The Diagnostic Performance of Transvaginal Ultrasound for Posterior Compartment Endometriosis Compared to Laparoscopic and Histopathological Findings: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Full-text available in languages other than English.
- Designed as a systematic review, meta-analysis, comment, letter to the editor, or conference abstract.
- Published before 2015.
- Conducted on animal or experimental models.
- Protocols focused on imaging techniques other than transvaginal ultrasound.
- Solely targeted treatment options or fertility assessment.
- Did not evaluate endometriosis of the posterior pelvic compartment.
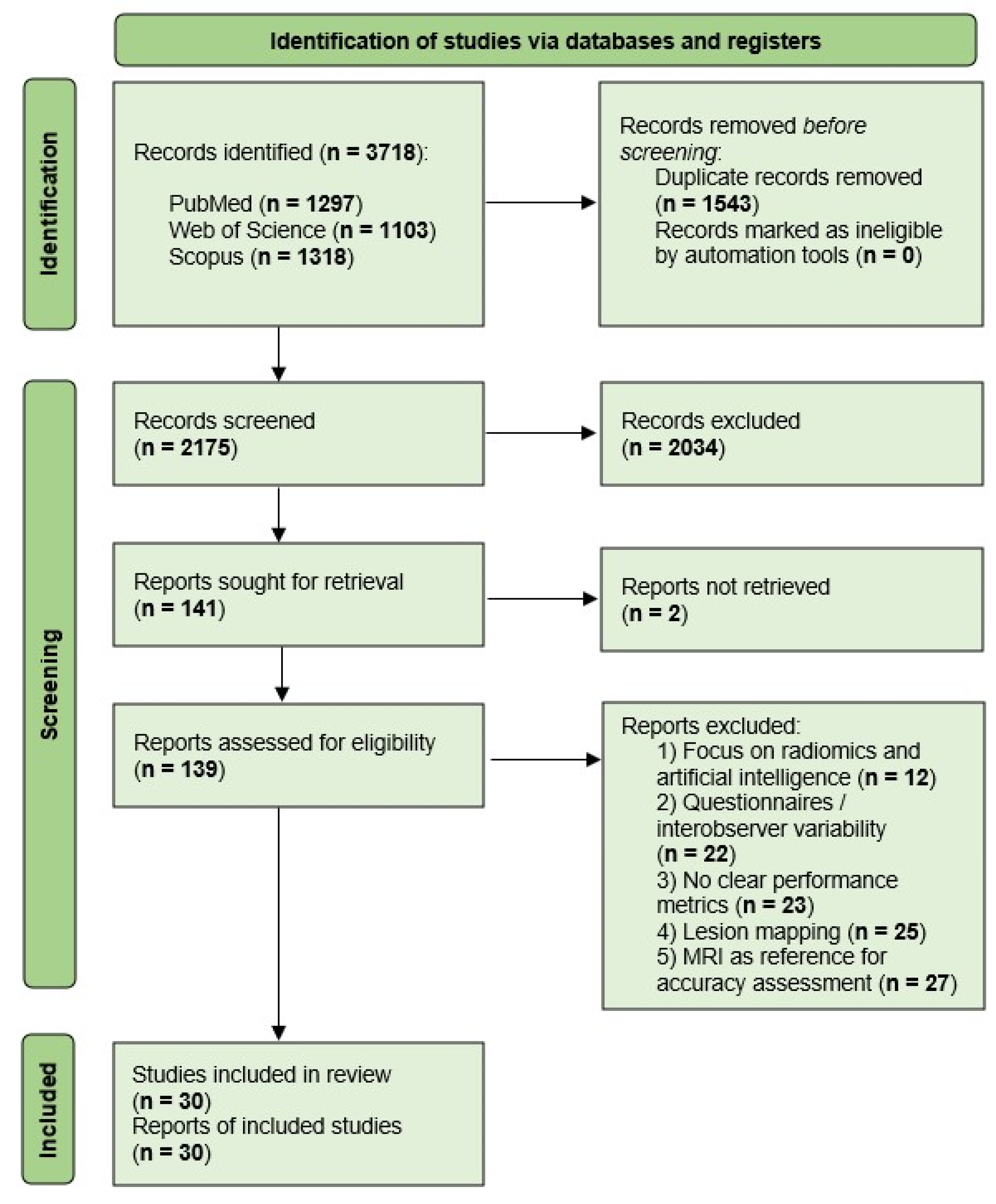
3. Results
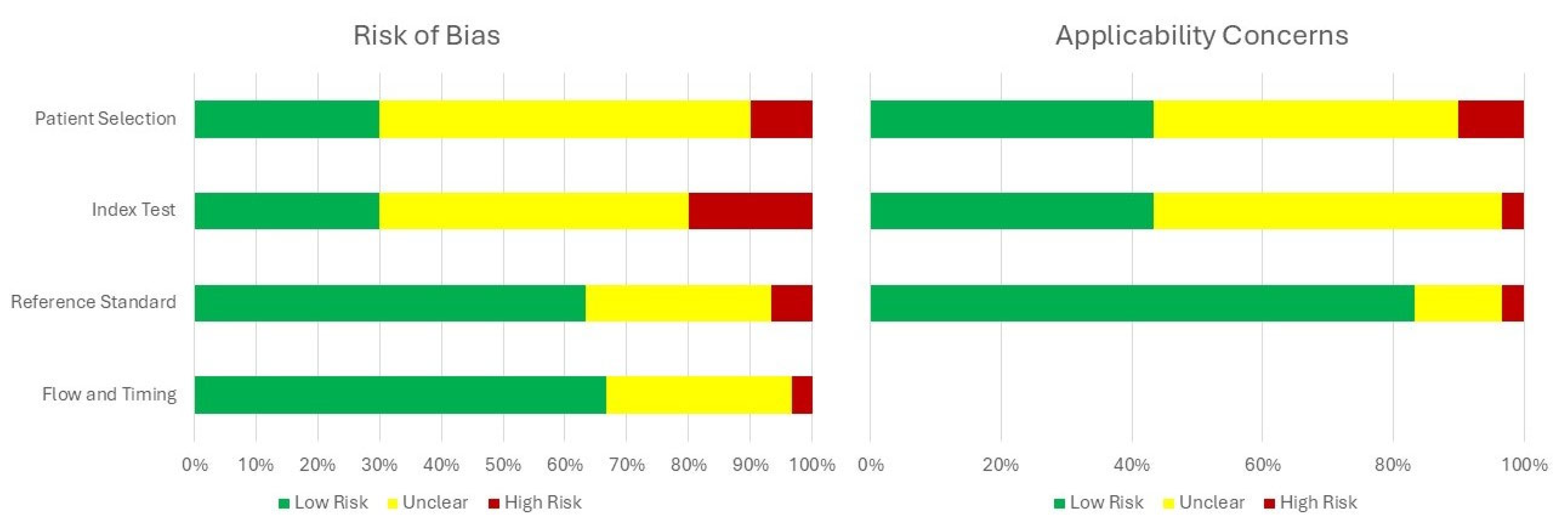
3.1. Study Selection and Characteristics
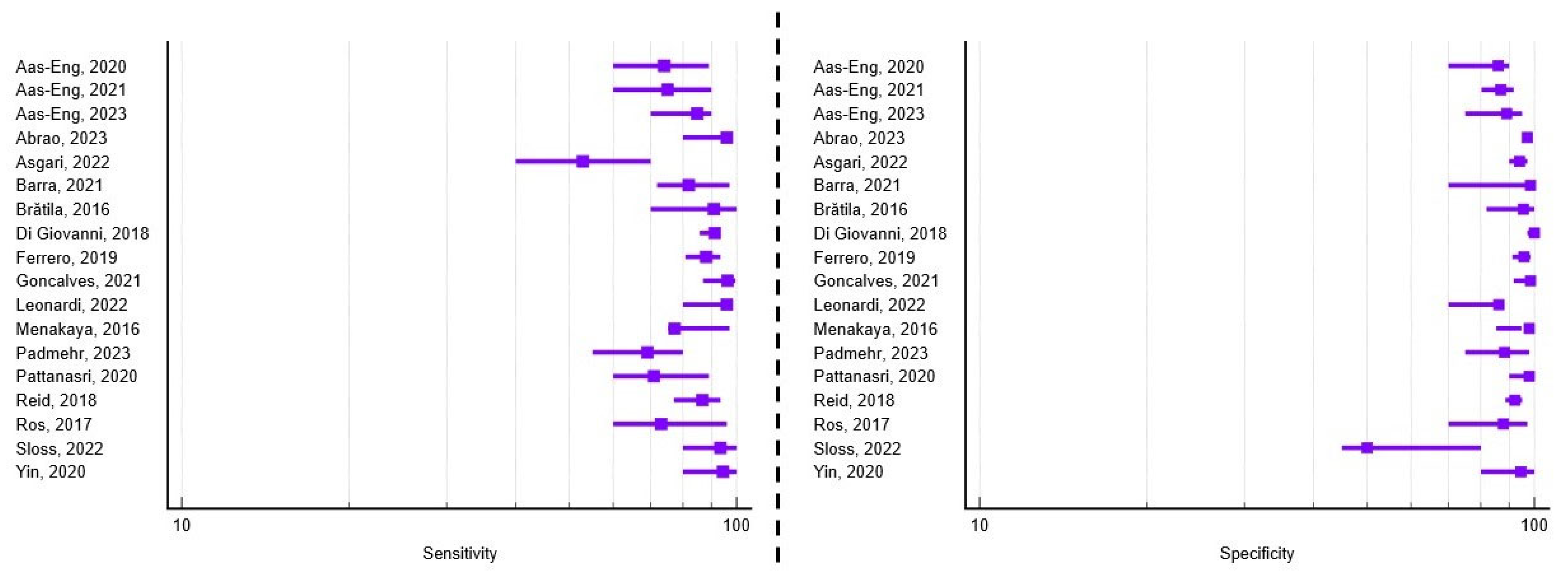
3.2. Diagnostic Performance of TVUS for Rectosigmoid Endometriosis
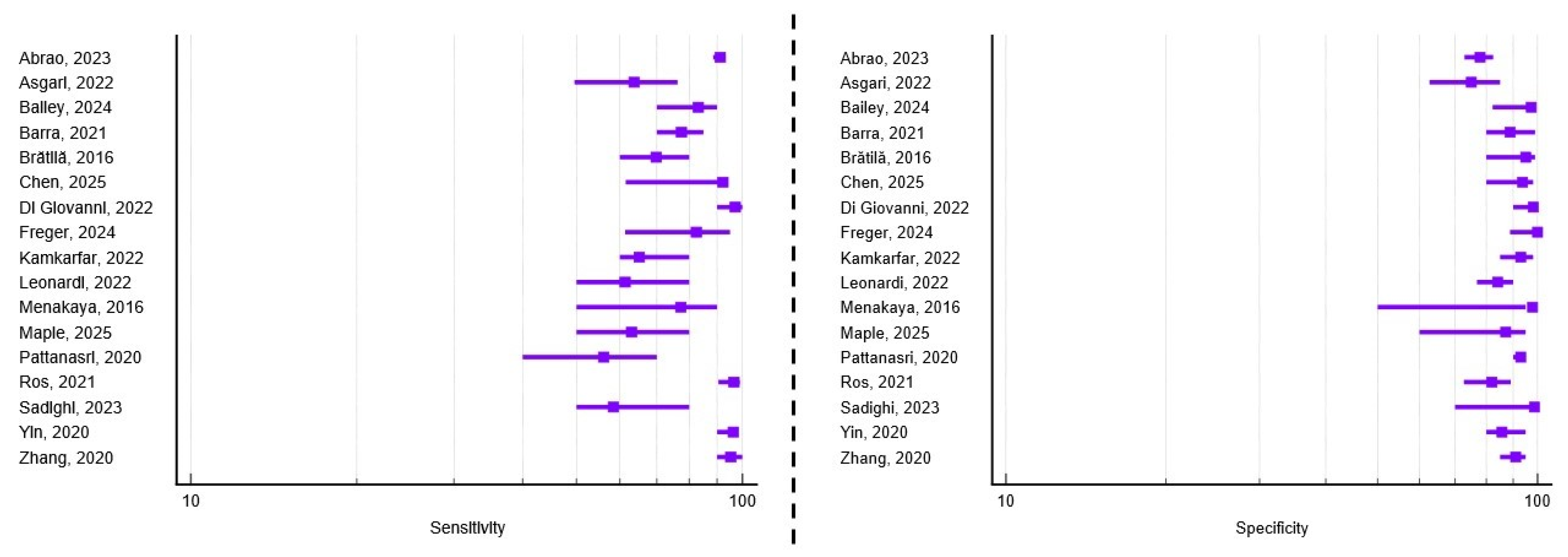
3.3. Diagnostic Performance of TVUS for Uterosacral Ligament Involvement
3.4. Diagnostic Performance of TVUS for Pouch of Douglas Obliteration
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acc | Accuracy |
| ADC | Apparent Diffusion Coefficient |
| AUC | Area Under the Curve |
| DIE | Deep Infiltrating Endometriosis |
| DWI | Diffusion-Weighted Imaging |
| ESHRE | European Society of Human Reproduction and Embryology |
| IDEA | International Deep Endometriosis Analysis |
| MRI | Magnetic Resonance Imaging |
| N/A | Not Applicable |
| NICE | National Institute for Health and Care Excellence |
| PIRT | Participants, Index test, Reference standard, Target condition |
| PDO | Pouch of Douglas Obliteration |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QUADAS-2 | Quality Assessment of Diagnostic Accuracy Studies, Version 2 |
| RS | Rectosigmoid Endometriosis |
| Se | Sensitivity |
| SOGC | Society of Obstetricians and Gynecologists of Canada |
| Sp | Specificity |
| TVUS | Transvaginal Ultrasound |
| UBESS | Ultrasound-Based Endometriosis Staging System |
| USL | Uterosacral Ligament |
References
- As-Sanie, S.; Mackenzie, S.C.; Morrison, L.; Schrepf, A.; Zondervan, K.T.; Horne, A.W.; Missmer, S.A. Endometriosis: A Review. JAMA 2025, 334, 64–78. [Google Scholar] [CrossRef]
- Moradi, Y.; Shams-Beyranvand, M.; Khateri, S.; Gharahjeh, S.; Tehrani, S.; Varse, F.; Tiyuri, A.; Najmi, Z. A systematic review on the prevalence of endometriosis in women. Indian J. Med. Res. 2021, 154, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Darbà, J.; Marsà, A. Economic Implications of Endometriosis: A Review. PharmacoEconomics 2022, 40, 1143–1158. [Google Scholar] [CrossRef]
- Soliman, A.M.; Taylor, H.S.; Bonafede, M.; Nelson, J.K.; Castelli-Haley, J. Incremental direct and indirect cost burden attributed to endometriosis surgeries in the United States. Fertil. Steril. 2017, 107, 1181–1190.e2. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.P.; Andres, M.P.; Carmona, F.; Baracat, E.C.; Abrão, M.S. Deep Endometriosis: The Involvement of Multiple Pelvic Compartments Is Associated with More Severe Pain Symptoms and Infertility. Reprod. Sci. 2023, 30, 1668–1675. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Eguez, P.M.; Forcada, P.; Ternero, E.; Martínez, C.; Pascual, M.Á.; Guerriero, S. Diagnostic accuracy of sliding sign for detecting pouch of Douglas obliteration and bowel involvement in women with suspected endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 60, 477–486. [Google Scholar] [CrossRef]
- Moura, A.P.C.; Ribeiro, H.S.A.A.; Bernardo, W.M.; Simões, R.; Torres, U.S.; D’Ippolito, G.; Bazot, M.; Ribeiro, P.A.A.G. Accuracy of transvaginal sonography versus magnetic resonance imaging in the diagnosis of rectosigmoid endometriosis: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0214842. [Google Scholar]
- Nisenblat, V.; Prentice, L.; Bossuyt, P.M.M.; Farquhar, C.; Hull, M.L.; Johnson, N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 7, CD012281. [Google Scholar] [CrossRef] [PubMed]
- Young, S.W.; Jha, P.; Chamié, L.; Rodgers, S.; Kho, R.M.; Horrow, M.M.; Glanc, P.; Feldman, M.; Groszmann, Y.; Khan, Z.; et al. Society of Radiologists in Ultrasound Consensus on Routine Pelvic US for Endometriosis. Radiology 2024, 311, e232191. [Google Scholar] [CrossRef]
- Condous, G.; Gerges, B.; Thomassin-Naggara, I.; Becker, C.; Tomassetti, C.; Krentel, H.; van Herendael, B.J.; Malzoni, M.; Abrao, M.S.; Saridogan, E.; et al. Non-invasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An International Consensus Statement. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2024, 64, 129–144. [Google Scholar] [CrossRef]
- Leonardi, M.; Uzuner, C.; Mestdagh, W.; Lu, C.; Guerriero, S.; Zajicek, M.; Dueckelmann, A.; Filippi, F.; Buonomo, F.; Pascual, M.A.; et al. Diagnostic accuracy of transvaginal ultrasound for detection of endometriosis using International Deep Endometriosis Analysis (IDEA) approach: Prospective international pilot study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 60, 404–413. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Aas-Eng, M.K.; Dauser, B.; Lieng, M.; Diep, L.M.; Leonardi, M.; Condous, G.; Hudelist, G. Transvaginal sonography accurately measures lesion-to-anal-verge distance in women with deep endometriosis of the rectosigmoid. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2020, 56, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Aas-Eng, M.K.; Lieng, M.; Dauser, B.; Diep, L.M.; Leonardi, M.; Condous, G.; Hudelist, G. Transvaginal sonography determines accurately extent of infiltration of rectosigmoid deep endometriosis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 58, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Aas-Eng, M.K.; Young, V.S.; Dormagen, J.B.; Pripp, A.H.; Hudelist, G.; Lieng, M. Lesion-to-anal-verge distance in rectosigmoid endometriosis on transvaginal sonography vs magnetic resonance imaging: Prospective study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2023, 61, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Abrao, M.S.; Andres, M.P.; Gingold, J.A.; Rius, M.; Neto, J.S.; Goncalves, M.O. Di Giovanni, A.; Malzoni, M.; Carmona, F. Preoperative Ultrasound Scoring of Endometriosis by AAGL 2021 Endometriosis Classification Is Concordant with Laparoscopic Surgical Findings and Distinguishes Early from Advanced Stages. J. Minim. Invasive Gynecol. 2023, 30, 363–373. [Google Scholar] [CrossRef]
- Arion, K.; Aksoy, T.; Allaire, C.; Noga, H.; Williams, C.; Bedaiwy, M.A.; Yong, P.J. Prediction of Pouch of Douglas Obliteration: Point-of-care Ultrasound Versus Pelvic Examination. J. Minim. Invasive Gynecol. 2019, 26, 928–934. [Google Scholar] [CrossRef]
- Asgari, Z.; Hosseini, R.; Sepidarkish, M.; Nabati, A. Accuracy of transvaginal and transrectal ultrasounds in the diagnosis of endometriosis: A retrospective cohort study. Int. J. Reprod. Biomed. 2022, 20, 365–376. [Google Scholar] [CrossRef]
- Bailey, F.; Gaughran, J.; Mitchell, S.; Ovadia, C.; Holland, T.K. Diagnosis of superficial endometriosis on transvaginal ultrasound by visualization of peritoneum of pouch of Douglas. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2024, 63, 105–112. [Google Scholar] [CrossRef]
- Barra, F.; Leone Roberti Maggiore, U.; Evangelisti, G.; Scala, C.; Alessandri, F.; Vellone, V.G.; Stabilini, C.; Ferrero, S. A prospective study comparing rectal water contrast-transvaginal ultrasonography with sonovaginography for the diagnosis of deep posterior endometriosis. Acta Obstet. Gynecol. Scand. 2021, 100, 1700–1711. [Google Scholar] [CrossRef]
- Brătilă, E.; Comandaşu, D.E.; Coroleucă, C.; Cîrstoiu, M.M.; Berceanu, C.; Mehedintu, C.; Bratila, P.; Vladareanu, S. Diagnosis of endometriotic lesions by sonovaginography with ultrasound gel. Med. Ultrason. 2016, 18, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, L.; Wang, S.; Chen, M.; Zhang, C.; Fang, Y.; Deng, M.; Jin, C. Douglas Pouch Fluid Improves the Accuracy of Transvaginal Ultrasound in the Diagnosis of Uterosacral Ligaments Deep Infiltration Endometriosis: A Prospective Study. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2025, 44, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, A.; Casarella, L.; Coppola, M.; Iuzzolino, D.; Rasile, M.; Malzoni, M. Combined Transvaginal/Transabdominal Pelvic Ultrasonography Accurately Predicts the 3 Dimensions of Deep Infiltrating Bowel Endometriosis Measured after Surgery: A Prospective Study in a Specialized Center. J. Minim. Invasive Gynecol. 2018, 25, 1231–1240. [Google Scholar] [CrossRef]
- Di Giovanni, A.; Casarella, L.; Coppola, M.; Falcone, F.; Iuzzolino, D.; Rasile, M.; Malzoni, M. Ultrasound Evaluation of Retrocervical and Parametrial Deep Endometriosis on the Basis of Surgical Anatomic Landmarks. J. Minim. Invasive Gynecol. 2022, 29, 1140–1148. [Google Scholar] [CrossRef]
- Ferrero, S.; Scala, C.; Stabilini, C.; Vellone, V.G.; Barra, F.; Leone Roberti Maggiore, U. Transvaginal sonography with vs without bowel preparation in diagnosis of rectosigmoid endometriosis: Prospective study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2019, 53, 402–409. [Google Scholar] [CrossRef]
- Freger, S.M.; Turnbull, V.; McGowan, K.; Leonardi, M. Prospective diagnostic test accuracy of transvaginal ultrasound posterior approach for uterosacral ligament and torus uterinus deep endometriosis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2024, 63, 263–270. [Google Scholar] [CrossRef]
- Goncalves, M.O.; Siufi Neto, J.; Andres, M.P.; Siufi, D.; de Mattos, L.A.; Abrao, M.S. Systematic evaluation of endometriosis by transvaginal ultrasound can accurately replace diagnostic laparoscopy, mainly for deep and ovarian endometriosis. Hum. Reprod. 2021, 36, 1492–1500. [Google Scholar] [CrossRef]
- Kamkarfar, P.; Shahriyaripoor, R.; Rokhgireh, S.; Mostafavi, S.R.S.; Chaichian, S.; Mehdizadeh Kashi, A. Comparison of diagnostic values of transvaginal sonography with laparoscopic and histological results in the evaluation of uterosacral ligaments’ involvement in endometriosis patients. Casp. J. Intern. Med. 2022, 13, 705–712. [Google Scholar]
- Leonardi, M.; Robledo, K.P.; Espada, M.; Vanza, K.; Condous, G. SonoPODography: A new diagnostic technique for visualizing superficial endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 124–131. [Google Scholar] [CrossRef]
- Menakaya, U.; Reid, S.; Lu, C.; Gerges, B.; Infante, F.; Condous, G. Performance of ultrasound-based endometriosis staging system (UBESS) for predicting level of complexity of laparoscopic surgery for endometriosis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2016, 48, 786–795. [Google Scholar] [CrossRef]
- Maple, S.; Bezak, E.; Chalmers, K.J.; Parange, N. Ultrasound of the uterosacral ligaments: A reliability study for diagnosing endometriosis in Australian non-specialised medical imaging and radiology settings. Australas. J. Ultrasound Med. 2025, 28, e12420. [Google Scholar] [CrossRef] [PubMed]
- Padmehr, R.; Shadjoo, K.; Mohazzab, A.; Gorgin, A.; Karegar, R.; Jaberipour, P.; Sehat, Z.; Maleki, N. Transvaginal sonography and surgical findings in the diagnosis of endometriosis individuals: A cross-sectional study. Int. J. Reprod. Biomed. 2023, 21, 471–480. [Google Scholar] [CrossRef]
- Pattanasri, M.; Ades, A.; Nanayakkara, P. Correlation between ultrasound findings and laparoscopy in prediction of deep infiltrating endometriosis (DIE). Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 946–951. [Google Scholar] [CrossRef]
- Reid, S.; Espada, M.; Lu, C.; Condous, G. To determine the optimal ultrasonographic screening method for rectal/rectosigmoid deep endometriosis: Ultrasound “sliding sign,” transvaginal ultrasound direct visualization or both? Acta Obstet. Gynecol. Scand. 2018, 97, 1287–1292. [Google Scholar] [CrossRef]
- Ros, C.; Martínez-Serrano, M.J.; Rius, M.; Abrao, M.S.; Munrós, J.; Martínez-Zamora, M.Á.; Gracia, M.; Carmona, F. Bowel Preparation Improves the Accuracy of Transvaginal Ultrasound in the Diagnosis of Rectosigmoid Deep Infiltrating Endometriosis: A Prospective Study. J. Minim. Invasive Gynecol. 2017, 24, 1145–1151. [Google Scholar] [CrossRef]
- Ros, C.; de Guirior, C.; Mension, E.; Rius, M.; Valdés-Bango, M.; Tortajada, M.; Matas, I.; Martínez-Zamora, M.Á.; Gracia, M.; Carmona, F. Transvaginal ultrasound for diagnosis of deep endometriosis involving uterosacral ligaments, torus uterinus and posterior vaginal fornix: Prospective study. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021, 58, 926–932. [Google Scholar] [CrossRef]
- Sadighi, N.; Moradi, B.; Gity, M.; Boroujerdirad, B.; Tanha, F.; Daneshvar, R.; Azadbakht, J. Diagnostic Accuracy of Transvaginal Sonography for Deeply Infiltrating Endometriosis and Pouch of Douglas Obliteration in the Presence or Absence of Ovarian Endometrioma. Iran. J. Radiol. 2023, 19, e127068. [Google Scholar] [CrossRef]
- Sloss, S.; Mooney, S.; Ellett, L.; Readman, E.; Ma, T.; Brouwer, R.; Yang, N.; Ireland-Jenkin, K.; Stone, K.; Maher, P. Preoperative Imaging in Patients with Deep Infiltrating Endometriosis: An Important Aid in Predicting Depth of Infiltration in Rectosigmoid Disease. J. Minim. Invasive Gynecol. 2022, 29, 633–640. [Google Scholar] [CrossRef]
- Venkatesh, S.; Anjali, M.; Vasudeva, A.; Kumar, P. Sliding Sign and Gel Sonovaginography: A Sneak Peek Prior to Laparoscopy in Patients with Endometriosis. J. Hum. Reprod. Sci. 2020, 13, 26–30. [Google Scholar] [CrossRef]
- Yin, S.; Lin, Q.; Xu, F.; Xu, J.; Zhang, Y. Diagnosis of Deep Infiltrating Endometriosis Using Transvaginal Ultrasonography. Front. Med. 2020, 7, 567929. [Google Scholar] [CrossRef]
- Zhang, X.; He, T.; Shen, W. Comparison of physical examination, ultrasound techniques and magnetic resonance imaging for the diagnosis of deep infiltrating endometriosis: A systematic review and meta-analysis of diagnostic accuracy studies. Exp. Ther. Med. 2020, 20, 3208–3220. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.; Xu, F.; Lin, Q.; Xu, J.; Du, B. Evaluation of Uterosacral Ligament Involvement in Deep Endometriosis by Transvaginal Ultrasonography. Front. Pharmacol. 2019, 10, 374. [Google Scholar] [CrossRef]
- Deslandes, A.; Parange, N.; Childs, J.T.; Osborne, B.; Bezak, E. Current Status of Transvaginal Ultrasound Accuracy in the Diagnosis of Deep Infiltrating Endometriosis Before Surgery: A Systematic Review of the Literature. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2020, 39, 1477–1490. [Google Scholar] [CrossRef]
- Guerriero, S.; Saba, L.; Pascual, M.A.; Ajossa, S.; Rodriguez, I.; Mais, V.; Alcazar, J.L. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 51, 586–595. [Google Scholar] [CrossRef]
- Thomassin-Naggara, I.; Dolciami, M.; Chamie, L.P.; Guerra, A.; Bharwani, N.; Freeman, S.; Rousset, P.; Manganaro, L.; The ESUR Endometriosis Working Group; Avesani, G.; et al. ESUR consensus MRI for endometriosis: Indications, reporting, and classifications. Eur. Radiol. 2025, 27, 1–9. [Google Scholar] [CrossRef]
- Hofmann, B.; Brandsaeter, I.Ø.; Kjelle, E. Variations in wait times for imaging services: A register-based study of self-reported wait times for specific examinations in Norway. BMC Health Serv. Res. 2023, 23, 1287. [Google Scholar]
- Expert Panel on GYN and OB Imaging; Feldman, M.K.; Wasnik, A.P.; Adamson, M.; Dawkins, A.A.; Dibble, E.H.; Jones, L.P.; Joshi, G.; Melamud, K.; Patel-Lippmann, K.K.; et al. ACR Appropriateness Criteria® Endometriosis. J. Am. Coll. Radiol. 2024, 21, S384–S395. [Google Scholar]
- Ebrahimi, M.; Naghdi, S.; Davari-Tanha, F.; Moradi, B.; Feizabad, E.; Majidi, K. Value of ultrasound-based endometriosis staging system in anticipating complexity of laparoscopic surgery. Fertil. Steril. 2025, 123, 893–898. [Google Scholar] [CrossRef]
- Guerriero, S.; Saba, L.; Ajossa, S.; Peddes, C.; Angiolucci, M.; Perniciano, M.; Melis, G.B.; Alcázar, J.L. Three-dimensional ultrasonography in the diagnosis of deep endometriosis. Hum. Reprod. 2014, 29, 1189–1198. [Google Scholar] [CrossRef]
- Guerriero, S.; Alcázar, J.L.; Pascual, M.A.; Ajossa, S.; Perniciano, M.; Piras, A.; Mais, V.; Piras, B.; Schirru, F.; Benedetto, M.G.; et al. Deep Infiltrating Endometriosis: Comparison Between 2-Dimensional Ultrasonography (US), 3-Dimensional US, and Magnetic Resonance Imaging. J. Ultrasound Med. 2018, 37, 1511–1521. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

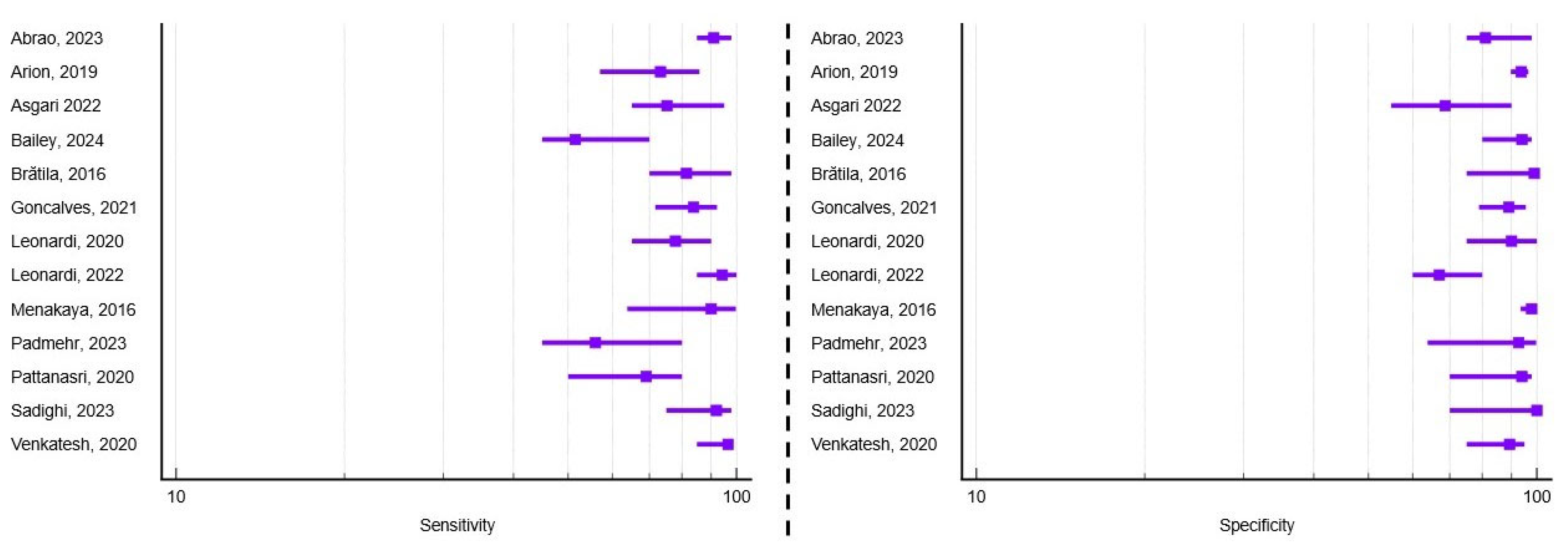
| No. | First Author and Year | Country | Study Design | Sample Size | Mean Age (Years) | Transducer Frequency (MHz) | Type of Surgical intervention | RS Performance (%) | USL Performance (%) | PDO Performance (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Aas-Eng, 2020 [13] | Austria Australia Norway | Prospective Multicenter | 147 | 35.3 | 5–9 | Bowel resection | Se = 74 Sp = 86 | N/A | N/A |
| 2. | Aas-Eng, 2021 [14] | Austria Australia Norway | Prospective Multicenter | 207 | 35.7 | 5–9 | Bowel resection | Se = 75 Sp = 87 | N/A | N/A |
| 3. | Aas-Eng, 2023 [15] | Norway | Prospective Single-center | 75 | 38.3 | 5–9 | Bowel resection | Se = 85 Sp = 89 | N/A | N/A |
| 4. | Abrao, 2023 [16] | Brazil Italy Spain | Retrospective Multicenter | 878 | 37.3 | 4–9 | Bowel resection Electroablation | Se = 96.1 Sp = 97 Acc = 96.7 | Se = 91.1 Sp = 77.9 Acc = 86.5 | Se = 93 Sp = 81 Acc = 85.9 |
| 5. | Arion, 2019 [17] | Canada | Prospective Single-center | 269 | 34.4 | 5–9 | Lesion excision | N/A | N/A | Se = 73.2 Sp = 93.9 |
| 6. | Asgari, 2022 [18] | Iran | Retrospective Single-center | 119 | 35.4 | 5–9 | Bowel resection/shaving Lesion excision | Se = 52.9 Sp = 94.1 Acc = 73.5 | Se = 63.6 Sp = 75.1 Acc = 69.3 | Se = 75.1 Sp = 68.6 Acc = 71.8 |
| 7. | Bailey, 2024 [19] | UK | Retrospective Single-center | 100 | 35.2 | 5–9 | Lesion excision | N/A | Se = 83.3 Sp = 97.4 Acc = 81.6 | Se = 51.5 Sp = 94 Acc = 87.4 |
| 8. | Barra, 2021 [20] | Brazil | Prospective Single-center | 281 | 36.8 | 6–9 | Bowel resection Lesion excision | Se = 82 Sp = 98.5 Acc = 93.6 | Se = 77.6 Sp = 88.8 Acc = 82.6 | N/A |
| 9. | Brătilă, 2016 [21] | Romania | Prospective Multicenter | 193 | 32 | 7.5 | Bowel shaving Lesion excision | Se = 91 Sp = 95.5 | Se = 69.7 Sp = 95 | Se = 81.5 Sp = 99 |
| 10. | Chen, 2025 [22] | China | Prospective Single-center | 42 | 36.4 | 6–12 | Laparoscopy Biopsy | N/A | Se = 92.3 Sp = 93.8 Acc = 92.9 | N/A |
| 11. | Di Giovanni, 2018 [23] | Italy | Prospective Single-center | 328 | 34.9 | 5–9 | Bowel resection/shaving | Se = 91.4 Sp = 100 Acc = 93 | N/A | N/A |
| 12. | Di Giovanni, 2022 [24] | Italy | Prospective Single-center | 4983 | Not reported * | 5–9 | Lesion excision | N/A | Se = 97 Sp = 98 | N/A |
| 13. | Ferrero, 2019 [25] | Italy | Prospective Single-center | 262 | 34.1 | 6–12 | Bowel resection | Se = 88.1 Sp = 95.8 Acc = 92.3 | N/A | N/A |
| 14. | Freger, 2024 [26] | Canada | Prospective Single-center | 54 | 35.2 | 4–9 | Laparoscopy Biopsy | N/A | Se = 82.6 Sp = 100 Acc = 92.6 | N/A |
| 15. | Goncalves, 2021 [27] | Brazil | Prospective Single-center | 120 | 33.6 | 6–12 | Bowel resection/shaving Lesion excision | Se = 96.2 Sp = 98.5 Acc = 97.1 | N/A | Se = 83.9 Sp = 89.1 Acc = 86.7 |
| 16. | Kamkarfar, 2022 [28] | Iran | Prospective Single-center | 80 | 34.4 | 5–9 | Lesion excision Biopsy | N/A | Se = 65 Sp = 93 | N/A |
| 17. | Leonardi, 2020 [29] | Australia | Prospective Single-center | 42 | Not reported * | 5–9 | Laparoscopy Biopsy | N/A | N/A | Se = 77.7 Sp = 100 Acc = 83.3 |
| 18. | Leonardi, 2022 [11] | Australia Austria Germany Israel Italy Spain | Prospective Multicenter | 273 | 34 | Not reported individually for each center * | Bowel resection/shaving Lesion excision Biopsy only | Se = 96 Sp = 86.2 Acc = 89.8 | Se = 61.2 Sp = 84.2 Acc = 74.6 | Se = 94.2 Sp = 66.9 Acc = 77.3 |
| 19. | Menakaya, 2016 [30] | Australia | Combined Multicenter | 192 | 23.7 | 7.5 | Laparoscopy Biopsy | Se = 83.3 Sp = 90.8 Acc = 89.4 | Se = 77.3 Sp = 97.8 Acc = 92 | Se = 90.2 Sp = 98 Acc = 96 |
| 20. | Maple, 2025 [31] | Australia | Retrospective Single-center | 42 | 33 | 3–11 | Laparoscopy Biopsy | N/A | Se = 63 Sp = 87 | N/A |
| 21. | Padmehr, 2023 [32] | Iran | Retrospective Single-center | 170 | 34.4 | Not reported * | Bowel resection Lesion excision | Se = 69 Sp = 88.3 Acc = 84.8 | N/A | Se = 56 Sp = 92.8 Acc = 88.9 |
| 22. | Pattanasri, 2020 [33] | Australia | Retrospective Single-center | 119 | 36 | Not reported * | Bowel resection Lesion excision | Se = 71 Sp = 98 Acc = 85 | Se = 56 Sp = 93 Acc = 75 | Se = 69 Sp = 94 Acc = 82 |
| 23. | Reid, 2018 [34] | Australia | Prospective Multicenter | 376 | Not reported * | 7.5 | Bowel resection | Se = 86.8 Sp = 92.3 Acc = 87 | N/A | N/A |
| 24. | Ros, 2017 [35] | Spain | Retrospective Single-center | 40 | 36.8 | 5–9 | Bowel resection | Se = 73 Sp = 88 Acc = 82.5 | N/A | N/A |
| 25. | Ros, 2021 [36] | Spain | Prospective Single-center | 172 | 38.3 | 5–9 | Laparoscopy Biopsy | N/A | Se = 96.6 Sp = 82.1 Acc = 89.5 | N/A |
| 26. | Sadighi, 2023 [37] | Iran | Retrospective Single-center | 110 | 37.2 | Not reported * | Lesion excision Biopsy | N/A | Se = 58.3 Sp = 98.7 Acc = 89.5 | Se = 92 Sp = 100 Acc = 93.3 |
| 27. | Sloss, 2022 [38] | Australia | Retrospective Single-center | 135 | 36.7 | 5–9 | Bowel resection/shaving Biopsy only | Se = 93.6 Sp = 50 | N/A | N/A |
| 28. | Venkatesh, 2020 [39] | India | Prospective Single-center | 136 | 29.3 | 4–8 | Laparoscopy Biopsy | N/A | N/A | Se = 96.6 Sp = 89.5 Acc = 94.1 |
| 29. | Yin, 2020 [40] | China | Retrospective Single center | 198 | 35.3 | 5–9 | Lesion resection | Se = 94.4 Sp = 94.6 Acc = 94.9 | Se = 96.4 Sp = 85.7 Acc = 94.9 | N/A |
| 30. | Zhang, 2020 [41] | China | Retrospective Single-center | 118 | 35.2 | 5–9 | Laparoscopy Biopsy | N/A | Se = 95.3 Sp = 90.9 Acc = 94.1 | N/A |
| Clinical Scenario | Preferred Modality | Notes |
|---|---|---|
| Initial evaluation of suspected endometriosis | TVUS | Widely available, cost-effective, performed in routine gynecology visits |
| Assessment of rectosigmoid, uterosacral ligament, or pouch of Douglas involvement | TVUS | High diagnostic accuracy when performed by trained operators |
| Inconclusive or negative TVUS but high clinical suspicion | MRI | Provides complementary mapping |
| Preoperative assessment of complex/multifocal disease | MRI | Helpful for surgical planning, particularly with extrapelvic extension |
| Centers without specialized ultrasound expertise | MRI | Ensures comprehensive mapping |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capraș, R.-D.; Badea, I.C.; Moldovan, M.; Gaia-Oltean, A.I.; Badea, A.-F.; Telecan, T. The Diagnostic Performance of Transvaginal Ultrasound for Posterior Compartment Endometriosis Compared to Laparoscopic and Histopathological Findings: A Systematic Review. Healthcare 2025, 13, 2548. https://doi.org/10.3390/healthcare13202548
Capraș R-D, Badea IC, Moldovan M, Gaia-Oltean AI, Badea A-F, Telecan T. The Diagnostic Performance of Transvaginal Ultrasound for Posterior Compartment Endometriosis Compared to Laparoscopic and Histopathological Findings: A Systematic Review. Healthcare. 2025; 13(20):2548. https://doi.org/10.3390/healthcare13202548
Chicago/Turabian StyleCapraș, Roxana-Denisa, Iulia Clara Badea, Mădălina Moldovan, Adriana Ioana Gaia-Oltean, Alexandru-Florin Badea, and Teodora Telecan. 2025. "The Diagnostic Performance of Transvaginal Ultrasound for Posterior Compartment Endometriosis Compared to Laparoscopic and Histopathological Findings: A Systematic Review" Healthcare 13, no. 20: 2548. https://doi.org/10.3390/healthcare13202548
APA StyleCapraș, R.-D., Badea, I. C., Moldovan, M., Gaia-Oltean, A. I., Badea, A.-F., & Telecan, T. (2025). The Diagnostic Performance of Transvaginal Ultrasound for Posterior Compartment Endometriosis Compared to Laparoscopic and Histopathological Findings: A Systematic Review. Healthcare, 13(20), 2548. https://doi.org/10.3390/healthcare13202548







