Beetroot Supplementation as a Nutritional Strategy to Support Post-Exercise Autonomic Recovery in Postmenopausal Women: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Source, Search Strategy, and Study Selection
2.3. Data Collection and Data Extraction
2.4. Data Items
2.5. Assessment of the Risk of Bias in Individual Studies and Across Studies
2.6. Certainty Assessment (Levels of Evidence)
2.7. Qualitative Analysis (Systematic Review)
2.8. Synthesis of Results and Summary Measures
3. Results
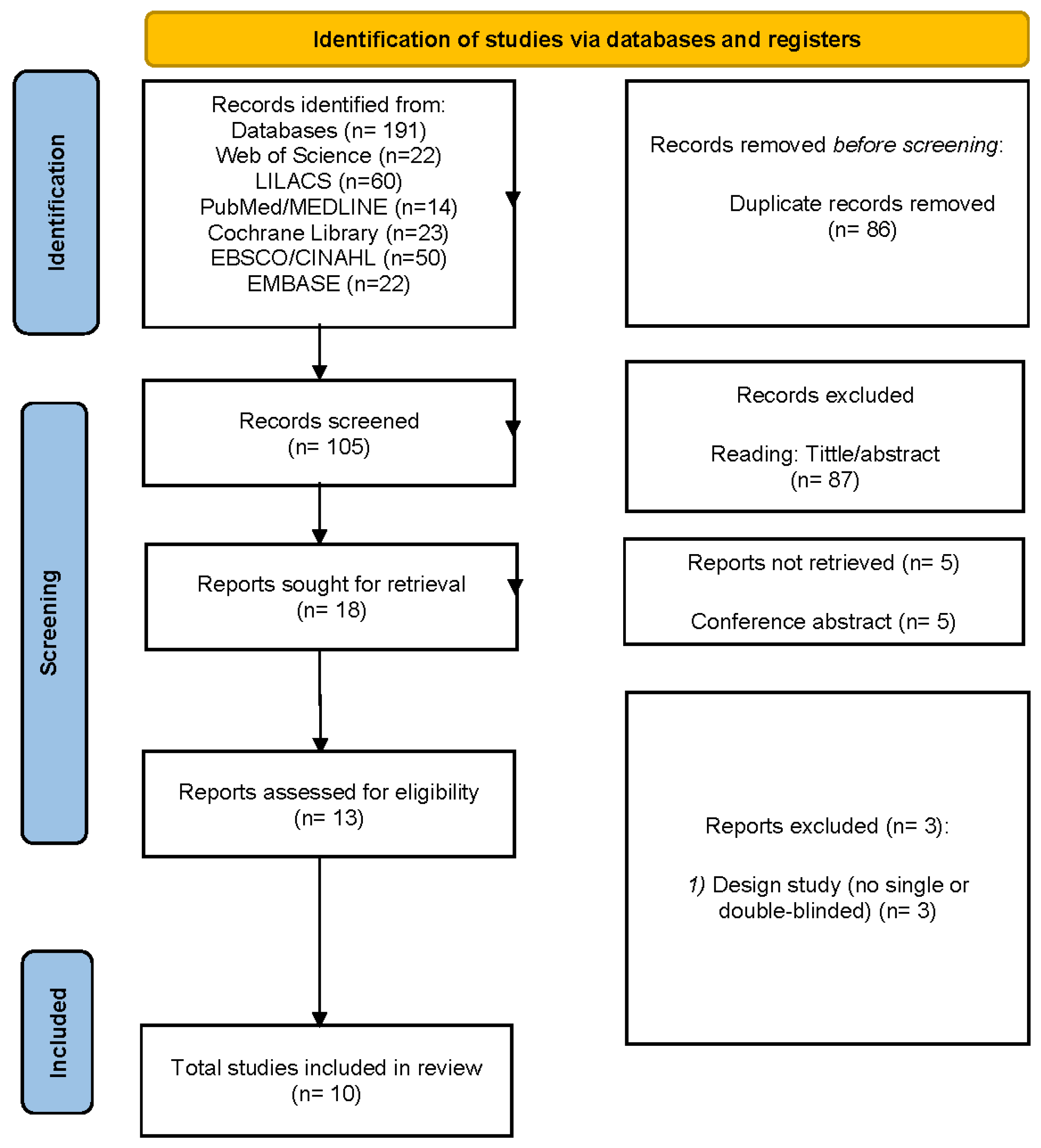
3.1. Study Selection
3.2. Study Characteristics
3.3. Results of Individual Studies
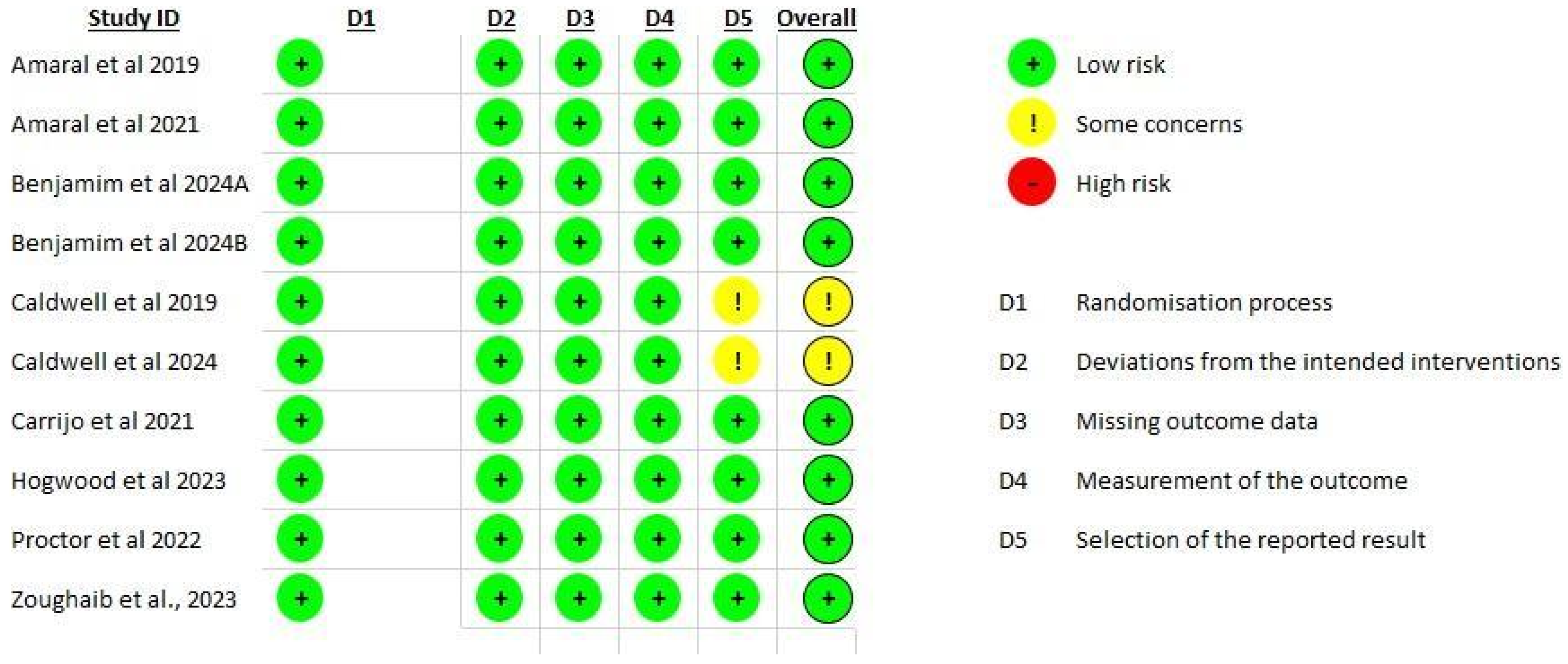
3.4. Risk of Bias
3.5. Randomization Process
3.6. Deviations from Intended Interventions
3.7. Missing Outcome Data
3.8. Measurement of Outcomes
3.9. Selection of Reported Results
3.10. Overall Bias
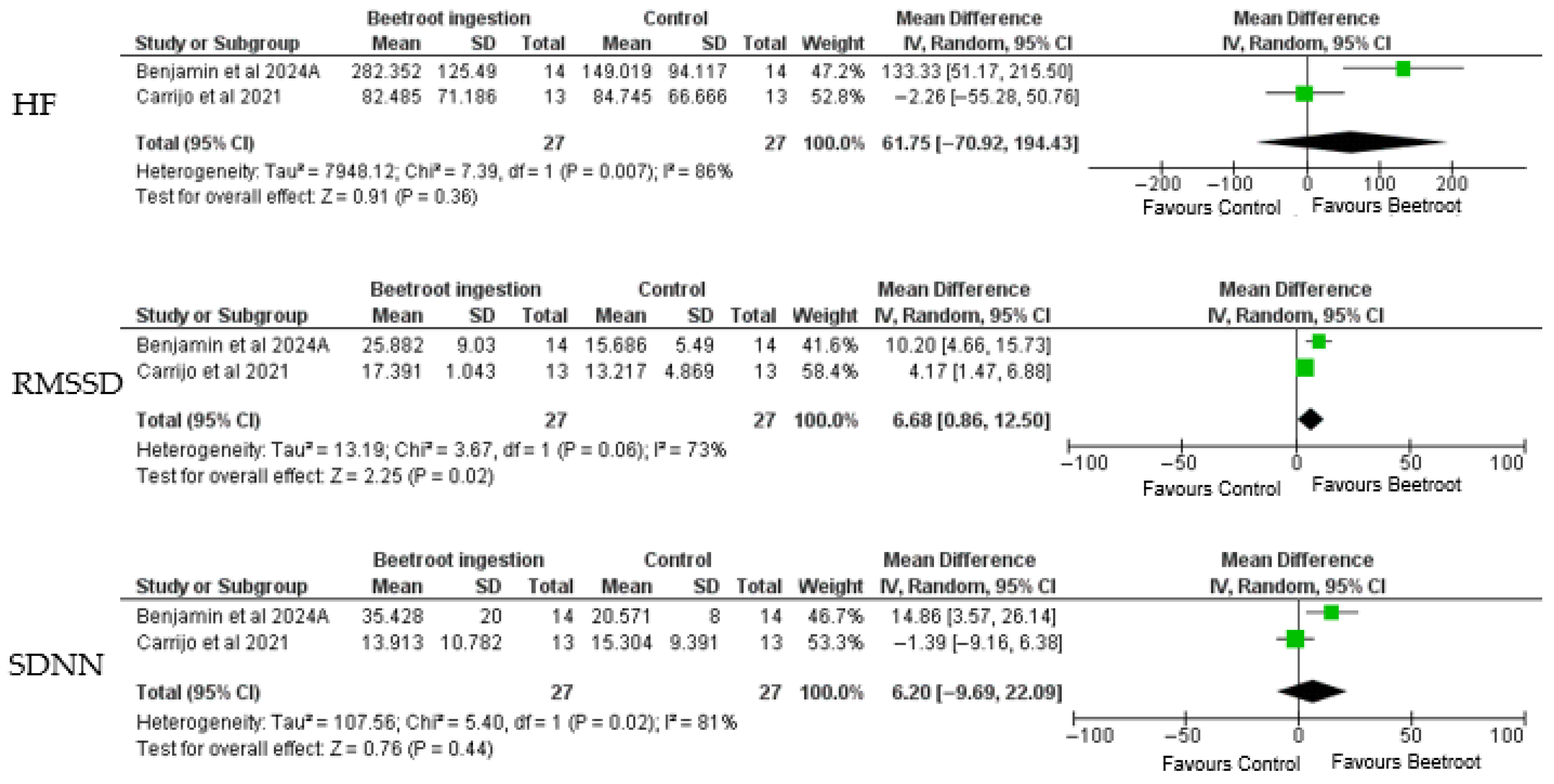
3.11. Synthesis of Results
3.12. GRADE Assessment
4. Discussion
- (1)
- Risk of bias specified some concerns for the selection of the reported result, but demonstrated low risk for the residual items.
- (2)
- GRADE established very low inevitability for HF, low certainty for SDNN, and moderate certainty for RMSSD.
4.1. Dose Considerations
4.2. Limitations
4.3. Practical Implications
4.4. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HRV | heart rate variability |
| VO2 | Oxygen consumption |
| RMSSD | Root Mean Square of Successive Differences |
| HF | High Frequency (power of heart rate variability) |
| SDNN | Standard Deviation of Normal-to-Normal Intervals |
| NOS | nitric oxide synthases |
| NO | nitric oxide |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MD | Mean difference |
| BMI | Body Mass Index |
| OFD | orange-flavored control |
| IL-6 | Interleukin-6 |
Appendix A
| Database | Search Strategy |
|---|---|
| General Boolean Syntax | ((“Beetroot supplementation” OR “beetroot juice” OR “beetroot extract” OR “dietary nitrate”) AND (“Postmenopausal women” OR “post-menopause” OR “menopause”) AND (“Exercise” OR “Physical Activity” OR “Strengthening Program” OR “Training” OR “Rehabilitation” OR “Habilitation” OR “heart rate variability”) |
| PUBMED | (((“Beetroot supplementation” [MeSH Terms] OR “beetroot juice” OR “beetroot extract” OR “dietary nitrate” [MeSH Terms]) AND (“Postmenopausal women” [MeSH Terms] OR “post-menopause” OR “menopause” [MeSH Terms]) AND (“Exercise” [MeSH Terms] OR “Physical Activity” [MeSH Terms] OR “Strengthening Program” OR “Training” [MeSH Terms] OR “Rehabilitation” [MeSH Terms] OR “Habilitation”) OR “heart rate variability”)) |
| EMBASE | ((‘beetroot supplementation’ OR ‘beetroot juice’ OR ‘beetroot extract’ OR ‘dietary nitrate’) AND (‘postmenopausal women’ OR ‘post-menopause’ OR ‘menopause’) AND (‘exercise’ OR ‘physical activity’ OR ‘strengthening program’ OR ‘training’ OR ‘rehabilitation’ OR ‘habilitation OR “heart rate variability”)) |
| LILACS | (“beetroot supplementation” OR “beetroot juice” OR “beetroot extract” OR “dietary nitrate”) AND (“postmenopausal women” OR “post-menopause” OR “menopause”) AND (“exercise” OR “physical activity” OR “strengthening program” OR “training” OR “rehabilitation” OR “habilitation” OR “heart rate variability”) |
| Cochrane Library | (“Beetroot supplementation” OR “beetroot juice” OR “beetroot extract” OR “dietary nitrate”) AND (“Postmenopausal women” OR “post-menopause” OR “menopause”) AND (“Exercise” OR “Physical Activity” OR “Strengthening Program” OR “Training” OR “Rehabilitation” OR “Habilitation”) |
| SCOPUS | ((“beetroot supplementation” OR “beetroot juice” OR “beetroot extract” OR “dietary nitrate”) AND (“postmenopausal women” OR “post-menopause” OR “menopause”) AND (“exercise” OR “physical activity” OR “strengthening program” OR “training” OR “rehabilitation” OR “habilitation” OR “heart rate variability”) |
| Web of Science | (“beetroot supplementation” OR “beetroot juice” OR “beetroot extract” OR “dietary nitrate”)AND (“postmenopausal women” OR “post-menopause” OR “menopause”) AND (“exercise” OR “physical activity” OR “strengthening program” OR “training” OR “rehabilitation” OR “habilitation” OR “heart rate variability”) |
| CINAHL | ((MH “Beetroot supplementation” OR “beetroot juice” OR “beetroot extract” OR “dietary nitrate”) AND (MH “Postmenopausal Women” OR “post-menopause” OR “menopause”) AND (MH “Exercise” OR “Physical Activity” OR “Strengthening Program” OR “Training” OR “Rehabilitation” OR “Habilitation”)) |
References
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44 (Suppl. 1), S35–S45. [Google Scholar] [CrossRef]
- Poon, E.T.; Iu, J.C.; Sum, W.M.; Wong, P.S.; Lo, K.K.; Ali, A.; Burns, S.F.; Trexler, E.T. Dietary Nitrate Supplementation and Exercise Performance: An Umbrella Review of 20 Published Systematic Reviews with Meta-analyses. Sports Med. 2025, 55, 1213–1231. [Google Scholar] [CrossRef]
- d’El-Rei, J.; Cunha, A.R.; Trindade, M.; Neves, M.F. Beneficial Effects of Dietary Nitrate on Endothelial Function and Blood Pressure Levels. Int. J. Hypertens. 2016, 2016, 6791519. [Google Scholar] [CrossRef] [PubMed]
- Benjamim, C.J.R.; Sousa, Y.B.A.; Porto, A.A.; de Moraes Pontes, Y.M.; Tavares, S.S.; da Silva Rodrigues, G.; da Silva, L.S.L.; da Silva Goncalves, L.; Guimaraes, C.S.; Rebelo, M.A.; et al. Nitrate-rich beet juice intake on cardiovascular performance in response to exercise in postmenopausal women with arterial hypertension: Study protocol for a randomized controlled trial. Trials 2023, 24, 94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary Nitrate and Physical Performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.C.; Tezini, G.C. Autonomic cardiovascular adjustments in postmenopausal women: A review. Aging Dis. 2013, 4, 320–328. [Google Scholar] [CrossRef]
- Bryan, N.S. Nitric oxide deficiency is a primary driver of hypertension. Biochem. Pharmacol. 2022, 206, 115325. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 19–26, Erratum in JAMA Cardiol. 2020, 5, 364. https://doi.org/10.1001/jamacardio.2020.0173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scuteri, A.; Morrell, C.H.; Orru, M.; Strait, J.B.; Tarasov, K.V.; Ferreli, L.A.P.; Loi, F.; Pilia, M.G.; Delitala, A.; Spurgeon, H.; et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 2014, 64, 1219–1227. [Google Scholar] [CrossRef]
- Venturelli, M.; Pedrinolla, A.; Boscolo Galazzo, I.; Fonte, C.; Smania, N.; Tamburin, S.; Muti, E.; Crispoltoni, L.; Stabile, A.; Pistilli, A.; et al. Impact of nitric oxide bioavailability on the progressive cerebral and peripheral circulatory impairments during aging and Alzheimer’s disease. Front. Physiol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.T.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef]
- Matovu, N.; Matovu, F.K.; Sseguya, W.; Tushemerirwe, F. Association of dietary intake and BMI among newly diagnosed type 2 diabetes patients attending diabetic clinics in Kampala. BMC Nutr. 2017, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Rojano-Ortega, D.; Peña Amaro, J.; Berral-Aguilar, A.J.; Berral-de la Rosa, F.J. Effects of Beetroot Supplementation on Recovery After Exercise-Induced Muscle Damage: A Systematic Review. Sports Health 2022, 14, 556–565. [Google Scholar] [CrossRef]
- Evangelista, J.F.; Meirelles, C.M.; Aguiar, G.S.; Alves, R.; Matsuura, C. Effects of Beetroot-Based Supplements on Muscular Endurance and Strength in Healthy Male Individuals: A Systematic Review and Meta-Analysis. J. Am. Nutr. Assoc. 2024, 43, 77–91. [Google Scholar] [CrossRef]
- Ferrada-Contreras, E.; Bonomini-Gnutzmann, R.; Jorquera-Aguilera, C.; MacmiIlan Kuthe, N.; Peña-Jorquera, H.; Rodríguez-Rodríguez, F. Does Co-Supplementation with Beetroot Juice and Other Nutritional Supplements Positively Impact Sports Performance? A Systematic Review. Nutrients 2023, 15, 4838. [Google Scholar] [CrossRef]
- Vitošević, B.; Filipović, M.; Popović, L.; Sterkowicz-Przybycień, K.; Purenović-Ivanović, T. Juice-Based Supplementation Strategies for Athletic Performance and Recovery: A Systematic Review. Sports 2025, 13, 269. [Google Scholar] [CrossRef]
- Amaral, A.L.; Mariano, I.M.; Carrijo, V.H.V.; de Souza, T.C.F.; Batista, J.P.; Mendonça, A.M.; de Souza, A.V.; Caixeta, D.C.; Teixeira, R.R.; Espindola, F.S.; et al. A Single Dose of Beetroot Juice Does Not Change Blood Pressure Response Mediated by Acute Aerobic Exercise in Hypertensive Postmenopausal Women. Nutrients 2019, 11, 1327. [Google Scholar] [CrossRef]
- Amaral, A.L.; Mariano, I.M.; Carrijo, V.H.V.; de Souza, T.C.F.; de Souza, A.V.; Caixeta, D.C.; Teixeira, R.R.; de Oliveira, E.P.; Espindola, F.S.; Puga, G.M. Antioxidant responses in hypertensive post-menopausal women after acute beetroot juice ingestion and aerobic exercise: Double blind and placebo-controlled crossover trial. Oxid. Med. Cell. Longev. 2021, 2021, 5579864. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; da Silva, L.S.L.; Sousa, Y.B.A.; Rodrigues, G.D.S.; Pontes, Y.M.M.; Rebelo, M.A.; Gonçalves, L.D.S.; Tavares, S.S.; Guimarães, C.S.; da Silva Sobrinho, A.C.; et al. Acute and short-term beetroot juice nitrate-rich ingestion enhances cardiovascular responses following aerobic exercise in postmenopausal women with arterial hypertension: A triple-blinded randomized controlled trial. Free Radic. Biol. Med. 2024, 211, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Benjamim, C.J.R.; da Silva, L.S.L.; da Silva Gonçalves, L.; Tasinafo Júnior, M.F.; Spellanzon, B.; Rebelo, M.A.; Tanus-Santos, J.E.; Bueno Júnior, C.R. The effects of dietary nitrate ingestion on physical performance tests in 50–65 years old postmenopausal women: A pilot randomized, double-blind, placebo-controlled, and crossover study. Clin. Nutr. 2024, 43, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.T.; Sutterfield, S.L.; Post, H.K.; Craig, J.C.; Baumfalk, D.R.; Copp, S.W.; Ade, C.J. Impact of Acute Dietary Nitrate Supplementation during Exercise in Hypertensive Women. Med. Sci. Sports Exerc. 2019, 51, 1014–1021. [Google Scholar] [CrossRef]
- Caldwell, J.T.; Koenke, A.; Zimmerman, L.; Wahl, A.E.; Fenn, S.A.; Grammer, E.E.; Stahl, M.E.; Allen, J.D.; Jaime, S.J. Acute impact of inorganic nitrate supplementation after ischemia and during small muscle mass exercise in postmenopausal females: A pilot study. Physiol. Rep. 2024, 12, e70076. [Google Scholar] [CrossRef]
- Carrijo, V.H.V.; Amaral, A.L.; Mariano, I.M.; de Souza, T.C.F.; Batista, J.P.; de Oliveira, E.P.; Puga, G.M. Beetroot juice intake with different amounts of nitrate does not change aerobic exercise-mediated responses in heart rate variability in hypertensive postmenopausal women: A randomized, crossover and double-blind study. J. Exerc. Sci. Fit. 2021, 19, 104–110. [Google Scholar] [CrossRef]
- Hogwood, A.C.; Ortiz de Zevallos, J.; Weeldreyer, N.; Clark, J.R.; Mazzella, V.; Cain, L.; Myaing, D.; Love, K.M.; Weltman, A.; Allen, J.D. The acute effects of exercise intensity and inorganic nitrate supplementation on vascular health in females after menopause. J. Appl. Physiol. 2023, 135, 1070–1081. [Google Scholar] [CrossRef]
- Proctor, D.N.; Neely, K.A.; Mookerjee, S.; Tucker, J.; Somani, Y.B.; Flanagan, M.; Kim-Shapiro, D.B.; Basu, S.; Muller, M.D.; Jin-Kwang Kim, D. Inorganic nitrate supplementation and blood flow restricted exercise tolerance in post-menopausal women. Nitric Oxide 2022, 122–123, 26–34. [Google Scholar] [CrossRef]
- Zoughaib, W.S.; Hoffman, R.L.; Yates, B.A.; Moorthi, R.N.; Lim, K.; Coggan, A.R. Short-term beetroot juice supplementation improves muscle speed and power but does not reduce blood pressure or oxidative stress in 65–79 y old men and women. Nitric Oxide 2023, 138–139, 34–41. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; updated August 2023; John Wiley & Sons: Chichester, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 19 May 2025).
- Nieman, D.C.; Sakaguchi, C.A.; Williams, J.C.; Mulani, F.A.; Shivprasad Suresh, P.; Omar, A.M.; Zhang, Q. Beet supplementation mitigates post-exercise inflammation. Front. Nutr. 2024, 11, 1408804. [Google Scholar] [CrossRef]
- Jaswal, P.; Bansal, S.; Chaudhary, R.; Basu, J.; Bansal, N.; Kumar, S. Nitric oxide: Potential therapeutic target in Heat Stress–induced Multiple Organ Dysfunction. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, J.; Ritchie, S.D.; Oddson, B.; Gagnon, D.D.; Mrozewski, T.; Little, J.; Nault, S. Using Heart Rate Variability Methods for Health-Related Outcomes in Outdoor Contexts: A Scoping Review of Empirical Studies. Int. J. Environ. Res. Public Health 2023, 20, 1330. [Google Scholar] [CrossRef] [PubMed]
- Vanderlei, L.C.M.; Pastre, C.M.; Hoshi, R.A.; Carvalo, T.D.D.E.; Godoy, M.F.D.E. Basic notions of heart rate variability and its clinical applicability. Rev. Bras. Cir. Cardiovasc. 2009, 24, 205–217. [Google Scholar] [CrossRef] [PubMed]
| Component | Description |
|---|---|
| (P) | Premenopausal or perimenopausal women, no restrictions on comorbidities. Studies involving men or mixed-gender populations without specific analysis for postmenopausal women were omitted. |
| (I) | The intervention group should receive beetroot-based interventions in any administration type, combined with any exercise modality. |
| (C) | Comparison groups included participants who received a placebo intervention—for example, nitrate-depleted beetroot juice or other inert substances—and engaged in an exercise training protocol of any type or intensity. |
| (O) | The primary outcomes assessed were cardiovascular parameters (including systolic and diastolic blood pressure, vascular function, and heart rate variability), besides measures of physical performance, such as peak oxygen uptake (VO2peak or VO2max) and functional fitness test results. |
| (S) | Involved studies included single or double-blind, randomized controlled trials or randomized crossover-controlled designs. This study was restricted to articles published in peer-reviewed scientific research journals. Conference papers, master’s theses, doctoral dissertations, descriptive studies, case studies, editorials, and reviews were omitted. |
| Author/ Years/Country | Study Design | Sample | Age (years) | Intervention | Control | Outcomes/Results | Limitations |
|---|---|---|---|---|---|---|---|
| Amaral et al. 2019/Brazil [19] | Double-blind, randomized, placebo-controlled, crossover trial. | 13 hypertensive postmenopausal women. | 58.1 ± 4.6 years. | Exercise Modality: Treadmill aerobic exercise. Intensity: 65–70% of heart rate reserve (HRR). Duration: 40 min. Beetroot Juice intervention: 35 mL of concentrated beetroot juice (Beet-It Sport Shot, Ashbocking, UK) diluted in 315 mL of distilled water with 6 g of non-caloric orange juice flavored powder (Clight, Brazil), totaling 350 mL of juice. Dose: 400 mg of nitrate (NO3−), equivalent to 20.78 mmol/kg of NO3−. | A nitrate-depleted version of the beetroot juice, prepared by filtering the BJ through an ion exchange resin to remove NO3− (3.86 mmol/kg of NO3−). | There were no differences in post-exercise blood pressure reduction between intervention and placebo groups. No significant differences were detected between the three experimental conditions for systolic blood pressure, diastolic blood pressure, or heart rate (p = 1.000), and no correlation was found between salivary nitrite and blood pressure (SBP p = 0.749; DBP p = 0.618). | Blood flow was not directly assessed, which limits the mechanistic interpretation of the hemodynamic responses observed. Second, the intervention was acute, based on a single dose of beetroot juice, thereby restricting the generalizability of the findings to chronic supplementation scenarios. Moreover, the participants were under heterogeneous pharmacological treatment with different classes of antihypertensive drugs. |
| Amaral et al. 2021/Brazil [20] | Crossover, randomized, double-blind, placebo-controlled trial. | 13 hypertensive postmenopausal women. | 58.1 ± 4.6 years. | Exercise Modality: Treadmill aerobic exercise. Intensity: 65–70% of heart rate reserve (HRR). Duration: 40 min. Beetroot Juice intervention: 35 mL of concentrated beetroot juice (Beet-It Sport Shot, UK) diluted in 315 mL of distilled water with 6 g of non-caloric orange juice flavored powder (Clight, Brazil), totaling 350 mL of juice. Dose: 400 mg of nitrate (NO3−), equivalent to 20.78 mmol/kg of NO3−. | A nitrate-depleted version of the beetroot juice, prepared by filtering the BJ through an ion exchange resin to remove NO3− (3.86 mmol/kg of NO3−). | There was a difference in decreasing catalase activity and GSH between the intervention and placebo groups. The catalase activity decreased after both high-nitrate and low-nitrate beetroot juice compared with the orange-flavored drink, with lower AUC values (p < 0.01). Salivary glutathione (GSH) also decreased only after the high-nitrate condition, with lower AUC compared to the control (p < 0.01). Time effects were observed for all variables: total protein increased significantly at 170′ compared to all other time points (p < 0.001); catalase was lower at 170′ than baseline (p < 0.001); GSH was higher at 130′ and 170′ versus baseline (p = 0.004); and FRAP was lower at 130′ and 260′ compared to baseline but higher at 170′ than 130′ (p < 0.001). No significant differences among conditions were found for FRAP responses (p = 0.680) | The participants were medicated hypertensive postmenopausal women, and the use of different antihypertensive drugs may have influenced the oxidative stress responses observed. Only the acute effects of beetroot juice ingestion in association with exercise were examined, without evaluation of chronic supplementation or the isolated impact of the beverages in the absence of exercise. |
| Benjamim et al. 2024/Brazil [21] | Randomized crossover, triple-blind, placebo-controlled trial. | 14 postmenopausal women with systemic arterial hypertension (SAH). | 59 ± 4 years. | Exercise Modality: Treadmill aerobic exercise. Intensity: 65–70% of the VO2peak. Duration: 30 min. Beetroot Juice Intervention: 70 mL of beetroot juice per bottle (Beet-It, Sports Shot, UL). Dose: 400 mg of nitrate (NO3−), equivalent to 6.4 mmol of NO3−. | A nitrate-depleted version of beetroot juice, prepared by filtering the BJ through an ion exchange resin to remove NO3− (0.38 mmol of NO3−). | Acute Ingestion: Significant reduction in systolic blood pressure (SBP) post-exercise (−9.28 mmHg; p = 0.019). Improved flow-mediated dilation (FMD) post-exercise (3.18%; p = 0.031). Enhanced recovery of parasympathetic modulation (Heart Rate Variability indices improved). Short-term Ingestion (One Week): Increased FMD values both before (4.5%; p = 0.005) and after exercise (4.2%; p = 0.004). Faster recovery of parasympathetic modulation after exercise. No significant reduction in SBP or Diastolic Blood Pressure (DBP) compared to placebo after one week. | The small sample size of hypertensive postmenopausal women limits statistical power and generalizability to other populations, such as men, younger adults, or physically active individuals. The intervention assessed only acute and short-term (one week) supplementation, preventing conclusions about long-term effects or sustained adaptations. |
| Benjamim et al. 2024/Brazil [22] | Randomized, double-blind, placebo-controlled crossover trial. | 15 postmenopausal women. | 59 ± 4 years. | Exercise Modality: Physical performance tests. Intensity: Handgrip strength (peak force measured with three attempts); arm curl test (2.3 Kg weight for 30 s, recording the number of completed moves); sit-to-stand test (as many times as possible within 30 s); agility and dynamic balance test (best time recorded in seconds); 6 min walk test (the greatest distance possible in a perimeter of 45.7 m within 6 min time frame). Beetroot Juice Intervention: 70 mL of beetroot juice (Beet It, Sport Nitrate 400, UK). Dose: 400 mg of nitrate (NO3−). | 70 mL of the nitrate-depleted version of beetroot juice. | The study suggests a slight improvement in the performance of the 6-min walk test (6MWT). NO2− plasma concentrations were consistently elevated in the NO3− condition at 0.41 (0.40) μM compared to the PLA at 0.18 (0.18) μM (p < 0.001). The 6MWT showed higher values in BRJ with NO3− condition (19.6 m [95%CI: 1.33 to 37.88]; p = 0.038), while the other physical performance tests did not show a significant difference between conditions (p > 0.05). | The sample size was small (n = 14 completers), which reduces statistical power and limits generalizability beyond the specific cohort of postmenopausal women aged 50–65. The intervention duration was relatively short (8 days), thus precluding conclusions regarding longer-term effects or sustainability of benefits. Third, while one physical performance test (6 min walk test) showed improvement, the other performance outcomes (handgrip strength, arm curl, sit-to-stand, agility/dynamic balance) did not differ between conditions, indicating that benefits may be limited to tests of longer duration/cardiorespiratory demand. |
| Caldwell et al. 2019/USA [23] | 2 d randomized, double-blind, placebo-controlled crossover. | 10 postmenopausal women. | 56 ± 1 years. | Exercise Modality: Isometric handgrip exercise. Intensity: 20% MVC. Duration: 7 min. Beetroot Juice intervention: 140 mL of concentrated NR (NO3−) beetroot juice supplement (Beet It Sport, James White Drinks Ltd., Ipswich, UK). Dose: 140 mL of concentrated NR (NO3−) containing 12.9 mmol NO3−. | 140 mL of nitrate placebo beetroot juice supplement (PL; James White Drinks Ltd.) containing negligible (NO3−). | Resting SBP, DBP, HR, MAP, and SVR were not significantly different between treatments. During the SS exercise, forearm blood flow (FBF) and forearm vascular conductance (FVC) were lower in the nitrate condition compared to placebo. Plasma nitrite levels were significantly higher after the nitrate-rich (NR) supplement compared to the placebo (NP) condition (809 ± 146 vs. 79 ± 19 nM; p < 0.001). Resting systolic, diastolic, mean arterial pressure, and heart rate did not differ between treatments (p > 0.05). During steady-state handgrip exercise, forearm blood flow (NR: 190 ± 16 vs. NP: 218 ± 17 mL·min−1; p = 0.03) and forearm vascular conductance (NR: 159 ± 12 vs. NP: 177 ± 12 mL·min−1·100 mmHg−1; p = 0.03) were significantly lower in the NR condition. | Only an acute dose of dietary nitrate supplementation was tested; therefore, the potential benefits of chronic supplementation remain unknown. The sympathetic nerve activity was not measured, which limits the mechanistic interpretation of the observed improvements in functional sympatholysis. |
| Caldwell et al. 2024/USA [24] | Randomized, double- blind, crossover study design | Twelve postmenopausal females. | 64 ± 5 years. | Exercise Modality: Isometric handgrip exercise. Intensity: ischemic exercise at 20% MVC and 20 contractions per min at 10, 15, and 20% MVC Duration: 3 min per stage and 3 min of ischemic exercise. Beetroot Juice intervention: nitrate-rich supplement in the form of beetroot juice. Dose: 140 mL of a concentrated beetroot juice supplement (Beet It Sport, James White Drinks Ltd., Ipswich, UK) containing [~12.9 mmol NO3−]. | Nitrate-poor supplement in the form of black currant [<0.2 mmol NO3−], (Jungle Powders, Pärnumaa, Estonia). | Acute supplementation did not change resting or ischemic exercise FMD. Further, no changes were seen with resting or ischemic exercise PORH. Plasma nitrate and nitrite concentrations significantly increased following nitrate-rich beetroot juice compared with placebo (p < 0.01). Mean arterial pressure was reduced after supplementation, both at rest (p < 0.05) and during incremental small muscle mass exercise (p < 0.05). | The small sample size (n = 12 postmenopausal females), the intervention was acute, using a single dose of nitrate-rich beetroot juice, which precludes conclusions about effects with repeated or long-term supplementation. |
| Carrijo et al. 2021/Brazil [25] | Crossover, randomized, and double-blind study. | 13 postmenopausal hypertensive women. | 58.1 ± 4.6 years | Exercise Modality: Treadmill aerobic exercise. Intensity: 65–70% of HR reserve Duration: 40 min. Beetroot Juice intervention: high-NO3− drink consisted of 35 mL concentrated beetroot juice diluted in 315 mL of distilled water with 6 g of non-caloric orange flavor powder (Clight, Mondelez International, Inc., São Paulo, Brazil), totaling 350 mL of juice. Dose: 400 mg of NO3− (Beet-It Sport Shot, James White Drinks Ltd., Ipswich, United Kingdom), containing 20.78 mmol/kg of NO3−. | A non-caloric orange-flavored drink (OFD) made by diluting 6 g of orange-flavored powder in 350 mL of distilled water, with no nitrate content. | No differences were found between the HR average of the sessions. SDNN increased significantly post-exercise compared with baseline (p < 0.05), while RMSSD and PNN50 did not differ (p > 0.05). In the frequency domain, LF and LF/HF ratio increased after exercise (p < 0.05), whereas HF showed no significant changes (p > 0.05). In the non-linear domain, SD1, SD2, and SD2/SD1 all increased after exercise across conditions (p < 0.05). Importantly, there were no interaction effects between time and session (p > 0.05), indicating that beetroot juice—whether high- or low-nitrate—did not modify HRV responses when compared to the orange-flavored control drink | The sample size was small (n = 13). All participants were hypertensive postmenopausal women under pharmacological treatment, and the use of different classes of antihypertensive drugs may have masked the potential effects of nitrate supplementation on heart rate variability (HRV). Furthermore, the study examined only the acute effects of a single dose of beetroot juice, which restricts conclusions regarding chronic supplementation. In addition, HRV was assessed for 90 min after exercise, and longer recovery windows might provide complementary insights. |
| Hogwood et al. 2023/USA [26] | Randomized, double-blind, placebo-controlled trial | 24 estrogen-deficient females postmenopausal | 61 ± 5 (Placebo) 59 ± 5 Nitrate-rich beetroot juice | Exercise modality: Cycle ergometer exercise test. Visits consisted of vascular health measures before (time point 0) and every 30 min after (time points 60, 90, 120, 150, and 180) calorically matched high-intensity exercise (HIE), moderate-intensity exercise (MIE). Dose: 13 mmol NO3− in the form of beetroot juice(BRJ; n = 12) or placebo (PL; n = 12) for 2 days before experimental visits and 2 h before testing. | The placebo was a nitrate-depleted beetroot juice with a similar appearance and taste to the nitrate-rich supplement. | Beetroot juice (BRJ) supplementation significantly increased plasma nitrate and nitrite and decreased serum endothelin-1 compared with placebo (all p < 0.001). Peak FMD improved with BRJ compared with placebo (p = 0.02), and exercise intensity also had a significant effect (p < 0.001), with a nonsignificant interaction (p = 0.11). Within-condition analyses showed that BRJ combined with high-intensity exercise (HIE) enhanced FMD compared with BRJ plus control (p = 0.05; p = 0.005 when baseline diameter was included as a covariate), while BRJ plus moderate-intensity exercise showed medium effect sizes but did not reach significance. Resting vascular measures, including blood pressure and pulse wave velocity, were not significantly affected by BRJ supplementation (p > 0.05), though systolic blood pressure decreased modestly after both placebo (p = 0.04) and BRJ (p = 0.01) supplementation | The relatively small sample size (n = 24, with 12 per treatment arm) was powered only for the primary outcome of peak flow-mediated dilation (FMD) and not for more complex interaction analyses. The majority of participants demonstrated relatively preserved vascular health, with only nine individuals showing impaired baseline FMD (<4.5%), potentially masking the magnitude of improvement achievable in less healthy cohorts. |
| Proctor et al. 2022/USA [27] | Double-blind, randomized crossover design | 13 postmenopausal women (57–76 yr) | 64 ± 1.4 | Exercise modality: Physical performance test. Performed rhythmic isometric handgrip contractions (10% MVC, 30 per min) during progressive forearm blood flow restriction (upper arm cuff gradually inflated 20 mmHg each min). Dose:140 mL of NO3− concentrated (9.7 mmol, 0.6 gm NO3−) | NO3− depleted beetroot juice | These results suggest that acute NO3− supplementation prolongs time-to-fatigue and speeds grip force development during progressive forearm muscle ischemia in postmenopausal women. Dietary nitrate supplementation significantly increased plasma nitrate and nitrite concentrations compared with placebo (p < 0.01). However, no significant differences were observed between conditions in flow-mediated dilation responses (p = 0.44) or resting blood pressure (systolic p = 0.21; diastolic p = 0.28). Heart rate also did not differ significantly between groups (p = 0.31). Subgroup analysis suggested that women with lower baseline FMD (<4.5%) tended to exhibit greater improvements with nitrate supplementation, but these changes did not reach statistical significance (p = 0.08). | The sample comprised only 13 postmenopausal women. The trial was short in duration, examining only acute supplementation and a 7-day intervention, which prevents conclusions about longer-term effects. Dietary intake beyond the supplementation protocol was not strictly controlled, and variability in background diet could have influenced nitrate bioavailability and vascular outcomes. |
| Zoughaib et al., 2023/USA [28] | Randomized, double-blind, placebo-controlled crossover design | Sixteen community-dwelling older men and women | 71 ± 5 years old | First phase: Participants were divided into groups for daily supplementation with BRJ for two weeks, with or without NO. After this period, blood samples were collected. Second phase: Prior to physical activity, blood pressure was measured, and new blood samples were collected. Participants were instructed to perform an acute intake of BRJ containing 18.2 ± 6.2 mmol of NO3−, while another group received BRJ without NO. Participants first performed three maximal 5 s isometric contractions, with 15 s of rest between each repetition. After a 2 min rest, isokinetic testing was conducted, during which each participant performed three maximal knee extensions at each velocity, with 2 min of rest between each set. They then underwent physical exercise with muscle performance assessed via isokinetic dynamometry. After a 10 min recovery period, blood pressure was measured again, and additional blood samples were collected. | Participants were initially assigned to one of two groups: BRJ with NO3− or BRJ without NO3− | Both acute and short-term supplementation with NO3−-rich BRJ have equally beneficial effects on muscle speed and power in older men and women. However, there were no changes in blood pressure or in plasma markers of oxidative stress Beetroot juice supplementation significantly elevated plasma nitrate and nitrite concentrations compared with the nitrate-depleted placebo (e.g., nitrite at 1 h, 2 h post-ingestion, and 10 min post-exercise; p = 0.0338, p = 0.0030, and p = 0.0030, respectively). Blood pressure outcomes (systolic, diastolic, and mean arterial pressure) showed only time (p = 0.0296) and duration (p = 0.0469) effects, with no treatment differences or interactions (all p ≥ 0.09). | The sample size was modest (n = 16 older men and women aged 65–79). Although increases in muscle speed and power were observed, the placebo/control (NO3− depleted beetroot juice) also showed some practice-or learning effects, which complicates attribution of changes uniquely to nitrate. The duration (2 weeks) for short-term supplementation is still relatively short. |
| Outcome | Number of Studies | Risk of Bias | Inconsistency | Indirectness | Imprecision | Certainty of Evidence |
|---|---|---|---|---|---|---|
| HF | 2 | Not serious | Very serious a | Not serious | Very serious b | Very low ⨁◯◯◯ |
| RMSSD | 2 | Not serious | Very serious c | Not serious | Not serious | Moderate ⨁⨁⨁◯ |
| SDNN | 2 | Not serious | Very serious d | Not serious | Serious e | Low ⨁⨁◯◯ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimundo, R.D.; Laurindo, L.F.; Gimenez, F.V.M.; Benjamim, J.; Gonzaga, L.A.; Barbosa, M.P.C.R.; Martins, M.d.M.; Ito, E.H.; Barroca, A.L.; Brito, G.d.J.; et al. Beetroot Supplementation as a Nutritional Strategy to Support Post-Exercise Autonomic Recovery in Postmenopausal Women: A Systematic Review and Meta-Analysis. Healthcare 2025, 13, 2496. https://doi.org/10.3390/healthcare13192496
Raimundo RD, Laurindo LF, Gimenez FVM, Benjamim J, Gonzaga LA, Barbosa MPCR, Martins MdM, Ito EH, Barroca AL, Brito GdJ, et al. Beetroot Supplementation as a Nutritional Strategy to Support Post-Exercise Autonomic Recovery in Postmenopausal Women: A Systematic Review and Meta-Analysis. Healthcare. 2025; 13(19):2496. https://doi.org/10.3390/healthcare13192496
Chicago/Turabian StyleRaimundo, Rodrigo D., Lucas Fornari Laurindo, Fabiana V. M. Gimenez, Jonas Benjamim, Luana A. Gonzaga, Marianne P. C. R. Barbosa, Marina de Morais Martins, Edson H. Ito, Alexandre L. Barroca, Giovanna de J. Brito, and et al. 2025. "Beetroot Supplementation as a Nutritional Strategy to Support Post-Exercise Autonomic Recovery in Postmenopausal Women: A Systematic Review and Meta-Analysis" Healthcare 13, no. 19: 2496. https://doi.org/10.3390/healthcare13192496
APA StyleRaimundo, R. D., Laurindo, L. F., Gimenez, F. V. M., Benjamim, J., Gonzaga, L. A., Barbosa, M. P. C. R., Martins, M. d. M., Ito, E. H., Barroca, A. L., Brito, G. d. J., Folegatti, D. R. M. A., Porto, A. A., Garner, D. M., Barbalho, S. M., & Valenti, V. E. (2025). Beetroot Supplementation as a Nutritional Strategy to Support Post-Exercise Autonomic Recovery in Postmenopausal Women: A Systematic Review and Meta-Analysis. Healthcare, 13(19), 2496. https://doi.org/10.3390/healthcare13192496