Medical and Financial Consequences of Using PCSK9 Inhibitors for Managing Hypercholesterolemia in Saudi Arabia: A Historical Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Source
2.2. Study Outcomes and Data Analysis
2.3. Sensitivity Analysis
3. Results
3.1. Patients’ Baseline Characteristics
3.2. The Effects of PCSK9 Inhibitors and the Combination of Statins with Ezetimibe on Lipid Profile
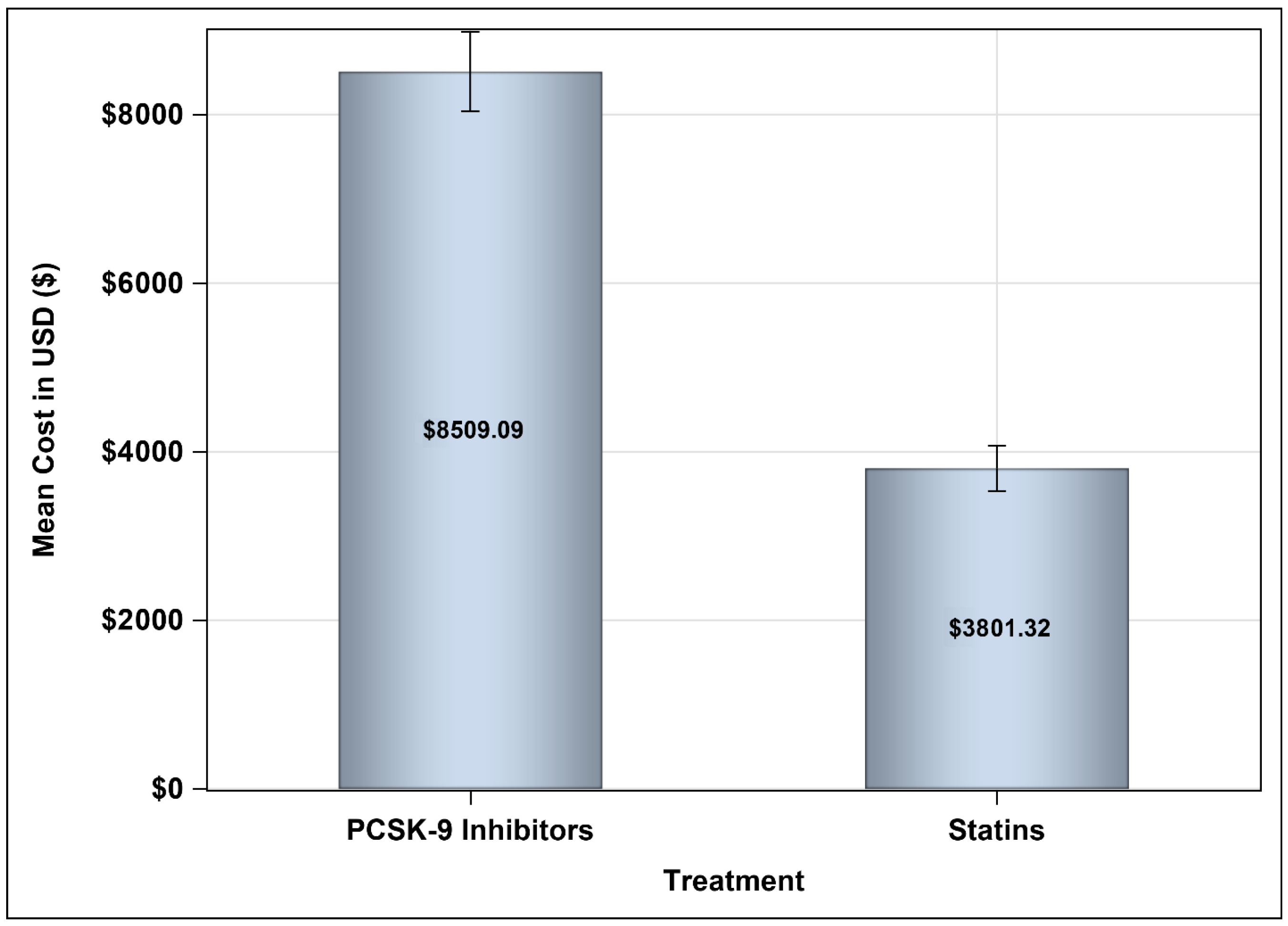
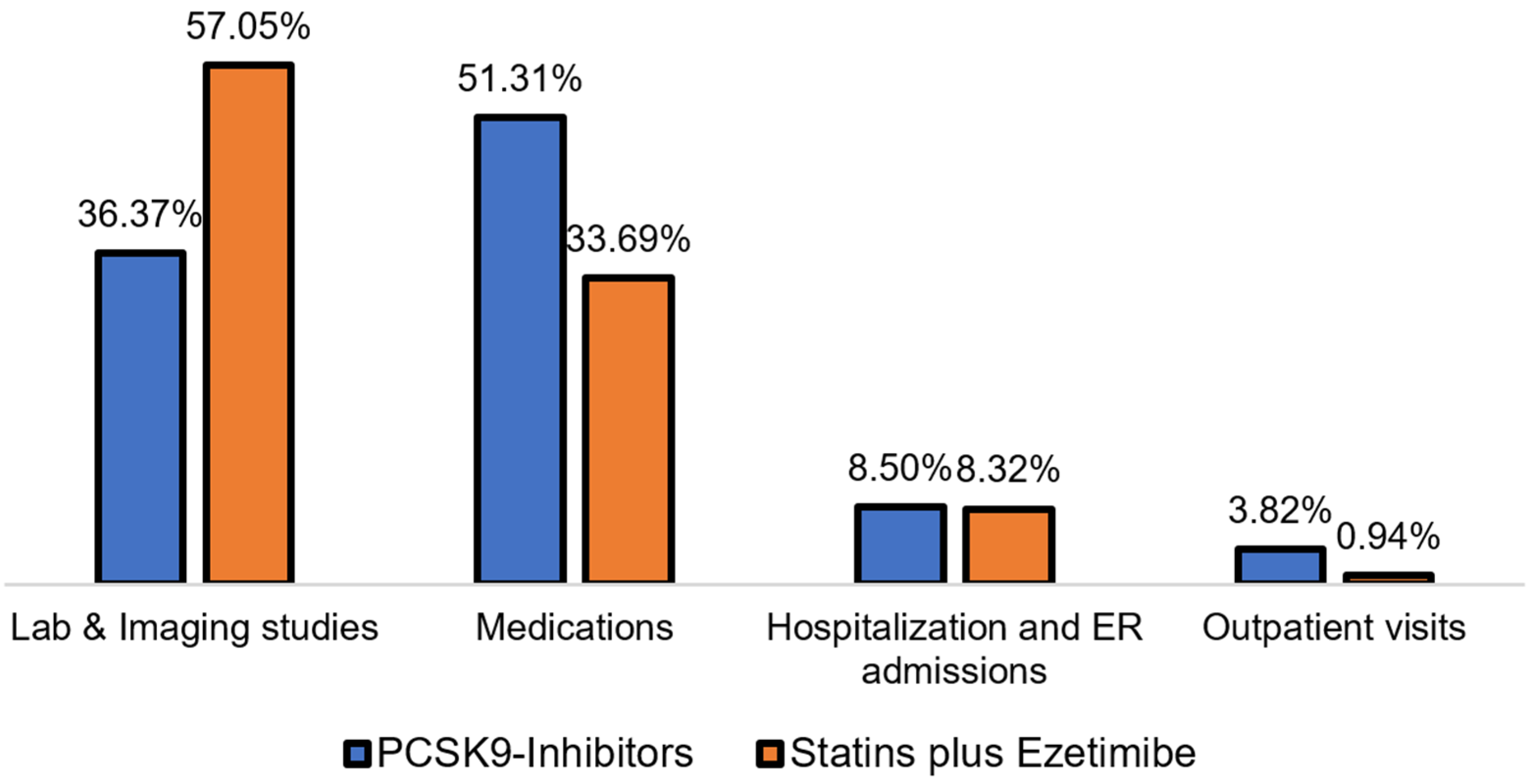
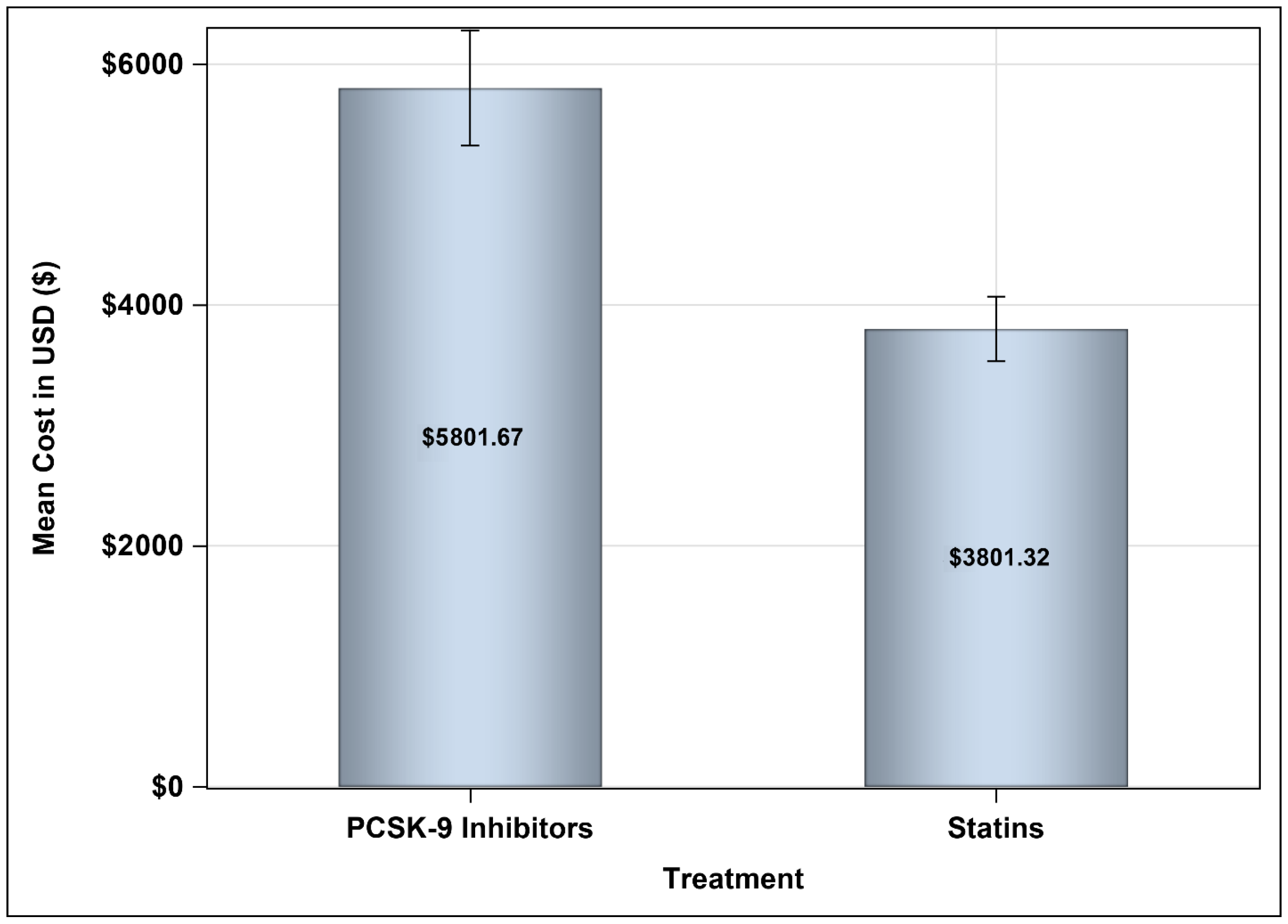
3.3. Medical Costs Associated with Treating Hypercholesterolemia with PCSK9 Inhibitors Versus Statins Plus Ezetimibe
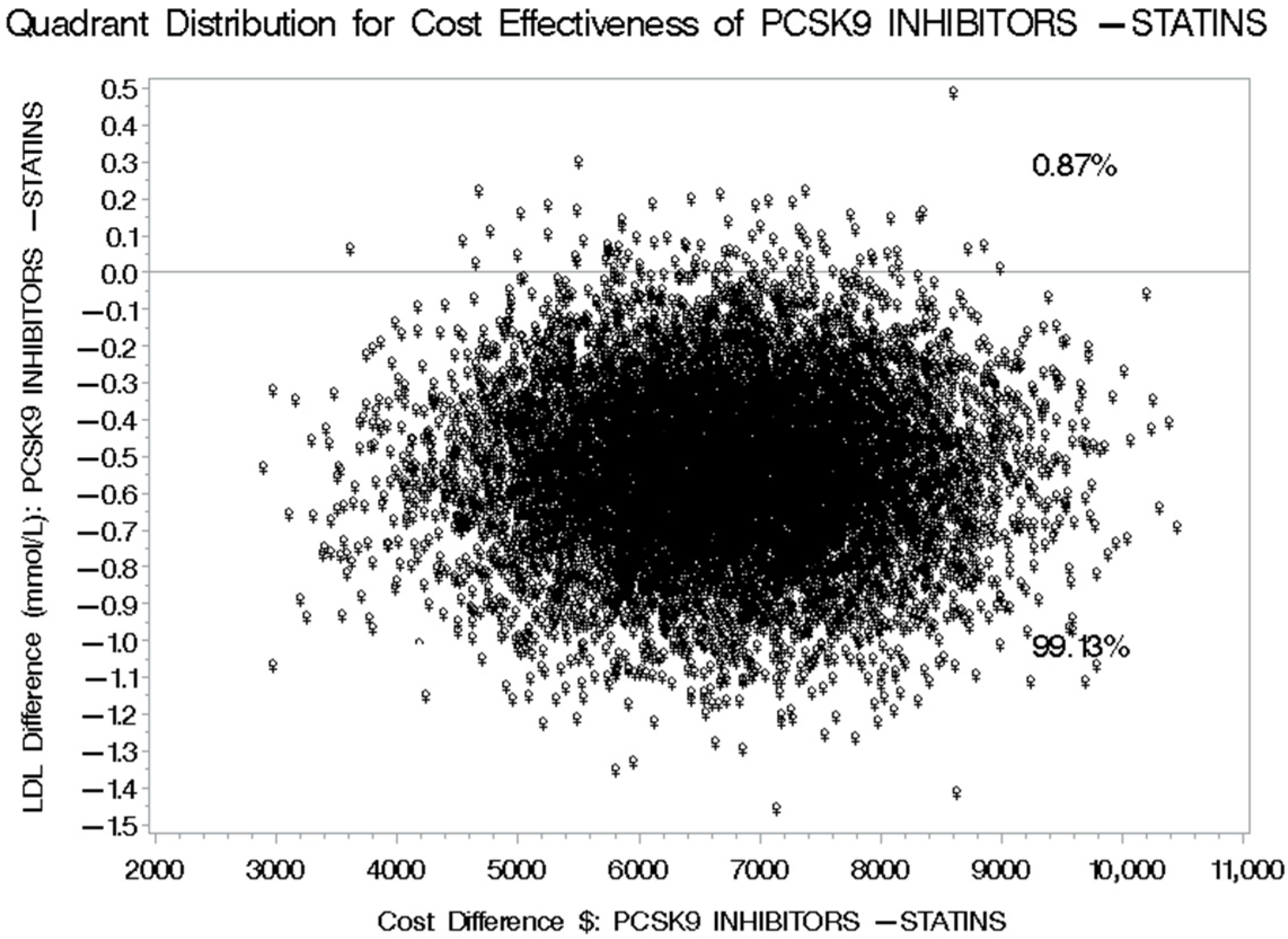
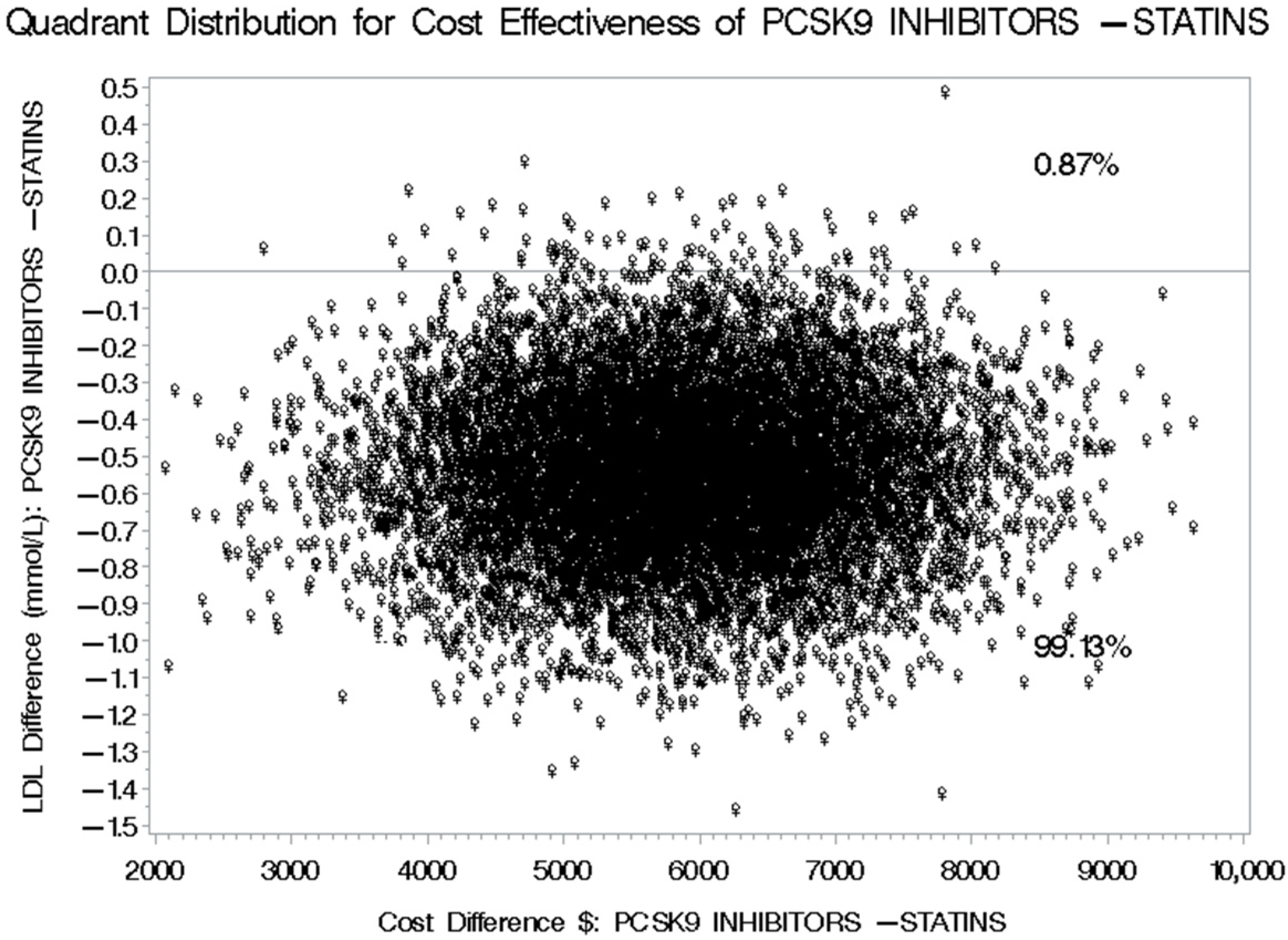
3.4. Cost-Effectiveness of PCSK9 Inhibitors Versus Statins Plus Ezetimibe for LDL-C Level Reduction
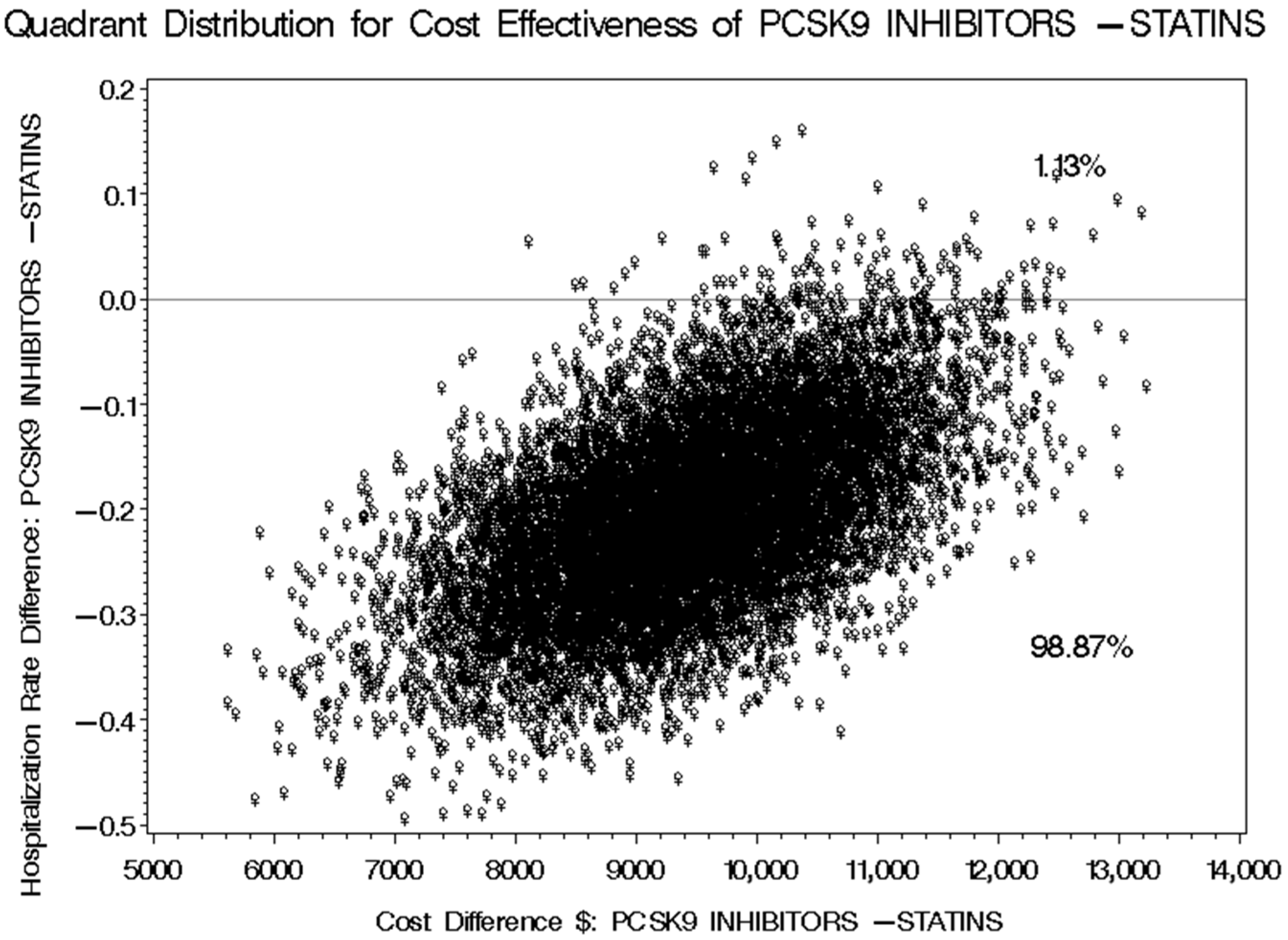
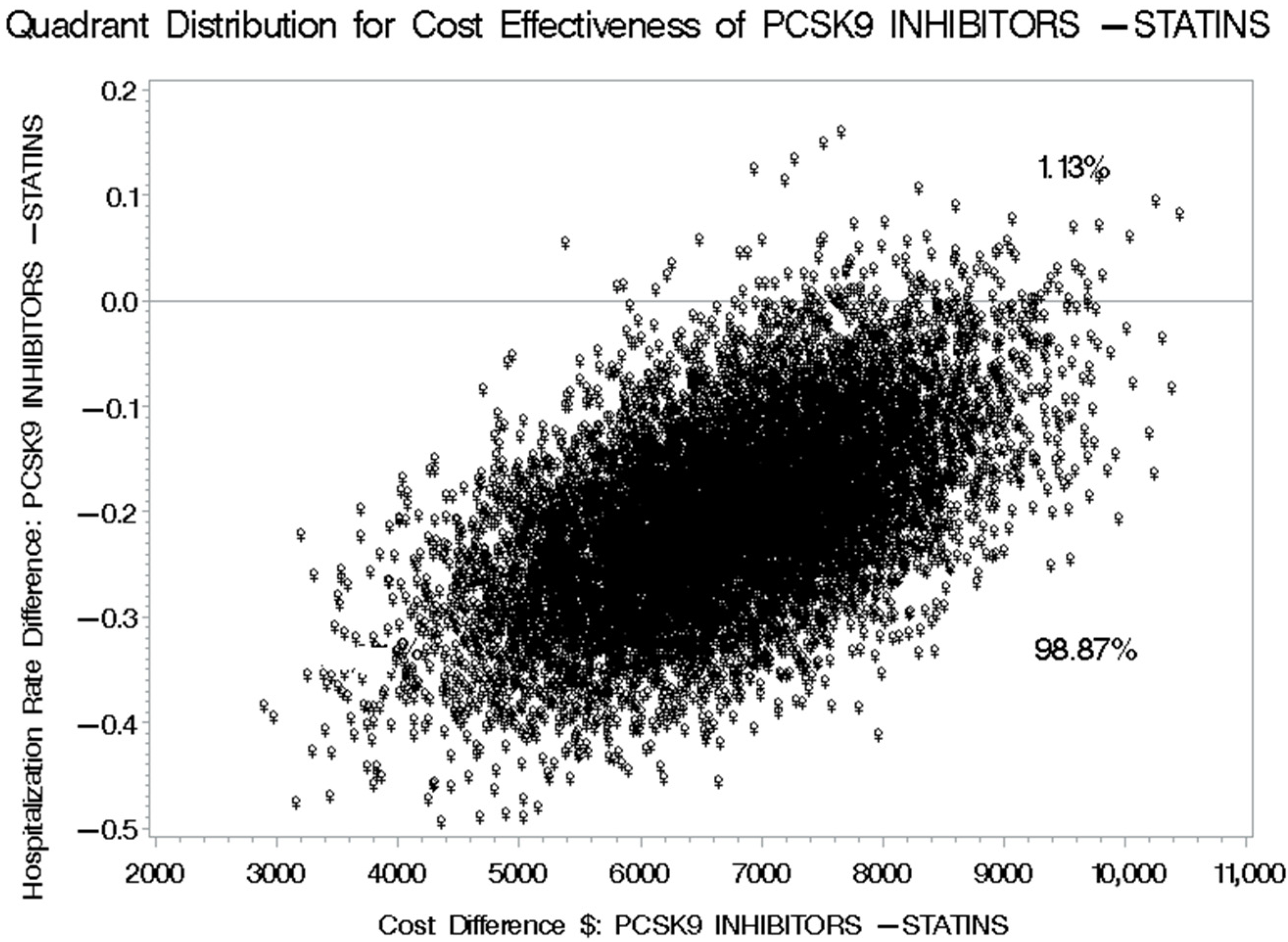
3.5. Cost-Effectiveness of PCSK9 Inhibitors Versus Statins Plus Ezetimibe for Reducing Cardiovascular-Related Hospitalization
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular disease |
| MACE | Major Adverse Cardiovascular Events |
| FH | Familial Hypercholesterolemia |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin type 9 |
| ANGPTL3 | Angiopoietin-like 3 |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| NNTs | Numbers Needed to Treat |
| EMRs | Electronic Medical Records |
| CKD | Chronic Kidney Disease |
| SFDA | Saudi Food and Drug Authority |
| HRQoL | Health-Related Quality of Life |
| mAbs | Monoclonal antibodies |
| USD | United States Dollars |
| QALY | Quality-adjusted life years |
| CSR | Clinical Study Report |
| NHS | National Health Service |
| CET | Cost-Effectiveness Threshold |
| ICER | Incremental Cost-Effectiveness Ratio |
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- Laranjo, L.; Lanas, F.; Sun, M.C.; Chen, D.A.; Hynes, L.; Imran, T.F.; Kazi, D.S.; Kengne, A.P.; Komiyama, M.; Kuwabara, M.; et al. World Heart Federation roadmap for secondary prevention of cardiovascular disease: 2023 update. Glob. Heart 2024, 19, 8. [Google Scholar] [CrossRef]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Turco, J.V.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D. The burden of hypercholesterolemia and ischemic heart disease in an ageing world. Pharmacol. Res. 2023, 193, 106814. [Google Scholar] [CrossRef] [PubMed]
- Alloubani, A.; Nimer, R.; Samara, R. Relationship between Hyperlipidemia, Cardiovascular Disease and Stroke: A Systematic Review. Curr. Cardiol. Rev. 2021, 17, e051121189015. [Google Scholar] [CrossRef] [PubMed]
- Tash, A.A.; Al-Bawardy, R.F. Cardiovascular Disease in Saudi Arabia: Facts and the Way Forward. J. Saudi Heart Assoc. 2023, 35, 148–162. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Hersi, A.; Mashhoud, W.; Arafah, M.R.; Abreu, P.C.; Al Rowaily, M.A.; Al-Mallah, M.H. Cardiovascular risk factors burden in Saudi Arabia: The Africa Middle East Cardiovascular Epidemiological (ACE) study. J. Saudi Heart Assoc. 2017, 29, 235–243. [Google Scholar] [CrossRef]
- World Health Organization. It’s Time to Walk the Talk: WHO Independent High-Level Commission on Noncommunicable Diseases-Final Report; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Alsaidan, A.A. Cardiovascular disease management and prevention in Saudi Arabia: Strategies, risk factors, and targeted interventions. Int. J. Clin. Pract. 2025, 2025, 7233591. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Su, L.; Mittal, R.; Ramgobin, D.; Jain, R.; Jain, R.; Wertz, P.W. Current Management Guidelines on Hyperlipidemia: The Silent Killer. J. Lipids 2021, 2021, 9883352. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Al Rifai, M.; Saeed, A.; Ballantyne, C.M.; Virani, S.S. PCSK9 Inhibitors in the Management of Cardiovascular Risk: A Practical Guidance. Vasc. Health Risk Manag. 2022, 18, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.-C.; Gipe, D.A.; et al. Evinacumab for Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 711–720. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, A.B.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Byrne, R.A.; Coughlan, J.J.; Rossello, X.; Ibanez, B.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Members of the Task Force for the 2023 ESC Guidelines for the management of acute coronary syndromes; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- AlRahimi, J.; AlSaif, S.; Alasnag, M.; Awan, Z.; Almutairi, F.; Al Mudaiheem, H.; Gencer, B.; Catapano, A.L.; Mach, F.; Tash, A. 2022 Saudi Guidelines for the Management of Dyslipidemia. Heart Views 2023, 24, 67–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Suo, Y.; Yang, L.; Zhang, X.; Yu, Q.; Zetng, M.; Zhalng, W.; Jiang, X.; Wang, Y.; Mancusi, C. Effect of PCSK9 Inhibitor on Blood Lipid Levels in Patients with High and Very-High CVD Risk: A Systematic Review and Meta-Analysis. Cardiol. Res. Pract. 2022, 2022, 8729003. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Skavdis, A.; Sourlas, A.; Papakonstantinou, E.J.; Genao, E.P.; Uceta, R.E.; Guzman, E. Safety and Tolerability of PCSK9 Inhibitors: Current Insights. Clin. Pharmacol. 2020, 12, 191–202. [Google Scholar] [CrossRef]
- Wang, H.F.; Mao, Y.-C.; Xu, X.-Y.; Zhao, S.-Y.; Han, D.-D.; Ge, S.-Y.; Song, K.; Geng, C.; Tian, Q.-B. Effect of alirocumab and evolocumab on all-cause mortality and major cardiovascular events: A meta-analysis focusing on the number needed to treat. Front. Cardiovasc. Med. 2022, 9, 1016802. [Google Scholar] [CrossRef]
- McDonagh, M.; Peterson, K.; Holzhammer, B.; Fazio, S. A Systematic Review of PCSK9 Inhibitors Alirocumab and Evolocumab. J. Manag. Care Spec. Pharm. 2016, 22, 641–653q. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.R.; Alshahrani, W.A.; Alhathlol, G.K.; Alsheikh, F.; Alakeel, A.; Al Yami, M.S.; Fouda, M.; Almohammed, O.A.; Alhamed, M.S.; Hafiz, A.; et al. Real-World safety and effectiveness of evolocumab in primary hypercholesterolemia and mixed dyslipidemia in Saudi Arabia. Saudi Pharm. J. 2024, 32, 102203. [Google Scholar] [CrossRef]
- Kholaif, N.; Alrumaih, S.; Vriz, O.; Mohamed, I.T.; Arabi, T.Z.; Makland, A.; Alkhatib, R.; Mohamed, G.; Di Michele, S.; Marra, A.M.; et al. Effectiveness of PCSK9 inhibitors in homozygous familial hypercholesterolemia: Insights from a Saudi Arabia genetic population. Eur. J. Prev. Cardiol. 2025, 32 (Suppl. S1), zwaf236.442. [Google Scholar] [CrossRef]
- Mercep, I.; Strikic, D.; Hrabac, P.; Pecin, I.; Reiner, Ž. PCSK9 inhibition: From effectiveness to cost-effectiveness. Front. Cardiovasc. Med. 2024, 11, 1339487. [Google Scholar] [CrossRef]
- Lin, P.L.; Wu, Y.-W.; Lin, C.-F.; Yeh, H.-I.; Chang, W.-T.; Charng, M.-J.; Huang, P.-H.; Lin, C.-C.; Lin, T.-H.; Lin, W.-W.; et al. Real-World Analyses of the Treatment Conditions in Patients Initiating Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitor in Taiwan. J. Atheroscler. Thromb. 2023, 30, 1123–1131. [Google Scholar] [CrossRef]
- Kodera, S.; Morita, H.; Kiyosue, A.; Ando, J.; Takura, T.; Komuro, I. Cost-Effectiveness of PCSK9 Inhibitor Plus Statin in Patients With Triple-Vessel Coronary Artery Disease in Japan. Circ. J. 2018, 82, 2602–2608. [Google Scholar] [CrossRef]
- Michaeli, D.T.; Michaeli, J.C.; Boch, T.; Michaeli, T. Cost-effectiveness of cholesterol-lowering drugs for secondary cardiovascular prevention in the UK: Ezetimibe, evolocumab, and alirocumab. Eur. Heart J. 2022, 43 (Suppl. 2), ehac544.2367. [Google Scholar] [CrossRef]
- Kumar, R.; Tonkin, A.; Liew, D.; Zomer, E. The cost-effectiveness of PCSK9 inhibitors—The Australian healthcare perspective. Int. J. Cardiol. 2018, 267, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Azari, S.; Rezapour, A.; Omidi, N.; Alipour, V.; Behzadifar, M.; Safari, H.; Tajdini, M.; Bragazzi, N.L. Cost-effectiveness analysis of PCSK9 inhibitors in cardiovascular diseases: A systematic review. Heart Fail. Rev. 2020, 25, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, A.; Hong, J.C.; Khera, R.; Virani, S.S.; Krumholz, H.M.; Nasir, K. Updated Cost-effectiveness Assessments of PCSK9 Inhibitors From the Perspectives of the Health System and Private Payers: Insights Derived From the FOURIER Trial. JAMA Cardiol. 2017, 2, 1369–1374. [Google Scholar] [CrossRef]
- Alghamdi, A.; Balkhi, B.; Altowaijri, A.; Al-Shehri, N.; Ralph, L.; Marriott, E.-R.; Urbich, M.; Aljanad, F.; Aziziyeh, R. Cost-Effectiveness Analysis of Evolocumab for the Treatment of Dyslipidemia in the Kingdom of Saudi Arabia. Pharmacoecon Open 2022, 6, 277–291. [Google Scholar] [CrossRef]
- Al-Jedai, A.H.; Lomas, J.; Almudaiheem, H.Y.; Al-Ruthia, Y.S.H.; Alghamdi, S.; Awad, N.; Alghamdi, A.; Alowairdhi, M.A.; Alabdulkarim, H.; Almadi, M.; et al. Informing a cost-effectiveness threshold for Saudi Arabia. J. Med. Econ. 2023, 26, 128–138. [Google Scholar] [CrossRef]
- Špacírová, Z.; Epstein, D.; García-Mochón, L.; Rovira, J.; Olry de Labry Lima, A.; Espín, J. A general framework for classifying costing methods for economic evaluation of health care. Eur. J. Health Econ. 2020, 21, 529–542. [Google Scholar] [CrossRef]
- Briggs, A.H.; Wonderling, D.E.; Mooney, C.Z. Pulling cost-effectiveness analysis up by its bootstraps: A non-parametric approach to confidence interval estimation. Health Econ. 1997, 6, 327–340. [Google Scholar] [CrossRef]
- Khan, I.; Neumann, P.J.; Radensky, P.W. Abstract 10485: Low Density Lipoprotein Cholesterol (LDL-C) Lowering in a Population Represented in a Meta-Analysis of Major Randomized Trials is Associated with a Positive Health-Economic Benefit. Circulation 2011, 124 (Suppl. 21), A10485. [Google Scholar]
- Erviti, J.; Wright, J.; Bassett, K.; Ben-Eltriki, M.; Jauca, C.; Saiz, L.C.; Leache, L.; Gutiérrez-Valencia, M.; Perry, T.L. Restoring mortality data in the FOURIER cardiovascular outcomes trial of evolocumab in patients with cardiovascular disease: A reanalysis based on regulatory data. BMJ Open 2022, 12, e060172. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shraie, N.; Alhammad, A.; Balkhi, B.; Al-Jedai, A. Implementation of risk-sharing agreements in Saudi Arabia: Comparison and reflection on the NICE model. Trop. J. Pharm. Res. 2023, 22, 1121–1131. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, S.; Zhong, X.; Li, M.; Liu, M.; Zhuang, X.; Liao, X. Real-World Assessment of the Association Between PCSK9i Adherence and LDL Reduction and Variability in a Chinese Clinical Practice. Clin. Epidemiol. 2025, 17, 537–546. [Google Scholar] [CrossRef]
- Muntner, P.; Ghazi, L.; Jones, J.; Dhalwani, N.; Poudel, B.; Wen, Y.; Chen, L.; Wang, Z.; Bittner, V.; Kalich, B.; et al. Persistence and Adherence to PCSK9 Inhibitor Monoclonal Antibodies Versus Ezetimibe in Real-World Settings. Adv. Ther. 2024, 41, 2399–2413. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | PCSK9 Inhibitors (N = 118) | Statins Plus Ezetimibe (N = 304) | p-Value | Total |
|---|---|---|---|---|
| Age (yrs.), mean ± SD | 50.96 ± 14.49 | 67.72 ± 13.79 | <0.001 | 63.04 ± 15.88 |
| Gender, n(%) | ||||
| Male | 79 (66.95) | 182 (59.87) | 0.179 | 261 (61.85) |
| Female | 39 (33.05) | 122 (40.13) | 161 (38.15) | |
| Duration of illness (yrs.), mean ± SD | 4.89 ± 2.69 | 5.39 ± 1.98 | 0.068 | 5.25 ± 2.22 |
| Body Mass Index (BMI), mean ± SD | 29.59 ± 5.92 | 30.59 ± 5.69 | 0.109 | 30.31 ± 2.69 |
| Duration of therapy (yrs.), mean ± SD | 3.03 ± 1.38 | 5.39 ± 1.98 | <0.001 | 4.73 ± 2.12 |
| Comorbidities, n (%) | ||||
| Chronic Kidney Disease (CKD) | 5 (4.24) | 61 (20.07) | <0.001 | 66 (15.64) |
| Diabetes | 51 (43.22) | 214 (70.39) | <0.001 | 265 (62.80) |
| Hypertension | 33 (27.97) | 161 (52.96) | <0.001 | 194 (45.97) |
| ST Elevation Myocardial Infarction (STEMI) | 14 (11.86) | 72 (23.68) | 0.006 | 86 (20.38) |
| Non-ST-elevation myocardial infarction (NSTEMI) | 28 (23.73) | 156 (51.32) | <0.001 | 184 (43.60) |
| Smoking status | ||||
| Smoker | 23 (19.49) | 1 (0.33) | <0.001 | 24 (5.69) |
| Ex-smoker | 6 (5.08) | 0 (0.0) | 6 (1.42) | |
| Non-smoker | 89 (75.42) | 303 (99.67) | 392 (92.89) | |
| Lab values | ||||
| LDL (mmol/L), mean ± SD | 5.19 ± 3.62 | 3.35 ± 1.89 | <0.001 | 3.87 ± 2.63 |
| Triglyceride (mmol/L), mean ± SD | 2.04 ± 2.29 | 1.81 ± 1.87 | 0.328 | 1.87 ± 1.99 |
| HDL (mmol/L), mean ± SD | 1.07 ± 0.29 | 0.99 ± 0.26 | 0.012 | 1.02 ± 0.27 |
| Total cholesterol (mmol/L), mean ± SD | 7.12 ± 3.74 | 4.96 ± 1.94 | <0.001 | 5.56 ± 2.75 |
| Variable | Mean at Baseline (mmol/L) ± SD | Mean at Follow-Up (mmol/L) ± SD | Mean Difference (mmol/L) (95% Confidence Interval) | p-Value |
|---|---|---|---|---|
| LDL for PCSK9 inhibitors | 5.19 ± 3.62 | 3.76 ± 3.16 | 1.432 (0.964–1.899) | <0.001 |
| LDL for statins plus ezetimibe | 3.35 ± 1.89 | 2.71 ± 1.45 | 0.644 (0.464–0.823) | <0.001 |
| Triglyceride for PCSK9 inhibitors | 2.04 ± 2.29 | 1.74 ± 1.04 | 0.307 (−0.031–0.645) | 0.074 |
| Triglyceride for statins plus ezetimibe | 1.81 ± 1.87 | 1.64 ± 1.27 | 0.168 (0.049–0.287) | 0.005 |
| HDL for PCSK9 inhibitors | 1.07 ± 0.29 | 1.07 ± 0.28 | −0.002 (−0.035–0.030) | 0.886 |
| HDL for statins plus ezetimibe | 0.99 ± 0.26 | 0.97 ± 0.189 | 0.022 (0.0006–0.0433) | 0.043 |
| Total cholesterol for PCSK9 inhibitors | 7.12 ± 3.74 | 5.48 ± 3.29 | 1.642 (1.156–2.129) | <0.001 |
| Total cholesterol for statins plus ezetimibe | 4.96 ± 1.94 | 4.27 ± 1.54 | 0.684 (0.497–0.872) | <0.001 |
| Variable | PCSK9 Inhibitors | Statins Plus Ezetimibe | Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| Cost of treatment (USD), mean ± SD | 19,061.95 ± 10,254.45 | 11,503.07 ± 12,834.15 | 7559 (7331.35–11,509.66) |
| Difference in LDL (mmol/L) | −1.43 ± 2.57 | −0.64 ± 1.59 | −0.79 (−1.46–−0.60) |
| Variable | PCSK9 Inhibitors | Statins Plus Ezetimibe | Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| Cost of treatment (USD), mean ± SD | 16,371.59 ± 10,277.46 | 11,496.45 ± 12,834.15 | 4875.14 (4623.54–8769.09) |
| Difference in LDL (mmol/L) | −1.43 ± 2.57 | −0.64 ± 1.59 | −0.79 (−1.46–−0.60) |
| Variable | PCSK9 Inhibitors | Statins Plus Ezetimibe | Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| Cost of treatment (USD), mean ± SD | 15,524.19 ± 10,260.67 | 11,500.72 ± 12,834.15 | 4023.5 (3786.80–7947.91) |
| Difference in LDL (mmol/L) | −1.43 ± 2.57 | −0.64 ± 1.59 | −0.79 (−1.46–−0.60) |
| Variable | PCSK9 Inhibitors | Statins Plus Ezetimibe | Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| Cost of treatment (USD), mean ± SD | 19,061.95 ± 10,254.45 | 11,503.07 ± 12,834.15 | 7559 (7331.35–11,509.66) |
| Mean number of cardiovascular-related hospitalizations | 0.645 ± 0.93 | 0.808 ± 0.82 | −0.163 (−0.287–−0.0601) |
| Variable | PCSK9 Inhibitors | Statins Plus Ezetimibe | Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| Cost of treatment (USD), mean ± SD | 16,371.59 ± 10,277.46 | 11,496.45 ± 12,834.15 | 4875.14 (4623.54–8769.09) |
| Mean number of cardiovascular-related hospitalizations | 0.645 ± 0.93 | 0.808 ± 0.82 | −0.163 (−0.287–0.0601) |
| Variable | PCSK9 Inhibitors | Statins Plus Ezetimibe | Mean Difference (95% Confidence Interval) |
|---|---|---|---|
| Cost of treatment (USD), mean ± SD | 15,524.19 ± 10,260.67 | 11,500.72 ± 12,834.15 | 4023.5 (3786.80–7947.91) |
| Mean number of cardiovascular-related hospitalizations | 0.645 ± 0.93 | 0.808 ± 0.82 | −0.163 (−0.287–0.0601) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlRuthia, Y.; Almutairi, K.K.; Aljammaz, N.A.; Alsuwayegh, A.; Alanazi, M.A.; AlSulaiman, R.F.; Alfaori, T.M.; Alabdan, N. Medical and Financial Consequences of Using PCSK9 Inhibitors for Managing Hypercholesterolemia in Saudi Arabia: A Historical Cohort Study. Healthcare 2025, 13, 2428. https://doi.org/10.3390/healthcare13192428
AlRuthia Y, Almutairi KK, Aljammaz NA, Alsuwayegh A, Alanazi MA, AlSulaiman RF, Alfaori TM, Alabdan N. Medical and Financial Consequences of Using PCSK9 Inhibitors for Managing Hypercholesterolemia in Saudi Arabia: A Historical Cohort Study. Healthcare. 2025; 13(19):2428. https://doi.org/10.3390/healthcare13192428
Chicago/Turabian StyleAlRuthia, Yazed, Khlood Khaled Almutairi, Norah Abdulaziz Aljammaz, Aseel Alsuwayegh, Miteb A. Alanazi, Rasha Fahad AlSulaiman, Tareq Majed Alfaori, and Numan Alabdan. 2025. "Medical and Financial Consequences of Using PCSK9 Inhibitors for Managing Hypercholesterolemia in Saudi Arabia: A Historical Cohort Study" Healthcare 13, no. 19: 2428. https://doi.org/10.3390/healthcare13192428
APA StyleAlRuthia, Y., Almutairi, K. K., Aljammaz, N. A., Alsuwayegh, A., Alanazi, M. A., AlSulaiman, R. F., Alfaori, T. M., & Alabdan, N. (2025). Medical and Financial Consequences of Using PCSK9 Inhibitors for Managing Hypercholesterolemia in Saudi Arabia: A Historical Cohort Study. Healthcare, 13(19), 2428. https://doi.org/10.3390/healthcare13192428





