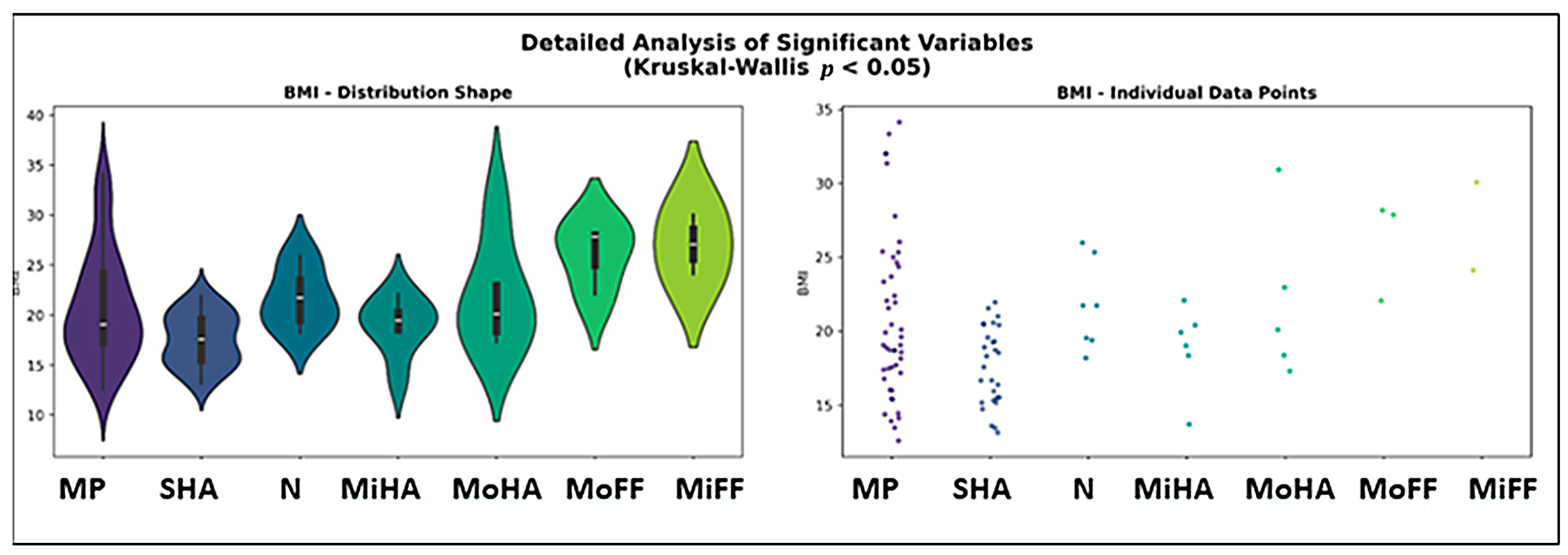
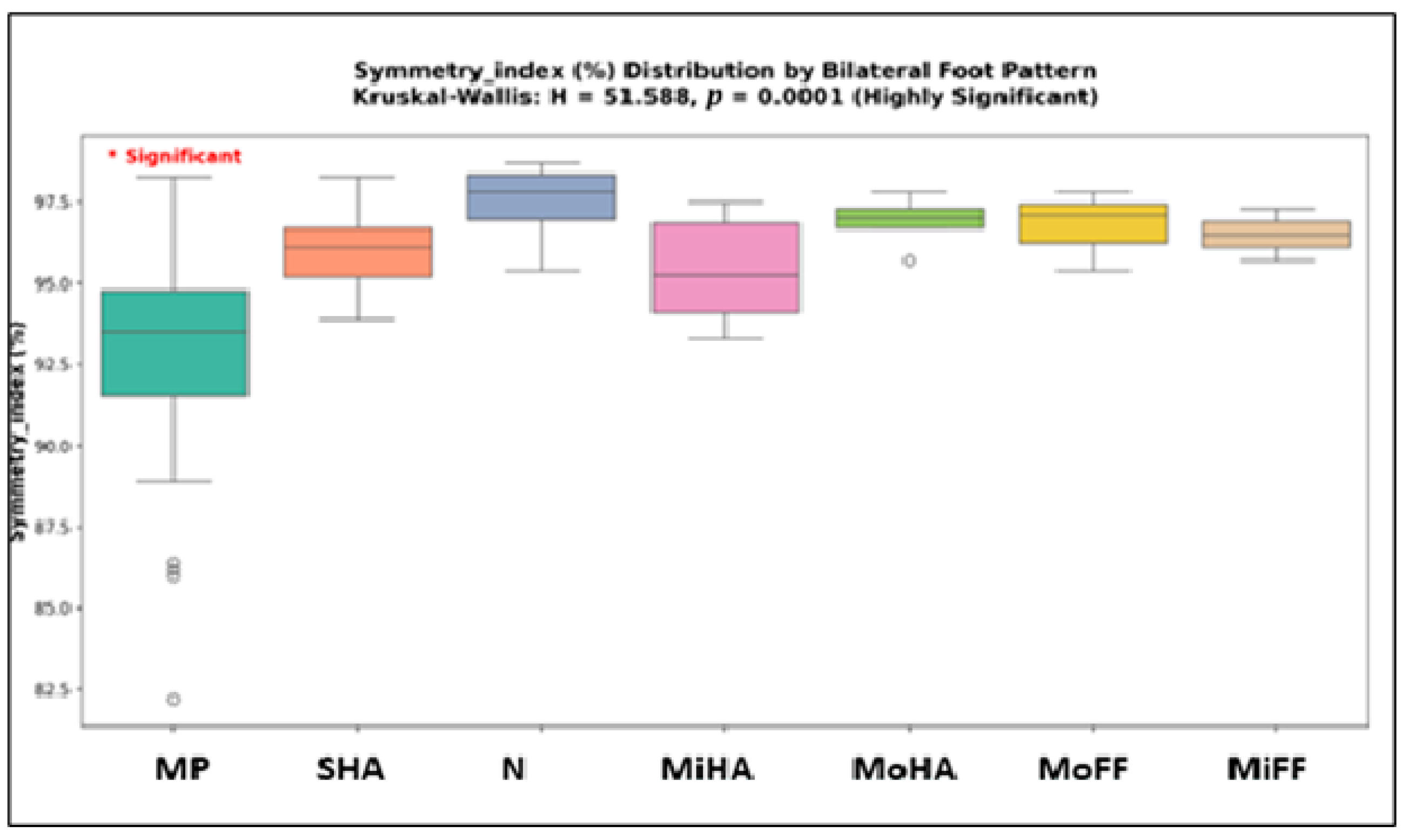
3.5. Foot Morphology Analysis
- Overweight children: higher than expected prevalence of moderate flatfoot (residual ≈ +3.29).
- Obese children: higher than expected prevalence of minimal flatfoot (residual ≈ +2.27) and moderate high arch (residual ≈ +1.08).
- Normal-weight children: slightly higher frequencies of severe high arch (residual ≈ +0.96) and minimal high arch (residual ≈ +0.44).
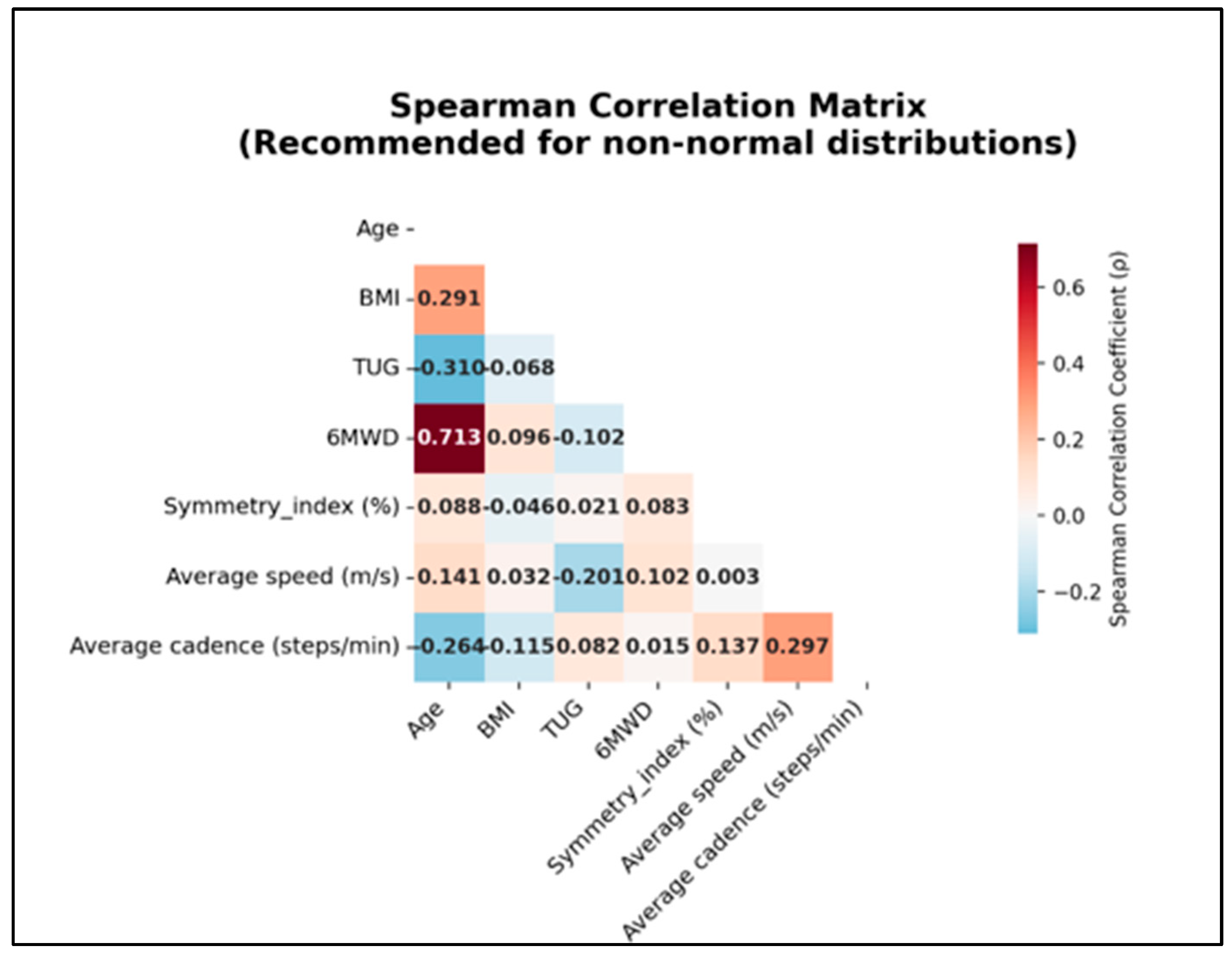
3.6. Correlation Analysis
3.7. Correlation Analysis (Spearman)
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Mei, Q.; Yu, P.; Gao, Z.; Hu, Q.; Fekete, G.; István, B.; Gu, Y. Biomechanical Characteristics of the Typically Developing Toddler Gait: A Narrative Review. Children 2022, 9, 406. [Google Scholar] [CrossRef]
- Resende, R.A.; Pinheiro, L.S.P.; Ocarino, J.M. Effects of Foot Pronation on the Lower Limb Sagittal Plane Biomechanics during Gait. Gait Posture 2019, 68, 130–135. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.L. Balance Control during Gait Initiation: State-of-the-Art and Research Perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Seyedahmadi, M.; Khalaghi, K.; Hazrati, S.; Keavanloo, F. Effect of Medial Longitudinal Arch Height of the Foot on Static and Dynamic Balance in 7–10-Year-Old Boy Gymnasts. Arch. Bone Jt. Surg. 2024, 12, 846–853. [Google Scholar] [PubMed] [PubMed Central]
- Wang, S.; Cui, H.; Tang, T.; Zhang, L.; Li, J.; Wu, M.; Hou, Y. Key Points of Development of Motor Skills in Childhood Embodied in Gait Parameters. Gait Posture 2023, 104, 51–57. [Google Scholar] [CrossRef]
- Cowley, E.; Marsden, J. The Effects of Prolonged Running on Foot Posture: A Repeated Measures Study of Half Marathon Runners Using the Foot Posture Index and Navicular Height. J. Foot Ankle Res. 2013, 6, 20. [Google Scholar] [CrossRef]
- Chen, J.P.; Chung, M.J.; Wang, M.J. Flatfoot Prevalence and Foot Dimensions of 5–13-Year-Old Children in Taiwan. Foot Ankle Int. 2009, 30, 326–332. [Google Scholar] [CrossRef]
- Buldt, A.K.; Forghany, S.; Landorf, K.B.; Levinger, P.; Murley, G.S.; Menz, H.B. Foot Posture Is Associated with Plantar Pressure during Gait: A Comparison of Normal, Planus and Cavus Feet. Gait Posture 2018, 62, 235–240. [Google Scholar] [CrossRef]
- Varghese, R.A.; Rebello, G.; Shah, H.; Benjamin, J. Biomechanical Basis for Treatment of Pediatric Foot Deformities Part II: Pathomechanics of Common Foot Deformities: Current Concept Review. J. Pediatr. Orthop. Soc. N. Am. 2022, 4, 464. [Google Scholar] [CrossRef]
- López, L.D.; Bouza, P.M.A.; Requeijo, C.A.; Saleta, C.J.L.; Bautista, C.A.; Tajes, F.A. The Impact of Foot Arch Height on Quality of Life in 6–12 Year Olds. Colomb. Med. 2014, 45, 168–172. [Google Scholar] [PubMed]
- Squibb, M.; Sheerin, K.; Francis, P. Measurement of the Developing Foot in Shod and Barefoot Paediatric Populations: A Narrative Review. Children 2022, 9, 750. [Google Scholar] [CrossRef]
- Oerlemans, L.N.T.; Peeters, C.M.M.; Munnik-Hagewoud, R.; Wingbermühle, R.W.; Poeze, M.; Meijer, K.; Winkens, B.; Arts, J.J. Foot Orthoses for Flexible Flatfeet in Children and Adults: A Systematic Review and Meta-Analysis of Patient-Reported Outcomes. BMC Musculoskelet. Disord. 2023, 24, 16. [Google Scholar] [CrossRef]
- Xu, L.; Gu, H.; Zhang, Y.; Sun, T.; Yu, J. Risk Factors of Flatfoot in Children: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8247. [Google Scholar] [CrossRef]
- Bogut, I.; Popović, Ž.; Tomac, Z.; Matijević, V.; Radmilović, G. Prevalence of Foot Deformities in Young Schoolchildren in Slavonia. Acta Clin. Croat. 2019, 58, 288–294. [Google Scholar] [CrossRef]
- Pita-Fernandez, S.; Gonzalez-Martin, C.; Alonso-Tajes, F.; Seoane-Pillado, T.; Pértega-Díaz, S.; Pérez-García, S.; Seijo-Bestilleiro, R.; Balboa-Barreiro, V. Flat Foot in a Random Population and Its Impact on Quality of Life and Functionality. J. Clin. Diagn. Res. 2017, 11, LC22–LC27. [Google Scholar] [CrossRef] [PubMed]
- Gijon-Nogueron, G.; Martinez-Nova, A.; Alfageme-Garcia, P.; Cervera-Marin, J.A.; Morales-Asencio, J.M. International Normative Data for Paediatric Foot Posture Assessment: A Cross-Sectional Investigation. BMJ Open 2019, 9, e023341. [Google Scholar] [CrossRef]
- Ezema, C.I.; Abaraogu, U.O.; Okafor, G.O. Flat Foot and Associated Factors among Primary School Children: A Cross-Sectional Study. Hong Kong Physiother. J. 2014, 32, 13–20. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Kotz, R.; Ledl, T.; Hauser, G.; Sluga, M. Prevalence of Flat Foot in Preschool-Aged Children. Pediatrics 2006, 118, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Skowron, N.; Malak, R.; Mojs, E.; Samborski, W. Foot Arch Condition in Comparison with the Muscular Balance of Lower Limbs in Children at School Age of 6–14 Years. J. Med. Sci. 2015, 84, 85–89. [Google Scholar] [CrossRef]
- Jiang, H.; Mei, Q.; Wang, Y.; He, J.; Shao, E.; Fernandez, J.; Gu, Y. Understanding Foot Conditions, Morphologies and Functions in Children: A Current Review. Front. Bioeng. Biotechnol. 2023, 11, 1192524. [Google Scholar] [CrossRef]
- Farahpour, N.; Jafarnezhadgero, A.; Allard, P.; Majlesi, M. Muscle Activity and Kinetics of Lower Limb during Walking in Pronated Feet Individuals with and without Low Back Pain. J. Electromyogr. Kinesiol. 2018, 39, 35–41. [Google Scholar] [CrossRef]
- Betsch, M.; Schneppendahl, J.; Dor, L.; Jungbluth, P.; Grassmann, J.P.; Windolf, J.; Thelen, S.; Hakimi, M.; Rapp, W.; Wild, M. Influence of Foot Positions on the Spine and Pelvis. Arthritis Care Res. 2011, 63, 1758–1765. [Google Scholar] [CrossRef]
- Chouhan, K.; Sathe, P.K.; Parihar, S.S.; Shrivastava, V.K. Comparison of Biomechanical Factors between Normal, Flat Foot, and High Arched Foot in University Students. Physiotherapy-J. Indian Assoc. Physiother. 2024, 18, 167–174. [Google Scholar] [CrossRef]
- Chang, J.-H.; Wang, S.-H.; Kuo, C.-L.; Shen, H.-C.; Hong, Y.-W.; Lin, L.-C. Prevalence of Flexible Flatfoot in Taiwanese School-Aged Children in Relation to Obesity, Gender, and Age. Eur. J. Pediatr. 2010, 169, 447–452. [Google Scholar] [CrossRef]
- Shih, Y.F.; Chen, C.Y.; Chen, W.Y.; Lin, H.C.; Su, F.C. Lower Extremity Kinematic Characteristics of Flatfoot in Children during Walking: A Systematic Review and Meta-Analysis. Gait Posture 2019, 70, 262–271. [Google Scholar] [CrossRef]
- Uden, H.; Scharfbillig, R.; Causby, R. The Typically Developing Paediatric Foot: How Flat Should It Be? A Systematic Review. J. Foot Ankle Res. 2017, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Tudor, A.; Ruzic, L.; Sestan, B.; Sirola, L.; Prpic, T. Flat-Footedness Is Not a Disadvantage for Athletic Performance in Children Aged 11 to 15 Years. Pediatrics 2009, 123, e386–e392. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ye, Q.; Gao, Q.; Lu, Y.; Zhang, D. Symmetry Analysis of Gait between Left and Right Limb Using Cross-Fuzzy Entropy. Comput. Math. Methods Med. 2016, 2016, 1737953. [Google Scholar] [CrossRef]
- Winiarski, S.; Czamara, A. Evaluation of Gait Kinematics and Symmetry during the First Two Stages of Physiotherapy after Anterior Cruciate Ligament Reconstruction. Acta Bioeng. Biomech. 2012, 14, 83–89. [Google Scholar] [PubMed]
- Cimolin, V.; Cau, N.; Sartorio, A.; Merenda, E.; Galli, M.; Capodaglio, P. Symmetry of Gait in Underweight, Normal and Overweight Children and Adolescents. Sensors 2019, 19, 2054. [Google Scholar] [CrossRef]
- Viteckova, S.; Kutilek, P.; Svoboda, Z.; Krupicka, R.; Szabo, Z. Gait Symmetry Measures: A Review of Current and Prospective Methods. Biomed. Signal Process. Control 2018, 42, 89–100. [Google Scholar] [CrossRef]
- Bergamini, E.; Cereatti, A.; Pavei, G. Walking symmetry is speed and index dependent. Sci. Rep. 2024, 14, 19548. [Google Scholar] [CrossRef] [PubMed]
- Zifchock, R.A.; Davis, I.; Higginson, J.; Royer, T. The Symmetry Angle: A Novel, Robust Method of Quantifying Asymmetry. Gait Posture 2008, 27, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Black, S.E.; McIlroy, W.E. Evaluation of Gait Symmetry after Stroke: A Comparison of Current Methods and Recommendations for Standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef]
- Queen, R.; Dickerson, L.; Ranganathan, S.; Schmitt, D. A Novel Method for Measuring Asymmetry in Kinematic and Kinetic Variables: The Normalized Symmetry Index. J. Biomech. 2020, 99, 109531. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Liu, Y.; Zheng, W.; Wang, W.; Yan, S. Altered Gait Patterns during Arch Development in Children with Persistent Obesity: An Experimental Longitudinal Study. Gait Posture 2024, 111, 143–149. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Yang, G.Y.; Jin, K. Age-Related Dysfunction in Balance: A Comprehensive Review of Causes, Consequences, and Interventions. Aging Dis. 2024, 16, 714–737. [Google Scholar] [CrossRef]
- Izzo, R.; Bertoni, M.; Cejudo, A.; Giovannelli, M.; Hosseini Varde’I, C. The Global Symmetry Index, Symmetry Index, Quality Index and Kinematics of the Gait Cycle with the Contribution of Magnetic-Inertial and Electromyographic Technology. J. Phys. Educ. Sport 2022, 22, 1258–1270. [Google Scholar] [CrossRef]
- Widiani, N.K.; Pramita, I.; Vittala, G. Risk Factors of Flat Foot in Children. Kinesiol. Physiother. Compr. 2024, 3, 10–15. [Google Scholar] [CrossRef]
- Yazıcı, M.V.; Çobanoğlu, G.; Yazıcı, G. Test–Retest Reliability and Minimal Detectable Change for Measures of a Wearable Gait Analysis System (G-Walk) in Children with Cerebral Palsy. Turk. J. Med. Sci. 2022, 52, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Taniguchi, Y.; Seino, S.; Sakurai, R.; Osuka, Y.; Obuchi, S.; Watanabe, Y.; Kim, H.; Inagaki, H.; Kitamura, A.; et al. Reference Values of Gait Parameters Measured with a Plantar Pressure Platform in Community-Dwelling Older Japanese Adults. Clin. Interv. Aging 2019, 14, 1265–1276. [Google Scholar] [CrossRef]
- Giontella, A.; Tagetti, A.; Bonafini, S.; Marcon, D.; Cattazzo, F.; Bresadola, I.; Antoniazzi, F.; Gaudino, R.; Cavarzere, P.; Montagnana, M.; et al. Comparison of Performance in the Six-Minute Walk Test (6MWT) between Overweight/Obese and Normal-Weight Children and Association with Haemodynamic Parameters: A Cross-Sectional Study in Four Primary Schools. Nutrients 2024, 16, 356. [Google Scholar] [CrossRef] [PubMed]
- Verbecque, E.; Schepens, K.; Theré, J.; Schepens, B.; Klingels, K.; Hallemans, A. The Timed Up and Go Test in Children: Does Protocol Choice Matter? A Systematic Review. Pediatr. Phys. Ther. 2019, 31, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Hildenbrand, F.F.; Treder, U.; Fischler, M.; Keusch, S.; Speich, R.; Fasnacht, M. Reference Values for the 6-Minute Walk Test in Healthy Children and Adolescents in Switzerland. BMC Pulm. Med. 2013, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Nicolini-Panisson, R.D.A.; Donadio, M.V.F. Normative Values for the Timed ‘Up and Go’ Test in Children and Adolescents and Validation for Individuals with Down Syndrome. Dev. Med. Child Neurol. 2014, 56, 490–497. [Google Scholar] [CrossRef]
- Shah, B.; Tombeau-Cost, K.; Fuller, A.; Birken, C.S.; Anderson, L.N. Sex and Gender Differences in Childhood Obesity: Contributing to the Research Agenda. BMJ Nutr. Prev. Health 2020, 3, 387–390. [Google Scholar] [CrossRef]
- Giuca, G.; Marletta, D.A.; Zampogna, B.; Sanzarello, I.; Nanni, M.; Leonetti, D. Correlation between the Severity of Flatfoot and Risk Factors in Children and Adolescents: A Systematic Review. Osteology 2025, 5, 11. [Google Scholar] [CrossRef]
- Wang, X.; Piantadosi, S.; Le-Rademacher, J.; Mandrekar, S.J. Statistical Considerations for Subgroup Analyses. J. Thorac. Oncol. 2021, 16, 375–380. [Google Scholar] [CrossRef]
- Dowling, A.M.; Steele, J.R.; Baur, L.A. Does Obesity Influence Foot Structure and Plantar Pressure Patterns in Prepubescent Children? Int. J. Obes. Relat. Metab. Disord. 2001, 25, 845–852. [Google Scholar] [CrossRef]
- Kim, M.H.; Cha, S.; Choi, J.E.; Yang, S.-S. Relation of Flatfoot Severity with Flexibility and Isometric Strength of the Foot and Trunk Extensors in Children. Children 2023, 10, 19. [Google Scholar] [CrossRef]
- Popescu, C.; Matei, D.; Amzolini, A.M.; Trăistaru, M.R. Inflammation and Physical Performance in Overweight and Obese Schoolchildren. Life 2024, 14, 1583. [Google Scholar] [CrossRef]
- Chen, K.C.; Tung, L.C.; Yeh, C.J.; Yang, J.F.; Kuo, J.F.; Wang, C.H. Change in Flatfoot of Preschool-Aged Children: A 1-Year Follow-Up Study. Gait Posture 2020, 75, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Boyer, K.A.; Hayes, K.L.; Umberger, B.R.; Adamczyk, P.G.; Bean, J.F.; Brach, J.S.; Clark, B.C.; Clark, D.J.; Ferrucci, L.; Finley, J.; et al. Age-Related Changes in Gait Biomechanics and Their Impact on the Metabolic Cost of Walking: Report from a National Institute on Aging Workshop. Exp. Gerontol. 2023, 173, 112102. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.; Matei, D.; Trăistaru, R. The Role of Kinesiotherapy in Enhancing Physical Performance and Self-Esteem: A Prospective Observational Study in Obese Adolescents. Balneo PRM Res. J. 2024, 15, 759. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Wu, H.; Wang, Y.; Jiang, H.; Gao, Z.; Jánosi, E.; Fekete, G.; Mei, Q.; Gu, Y. Bilateral Asymmetries of Plantar Pressure and Foot Balance during Walking, Running, and Turning Gait in Typically Developing Children. Bioengineering 2025, 12, 151. [Google Scholar] [CrossRef]
- Matsuda, S.; Demura, S. Age-Related, Interindividual, and Right/Left Differences in Anterior–Posterior Foot Pressure Ratio in Preschool Children. J. Physiol. Anthropol. 2013, 32, 20. [Google Scholar] [CrossRef]
- Rusu, L.; Marin, M.I.; Geambesa, M.M.; Rusu, M.R. Monitoring the Role of Physical Activity in Children with Flat Feet by Assessing Subtalar Flexibility and Plantar Arch Index. Children 2022, 9, 427. [Google Scholar] [CrossRef]
- Birhanu, A.; Nagarchi, K.; Getahun, F.; Gebremichael, M.A.; Wondmagegn, H. Magnitude of Flat Foot and Its Associated Factors among School-Aged Children in Southern Ethiopia: An Institution-Based Cross-Sectional Study. BMC Musculoskelet. Disord. 2023, 24, 966. [Google Scholar] [CrossRef]
- Evans, A.M.; Rome, K.; Peet, L. The Foot Posture Index, Ankle Lunge Test, Beighton Scale and the Lower Limb Assessment Score in Healthy Children: A Reliability Study. J. Foot Ankle Res. 2012, 5, 1. [Google Scholar] [CrossRef]
- Büyükçelebi, H.; Açak, M.; Eken, Ö.; Doğaner, A.; Özen, G.; Ardigò, L.P. Association between Pediatric Obesity and Foot Morphology: Insights from a Large-Scale Cross-Sectional Study Using Photogrammetry. BMC Pediatr. 2025, 25, 628. [Google Scholar] [CrossRef]
- Chen, K.-C.; Yeh, C.-J.; Tung, L.-C.; Yang, J.-F.; Yang, S.-F.; Wang, C.-H. Relevant Factors Influencing Flatfoot in Preschool-Aged Children. Eur. J. Pediatr. 2011, 170, 931–936. [Google Scholar] [CrossRef]
- Banwell, H.A.; Paris, M.E.; Mackintosh, S.; Williams, C.M. Paediatric Flexible Flat Foot: How Are We Measuring It and Are We Getting It Right? A Systematic Review. J. Foot Ankle Res. 2018, 11, 21. [Google Scholar] [CrossRef]
- van Vlimmeren, L.A.; Helders, P.J.M.; van Adrichem, L.N.A.; Engelbert, R.H.H. Diagnostic Strategies for the Evaluation of Asymmetry in Infancy—A Review. Eur. J. Pediatr. 2004, 163, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Luciano, F.; Ruggiero, L.; Minetti, A.; Pavei, G. Comparison of Three-Dimensional Body Centre of Mass Trajectories during Locomotion through Zero- and One-Dimensional Statistics. Sci. Rep. 2022, 12, 17777. [Google Scholar] [CrossRef] [PubMed]
- Siebers, H.L.; Alrawashdeh, W.; Betsch, M.; Migliorini, F.; Hildebrand, F.; Eschweiler, J. Comparison of Different Symmetry Indices for the Quantification of Dynamic Joint Angles. BMC Sports Sci. Med. Rehabil. 2021, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Lachance, J.A.; Mazzocco, M.M. A Longitudinal Analysis of Sex Differences in Math and Spatial Skills in Primary School Age Children. Learn. Individ. Differ. 2006, 16, 195–216. [Google Scholar] [CrossRef]
- Popescu, C.; Matei, D.; Amzolini, A.M.; Trăistaru, M.R. Comprehensive Gait Analysis and Kinetic Intervention for Overweight and Obese Children. Children 2025, 12, 122. [Google Scholar] [CrossRef]
- Avramescu, E.T.; Amzolini, A.M.; Bumbea, A.M.; Patru, S.; Traistaru, M.R.; Neagoe, C.D.; Ionescu, G.; Munteanu, C.; Matei, D. Gait Analysis Technologies for Evaluating Biomechanical Deviations: Insights from a Pilot Study on Healthy Athletes. Balneo PRM Res. J. 2025, 16, 772–778. [Google Scholar] [CrossRef]
- Vladutu, B.M.; Matei, D.; Amzolini, A.M.; Kamal, C.; Traistaru, M.R. A Prospective Controlled Study on the Longitudinal Effects of Rehabilitation in Older Women with Primary Sarcopenia. Life 2025, 15, 609. [Google Scholar] [CrossRef]
- Vladutu, B.M.; Matei, D.; Genunche-Dumitrescu, A.; Kamal, C.; Traistaru, M.R. The Role of Rehabilitation Program in Managing the Triad of Sarcopenia, Obesity, and Chronic Pain. Life 2025, 15, 1174. [Google Scholar] [CrossRef]
- Cho, Y.; Park, J.W.; Nam, K. The Relationship between Foot Posture Index and Resting Calcaneal Stance Position in Elementary School Students. Gait Posture 2019, 74, 142–147. [Google Scholar] [CrossRef]
- Tsai, L.C.; Yu, B.; Mercer, V.S.; Gross, M.T. Comparison of Different Structural Foot Types for Measures of Standing Postural Control. J. Orthop. Sports Phys. Ther. 2006, 36, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Stodółka, J.; Stodółka, W.; Maćkała, K. Structural Asymmetry of Foot Arch Formation in Early School-Age Children. Biomed. Hum. Kinet. 2015, 7, 78–86. [Google Scholar] [CrossRef]
- Alfageme-García, P.; Calderón-García, J.F.; Martínez-Nova, A.; Hidalgo-Ruiz, S.; Martínez-Álvarez, M.; Rico-Martín, S. Backpacks Effect on Foot Posture in Schoolchildren with a Neutral Foot Posture: A Three-Year Prospective Study. Int. J. Environ. Res. Public Health 2020, 17, 7313. [Google Scholar] [CrossRef] [PubMed]
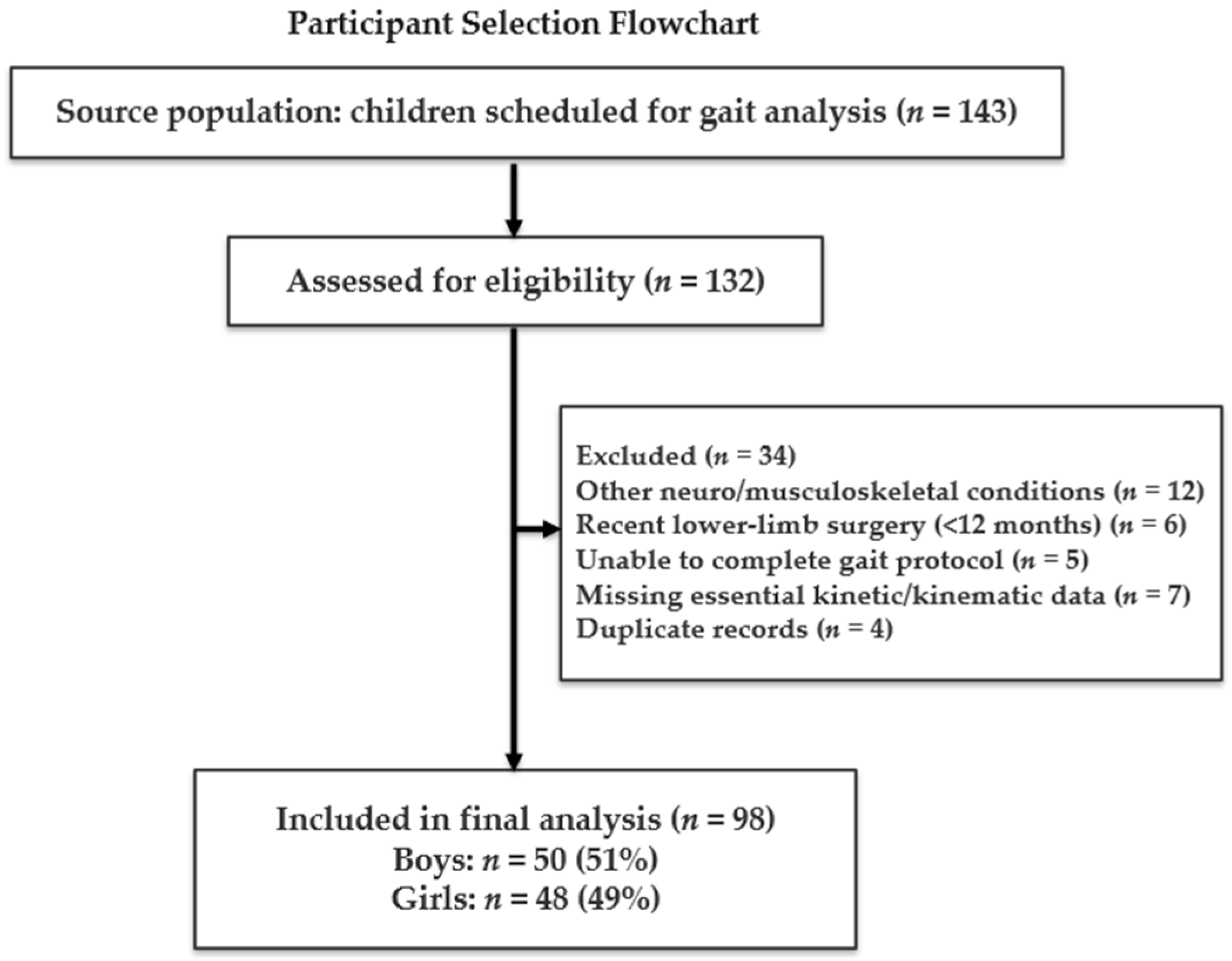
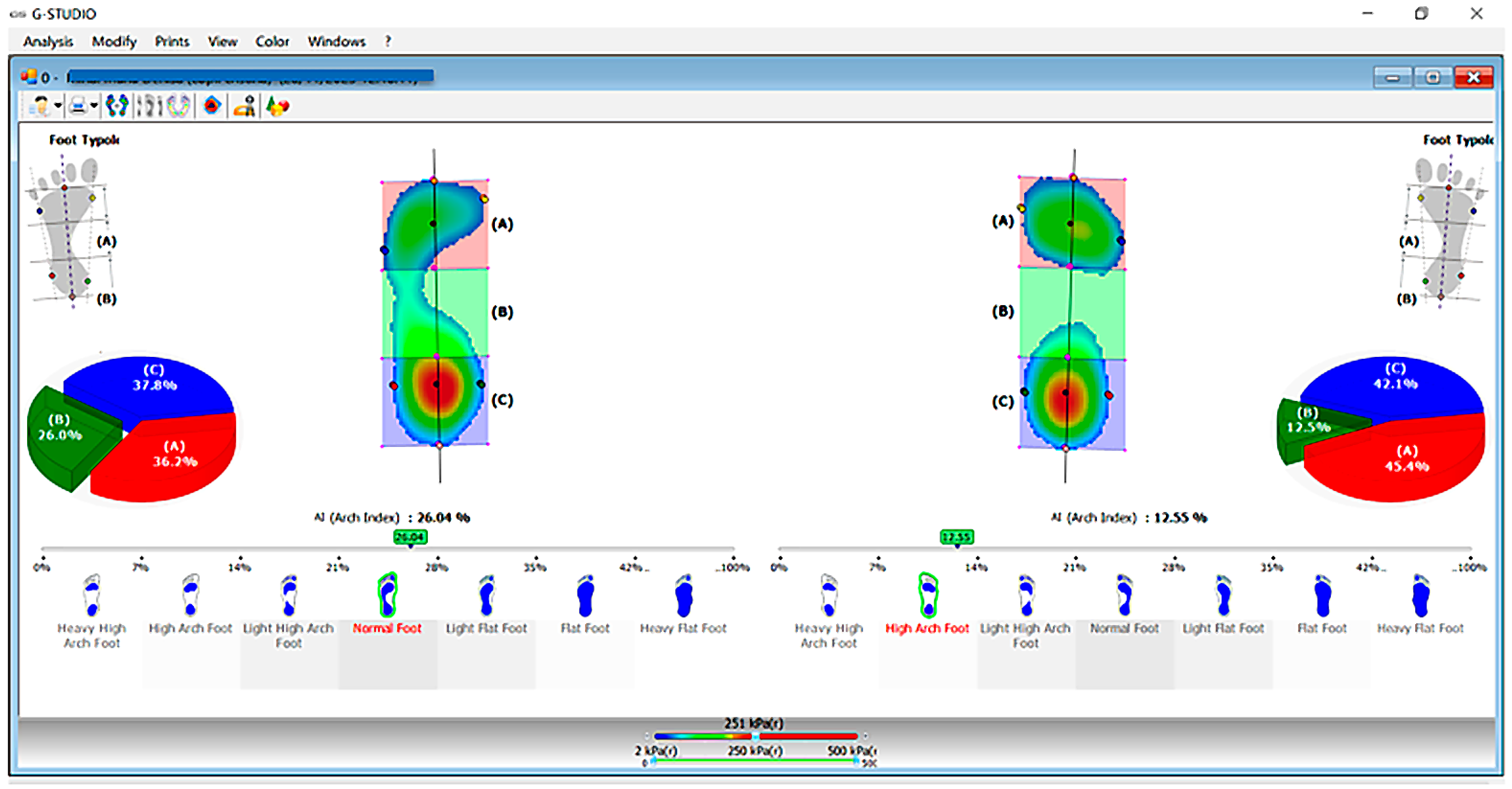
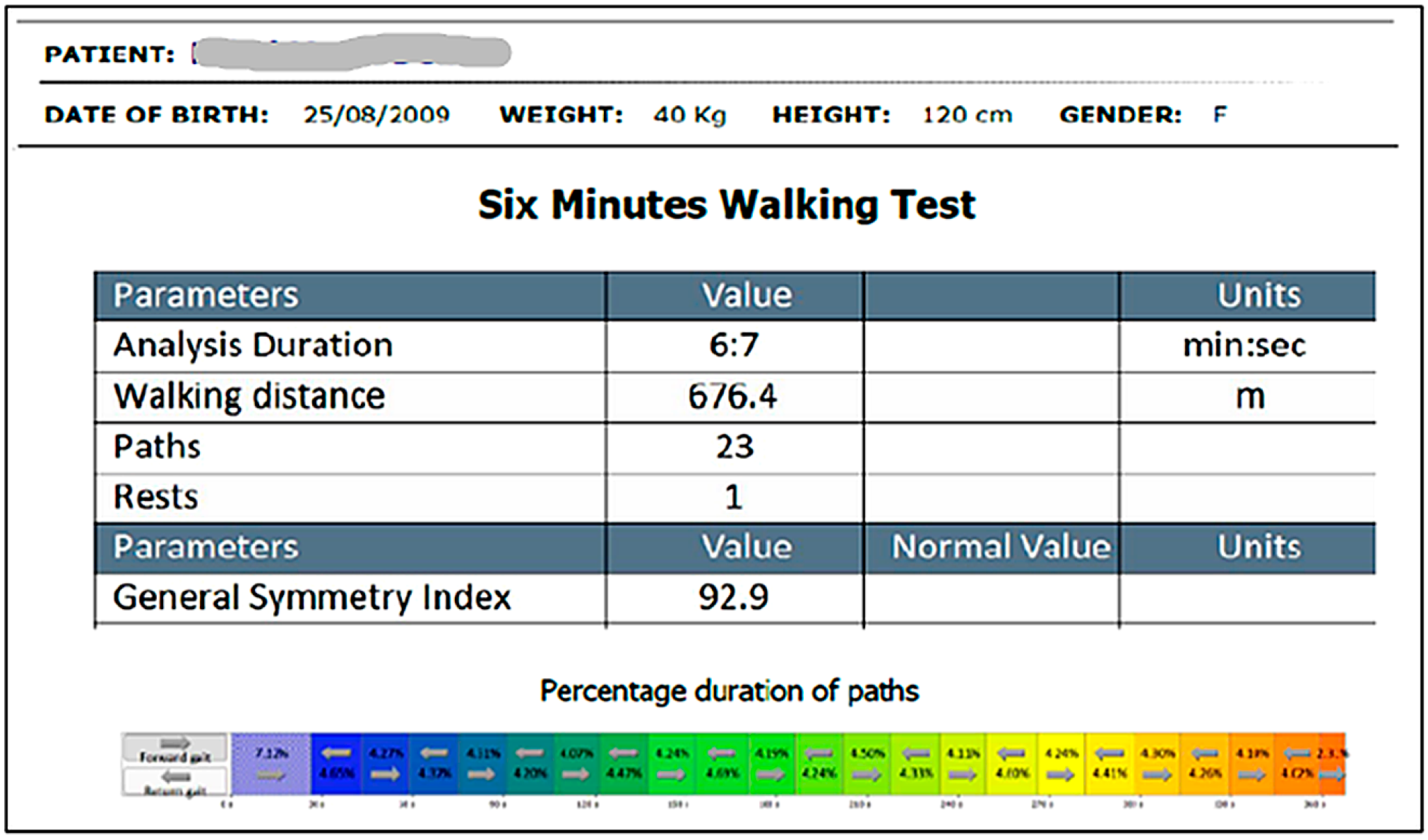

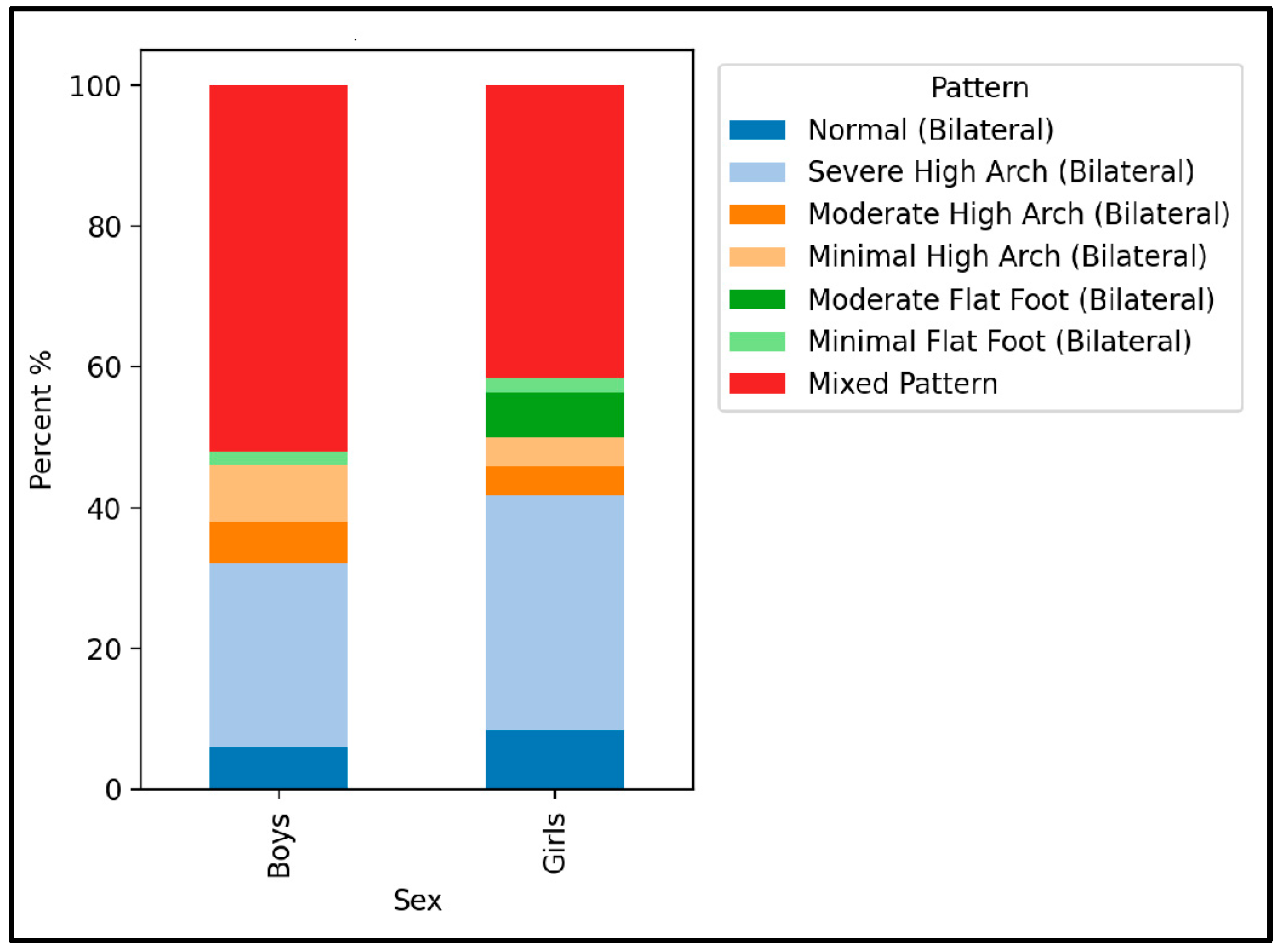

| Variable | Total (98) | Boys (50) | Girls (48) | p-Value |
|---|---|---|---|---|
| Age, years | 10.8 ± 1.47 (8–16) | 10.7 ± 1.30 (8–15) | 10.9 ± 1.64 (8–16) | 0.067 |
| Sex, n (%) | 98 (100%) | 50 (51%) | 48(49%) | 0.078 |
| BMI, kg/m2 | 20.1 ± 4.86 (12.58–34.13) | 19.96 ± 4.53 (13.69–34.13) | 20.29 ± 5.23 (12.58–32) | 0.062 |
| BMI categories, n (%) | 98 | 50 | 48 | |
| Normal weight | 82 (83.7%) | 45 (90%) | 37 (77%) | |
| Overweight | 9 (9.2%) | 3 (6%) | 6 (12.5%) | |
| Obese | 7 (7.1%) | 2 (4%) | 5 (10.5%) | |
| Living environment | Urban: 98 | Urban: 50 | Urban: 48 |
| Parameter | Count | Mean | Median | Std_Dev | Min | Max | Q1 | Q3 | Skewness | Kurtosis |
|---|---|---|---|---|---|---|---|---|---|---|
| TUG (seconds) | 98 | 14.16 | 13.83 | 2.59 | 9.64 | 22.29 | 12.2 | 15.9 | 0.599 | −0.026 |
| 6MWD (meters) | 98 | 568.37 | 561.15 | 70.68 | 401.3 | 781.8 | 510.2 | 611.85 | 0.149 | −0.157 |
| Symmetry_ index (%) | 98 | 94.61 | 95.05 | 2.91 | 82.2 | 98.7 | 93.55 | 96.68 | −1.551 | 3.425 |
| Average speed (m/s) | 98 | 1.15 | 1.19 | 0.28 | 0.14 | 1.68 | 1.05 | 1.31 | −1.367 | 3.298 |
| Average cadence (steps/min) | 98 | 117.03 | 115.75 | 10.4 | 90.7 | 145.7 | 110.6 | 124.15 | 0.216 | −0.068 |
| Parameter | Boys | Girls | ||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| TUG (seconds) | 50 | 13.81 | 2.23 | 48 | 14.53 | 2.89 |
| 6MWD (meters) | 50 | 573.79 | 73.81 | 48 | 562.73 | 67.58 |
| Symmetry index (%) | 50 | 93.99 | 3.01 | 48 | 95.26 | 2.69 |
| Average speed (m/s) | 50 | 1.16 | 0.31 | 48 | 1.14 | 0.24 |
| Average cadence (steps/min) | 50 | 118.44 | 10.13 | 48 | 115.57 | 10.57 |
| Parameter | BMI | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| Age (years) | Normal | 82 | 10.59 | 1.31 | 5 | 14 |
| Overweight | 9 | 11.22 | 1.3 | 10 | 14 | |
| Obese | 7 | 12.71 | 2.06 | 10 | 16 | |
| BMI (kg/m2) | Normal | 82 | 18.43 | 2.95 | 12.58 | 24.63 |
| Overweight | 9 | 26.32 | 1.26 | 25 | 28.17 | |
| Obese | 7 | 31.97 | 1.39 | 30.07 | 34.13 | |
| TUG (seconds) | Normal | 82 | 14.18 | 2.63 | 9.64 | 22.29 |
| Overweight | 9 | 14.03 | 2.96 | 11.49 | 19.44 | |
| Obese | 7 | 14.15 | 1.9 | 11.15 | 16.51 | |
| 6MWD (meters) | Normal | 82 | 566.13 | 68.84 | 401.3 | 703.6 |
| Overweight | 9 | 586.7 | 64.74 | 486.3 | 676.4 | |
| Obese | 7 | 571.07 | 103.09 | 500 | 781.8 | |
| Symmetry index (%) | Normal | 82 | 94.59 | 2.72 | 82.2 | 98.7 |
| Overweight | 9 | 95.91 | 2.35 | 91.3 | 98.6 | |
| Obese | 7 | 93.14 | 4.96 | 86 | 98.3 | |
| Average speed (m/s) | Normal | 82 | 1.14 | 0.29 | 0.14 | 1.59 |
| Overweight | 9 | 1.18 | 0.15 | 0.92 | 1.36 | |
| Obese | 7 | 1.26 | 0.32 | 0.86 | 1.68 | |
| Average cadence (steps/min) | Normal | 82 | 117.39 | 9.66 | 97.4 | 140.1 |
| Overweight | 9 | 119.12 | 9.7 | 107.1 | 139.5 | |
| Obese | 7 | 110.13 | 17.25 | 90.7 | 145.7 |
| Feet_Pattern | Parameter | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|
| Mixed Pattern | Age | 46 | 10.89 | 1.54 | 9 | 16 |
| BMI | 46 | 20.78 | 5.56 | 12.58 | 34.13 | |
| TUG | 46 | 14.25 | 2.27 | 9.64 | 19.44 | |
| 6MWD | 46 | 565.39 | 72.5 | 415.6 | 781.8 | |
| Symmetry index (%) | 46 | 92.72 | 3.05 | 82.2 | 98.3 | |
| Average speed (m/s) | 46 | 1.15 | 0.25 | 0.22 | 1.68 | |
| Average cadence (steps/min) | 46 | 115.89 | 11.06 | 90.7 | 140.1 | |
| Severe High Arch (Bilateral) | Age | 29 | 10.62 | 1.15 | 9 | 12 |
| BMI | 29 | 17.55 | 2.61 | 13.13 | 21.95 | |
| TUG | 29 | 13.66 | 2.67 | 9.81 | 20.3 | |
| 6MWD | 29 | 564.17 | 68.48 | 401.3 | 665.2 | |
| Symmetry index (%) | 29 | 96.01 | 1.22 | 93.9 | 98.3 | |
| Average speed (m/s) | 29 | 1.18 | 0.27 | 0.16 | 1.56 | |
| Average cadence (steps/min) | 29 | 118.5 | 9.08 | 102.1 | 137.5 | |
| Normal (Bilateral) | Age | 7 | 10.86 | 0.38 | 10 | 11 |
| BMI | 7 | 21.69 | 3 | 18.18 | 25.98 | |
| TUG | 7 | 14.18 | 2.03 | 11.49 | 17.78 | |
| 6MWD | 7 | 592.96 | 61.97 | 501.6 | 660.4 | |
| Symmetry index (%) | 7 | 97.5 | 1.17 | 95.4 | 98.7 | |
| Average speed (m/s) | 7 | 1.19 | 0.2 | 0.95 | 1.41 | |
| Average cadence (steps/min) | 7 | 119.9 | 7.49 | 107.5 | 126.8 | |
| Minimal Flat Foot (Bilateral) | Age | 2 | 10 | 2.83 | 8 | 12 |
| BMI | 2 | 27.09 | 4.21 | 24.11 | 30.07 | |
| TUG | 2 | 13.3 | 1.7 | 12.1 | 14.5 | |
| 6MWD | 2 | 480.45 | 27.65 | 460.9 | 500 | |
| Symmetry index (%) | 2 | 96.5 | 1.13 | 95.7 | 97.3 | |
| Average speed (m/s) | 2 | 1.3 | 0.33 | 1.06 | 1.53 | |
| Average cadence (steps/min) | 2 | 120.8 | 17.96 | 108.1 | 133.5 | |
| Minimal High Arch (Bilateral) | Age | 6 | 11.5 | 1.52 | 10 | 14 |
| BMI | 6 | 18.9 | 2.86 | 13.69 | 22.08 | |
| TUG | 6 | 13.11 | 2.34 | 10.3 | 16.65 | |
| 6MWD | 6 | 581.78 | 97.7 | 471.6 | 703.6 | |
| Symmetry index (%) | 6 | 95.4 | 1.74 | 93.3 | 97.5 | |
| Average speed (m/s) | 6 | 1.2 | 0.26 | 0.86 | 1.57 | |
| Average cadence (steps/min) | 6 | 110.65 | 9.22 | 101.3 | 121.2 | |
| Moderate High Arch (Bilateral) | Age | 5 | 11 | 1 | 10 | 12 |
| BMI | 5 | 21.92 | 5.47 | 17.29 | 30.92 | |
| TUG | 5 | 16.3 | 4.17 | 10.65 | 22.29 | |
| 6MWD | 5 | 605 | 45.58 | 533.3 | 654.2 | |
| Symmetry index (%) | 5 | 96.9 | 0.78 | 95.7 | 97.8 | |
| Average speed (m/s) | 5 | 1.02 | 0.45 | 0.43 | 1.67 | |
| Average cadence (steps/min) | 5 | 121.14 | 15.2 | 107.5 | 145.7 | |
| Moderate Flat Foot (Bilateral) | Age | 3 | 9.67 | 4.04 | 5 | 12 |
| BMI | 3 | 26.03 | 3.44 | 22.06 | 28.17 | |
| TUG | 3 | 16.82 | 4.1 | 12.19 | 19.97 | |
| 6MWD | 3 | 568.07 | 65.16 | 493.1 | 611.1 | |
| Symmetry index (%) | 3 | 96.77 | 1.23 | 95.4 | 97.8 | |
| Average speed (m/s) | 3 | 0.88 | 0.64 | 0.14 | 1.28 | |
| Average cadence (steps/min) | 3 | 117.13 | 7.96 | 110.6 | 126 |
| H-Statistic | p-Value | Significant | |
|---|---|---|---|
| Age (years) | 2.2295 | 0.8974 | no |
| BMI (kg/m2) | 18.2732 | 0.0056 | yes |
| TUG (seconds) | 5.9175 | 0.4325 | no |
| 6MWD (meters) | 7.1702 | 0.3054 | no |
| Symmetry index (%) | 51.5881 | 0.0000 | yes |
| Average speed (m/s) | 3.0578 | 0.8016 | no |
| Average cadence (steps/min) | 4.4042 | 0.6222 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traistaru, M.R.; Cealicu, M.; Matei, D.; Matei, M.A.; Anghelina, L.; Stoica, D. Chronic Implications of Bilateral Foot Pattern Variability in Schoolchildren. Healthcare 2025, 13, 2586. https://doi.org/10.3390/healthcare13202586
Traistaru MR, Cealicu M, Matei D, Matei MA, Anghelina L, Stoica D. Chronic Implications of Bilateral Foot Pattern Variability in Schoolchildren. Healthcare. 2025; 13(20):2586. https://doi.org/10.3390/healthcare13202586
Chicago/Turabian StyleTraistaru, Magdalena Rodica, Mihai Cealicu, Daniela Matei, Miruna Andreiana Matei, Liliana Anghelina, and Doru Stoica. 2025. "Chronic Implications of Bilateral Foot Pattern Variability in Schoolchildren" Healthcare 13, no. 20: 2586. https://doi.org/10.3390/healthcare13202586
APA StyleTraistaru, M. R., Cealicu, M., Matei, D., Matei, M. A., Anghelina, L., & Stoica, D. (2025). Chronic Implications of Bilateral Foot Pattern Variability in Schoolchildren. Healthcare, 13(20), 2586. https://doi.org/10.3390/healthcare13202586






