4.1. Cost-Effectiveness
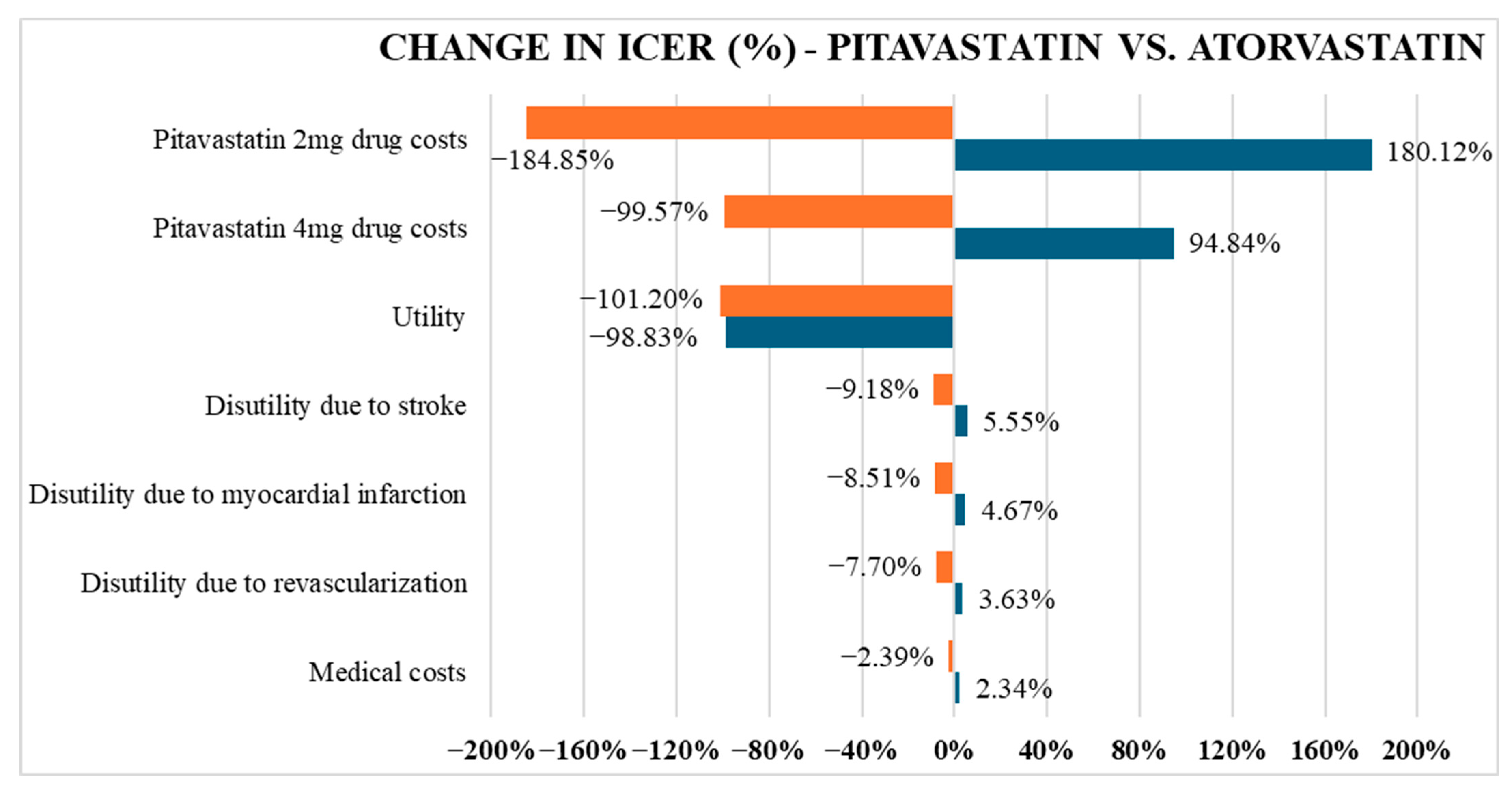
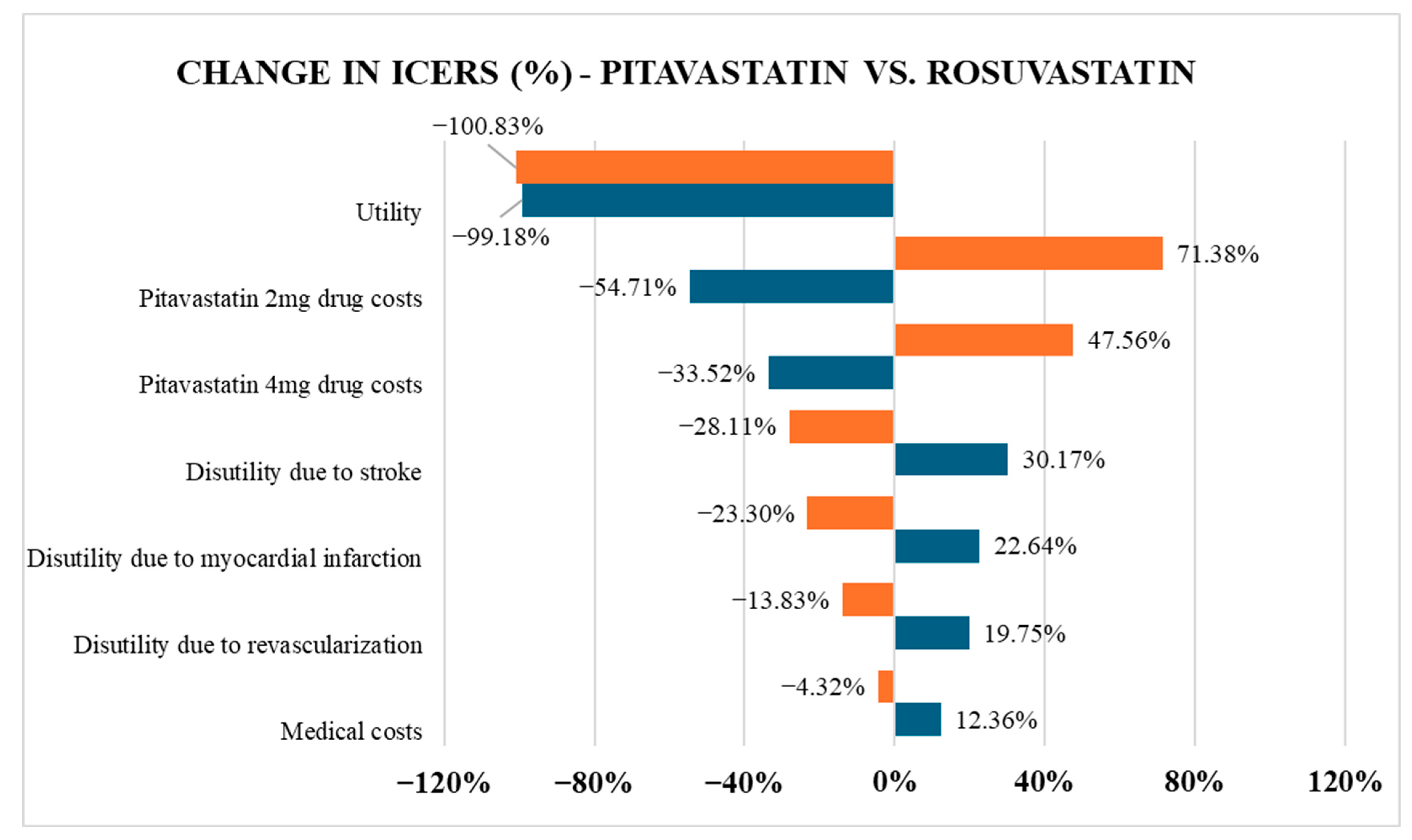
The study reveals that pitavastatin is more cost-effective than atorvastatin but more expensive than rosuvastatin over a lifetime horizon. Although pitavastatin yielded a slightly lower QALY, the difference was minor and offset by its lower cost compared to atorvastatin. In contrast, when compared to rosuvastatin, pitavastatin yielded a negative ICER (−1,242,167,485 VND/QALY), indicating that pitavastatin is both more effective and less costly, thereby establishing it as the dominant strategy in the current model. The divergence in cost-effectiveness trends when comparing pitavastatin to atorvastatin versus rosuvastatin stems primarily from differences in drug pricing, a factor confirmed by the one-way sensitivity analysis as having the most significant impact on ICER values. In Vietnam, rosuvastatin is widely available as a generic medication, while pitavastatin remains primarily confined to branded formulations. As a result, the price per tablet of pitavastatin 2–4 mg is at least 36% higher than that of rosuvastatin at a low dose of 5–10 mg.
Regarding the comparison with atorvastatin, the ICER of pitavastatin versus atorvastatin was within Vietnam’s willingness-to-pay threshold, suggesting pitavastatin would offer better economic benefit than atorvastatin. However, this conclusion holds only under the condition that the probability of achieving LDL-C targets remains high. In our study, a large proportion of patients successfully achieved LDL-C targets across all three treatment arms, with calculated rates of 94.22% for pitavastatin, 95.91% for atorvastatin, and 95.18% for rosuvastatin. These figures are consistent with previous estimates from a systematic review in Asia (which reported LDL-C goal attainment with pitavastatin ranging from 75% to 95%) [
34]. The values reported in our model are at the upper end of that range, raising questions about real-world plausibility. Achieving a 95% success rate in LDL-C control would require patients to strictly adhere to the prescribed regimen throughout the entire 12-week treatment period and to sustain that adherence over the subsequent 14 years. In reality, however, non-adherence to statin therapy is common, with many patients discontinuing treatment or missing doses, which could compromise long-term effectiveness and, consequently, the reliability of the ICER projections.
Meta-analyses have revealed that adherence to statin therapy for cardiovascular disease prevention remains low, typically ranging between 50% and 60% [
35]. Even for well-established statins like atorvastatin, which have been widely circulated in healthcare systems, adherence remains suboptimal, with real-world data showing a compliance rate of only 39%, and even lower (37%) among patients at high risk for cardiovascular disease [
36]. Notably, a large-scale 2019 study in Taiwan assessing long-term statin use in over 180,000 patients following hospital discharge demonstrated significant declines in adherence over time. While 71% of patients were considered adherent in the first six months, this rate dropped to 51% at one year, and further declined to 42% by the end of year seven [
37]. These findings underscore a substantial gap between the modelled assumption of sustained LDL-C target attainment and real-world treatment dynamics. As a result, the idealized ICER of pitavastatin, although favorable, may lack sufficient credibility to persuade healthcare providers or policymakers to shift from atorvastatin to pitavastatin, particularly when assessing long-term cost-effectiveness. Not to mention, Vietnam is classified as a lower-middle-income country [
38], where the government is more likely to allocate its limited healthcare budget to address acute conditions with immediate mortality risks, rather than invest in managing risk factors with delayed or invisible outcomes, such as lipid control. In this context, pitavastatin is a cost-effective option; however, it does not appear to be affordable for implementation on a large scale in clinical practice.
However, the demonstrated cost-effectiveness of pitavastatin over atorvastatin may still offer value as an alternative treatment option. Our results estimated that patients treated with pitavastatin 2 mg had around a 71.7% chance of achieving the LDL-C target, which is similar to a Japanese study that reported a 60–70% success rate depending on cardiovascular risk levels [
39]. As the Asian population is more sensitive to statins, lower doses already show strong LDL-C lowering; however, at the same time, patients are at a higher risk of musculoskeletal side effects [
40]. Hence, clinicians are likely to initiate therapy at low to moderate doses to minimize muscle-related adverse effects [
41,
42]. One notable benefit of pitavastatin is its high tolerability, as it can avoid metabolism by CYP3A4, a common pathway for many other statins, including atorvastatin [
43]. Metabolism via the CYP450 family often accelerates liver enzyme elevation and increases the risk of drug–drug interactions, making pitavastatin a promising and safer alternative to atorvastatin in older adults. Given the comparable LDL-C attainment rates between pitavastatin and atorvastatin, based on these findings, our analysis provides clinicians and healthcare specialists with a viable low-dose option that can achieve high LDL-C control rates for statin-naive individuals, particularly older adults or those at increased risk of intolerance.
Our conclusion about pitavastatin’s cost-effectiveness compared to the two most widely used statins was also observed in the Spanish cost-effectiveness analysis by Sales et al. in 2015 [
44]. The authors found that rosuvastatin was the most economical option over both atorvastatin and pitavastatin in patients with high and very high ASCVD risk [
44]. However, the context and the underlying decision question are distinct. Sales et al. applied a 25-year horizon and explicitly incorporated cardiovascular events into the model, which rendered pitavastatin non-cost-effective because it was less effective at preventing events and therefore failed to gain additional QALYs or reduce long-term CVD costs compared with rosuvastatin [
44]. In contrast, our analysis did not model CVD events. Instead, we focused on LDL-C target attainment as the measure of effectiveness, and on drug acquisition and monitoring costs under Vietnamese prices. This framing shifts the interpretation: pitavastatin becomes less favorable than rosuvastatin because its higher price cannot be justified by comparable LDL-C lowering efficacy. This framework is particularly relevant for minority groups such as hypercholesterolemia in HIV patients, where clinicians must consider which statin has the fewest drug–drug interactions with antiretroviral therapy. In the REPRIEVE trial (2023), pitavastatin reduced the risk of major CVD events by 35% compared with the placebo in HIV patients [
45]. Meanwhile, Yebyo et al. (2025) showed that other statins (rosuvastatin, atorvastatin, pravastatin, fluvastatin) provided about 21% CVD risk reduction in HIV patients but carried a 12% increased risk of type 2 diabetes [
46]. On the other hand, in the cost-effectiveness study by Boettiger et al. [
47,
48]. Pitavastatin was not cost-effective versus a non-statin strategy in the US or Thailand (ICER exceeded WTP), but still dominated simvastatin in Thailand [
47]. Taken together, this suggests that if the acquisition cost barrier can be reduced, pitavastatin could be a viable and cost-effective choice for achieving LDL-C goals in limited statin scenarios.
The added value of our study lies not only in providing evidence for pitavastatin as an economical and practical alternative for clinicians and patients, but also in supporting the Vietnamese healthcare system, particularly in hospital settings, in deciding which statins are most compatible with hospital budgets. In low- and middle-income country (LMIC) settings, where clinical efficacy and epidemiological data are often constrained, cost-effectiveness analyses become particularly valuable. The recent Indonesian study by Dewi et al. (2024) provided critical insights for LMIC health systems by demonstrating that, for ACS patients, the preventive effect of high-intensity statins on cardiovascular complications could outweigh the higher acquisition cost, justifying their inclusion in national health insurance schemes [
49]. In Vietnam, however, the pathway for a drug to be reimbursed under the national health insurance depends on the hospital tendering process. According to Circular 15/2019/TT-BYT, drugs that win the tender are reimbursed for one year [
50]. In this context, our finding that pitavastatin may be cost-effective compared with atorvastatin provides evidence to support its continued inclusion in future tenders, thereby offering an additional treatment option for patients who cannot use rosuvastatin or atorvastatin (such as those living with HIV). Given that pitavastatin most recently won a tender on 16 May 2024, our study provides policymakers with timely, updated information on the cost implications of maintaining pitavastatin as a reimbursed alternative.
4.2. Strengths and Limitations
The study employed a decision tree model to visualize the progression of health states, a strength when conducting a cost–utility analysis in dyslipidemia. Unlike Markov models, the decision tree assumes a one-way progression, where each health state occurs only once and does not repeat in cycles. This structure enables simplified assumptions and facilitates precise tracking of intervention effectiveness over a short-term horizon. In addition, the analysis compared pitavastatin with the two most widely used statins in clinical practice, providing clinicians with more insight into choosing the most effective statin for their patients. A further strength of our study is the focus on low-dose statin therapy rather than high-intensity regimens. This approach is consistent with prescribing trends in clinical practice in Vietnam. As a result, our findings are highly relevant to real-world treatment patterns and clinical expectations. Regarding cost, the drug prices were taken from Vietnam’s official databases, so the cost-effectiveness of pitavastatin calculated in this evaluation is relevant and applicable to the real-world healthcare context.
On the other hand, this study has several limitations that may affect the generalizability and practical applicability of its findings. First, our analysis did not incorporate cardiovascular events in the model, which we acknowledge as the main methodological drawback. As a result, the accumulated QALYs over a lifetime horizon may appear overly optimistic and do not fully reflect the actual health state of patients with hypercholesterolemia. The discrepancy between the dense evidence-based time-to-event data available for atorvastatin [
51,
52,
53] and rosuvastatin [
54,
55] can explain this limitation in the general population, but the data are still very scarce for pitavastatin. So far, pitavastatin clinical evidence has been restricted to narrower populations, such as patients with stable coronary artery disease (CAD) [
56] or those diagnosed with HIV [
45]. In such a scenario, developing a comprehensive model that includes ASCVD events would rely on heavy extrapolation, making the results even less generalizable. Second, most of the input data were derived from international studies (e.g., LDL-C reduction effectiveness, utility weights, and complication probabilities). In contrast, local data from Vietnam remains limited, particularly in terms of cost profiles and patient quality of life. Differences in population characteristics, treatment conditions, and the healthcare system may result in model outcomes that do not accurately reflect real-world clinical practice in Vietnam. Additionally, this study focused solely on direct medical costs and did not account for indirect costs, such as productivity loss or other societal expenses. These limitations were incorporated to facilitate better integration of real-world Vietnamese data and comprehensive health economic analysis, thereby enhancing the reliability and applicability of the findings for pharmaceutical policymaking and clinical practice.