Implementation of Medicalholodeck® for Augmented Reality Surgical Navigation in Microsurgical Mandibular Reconstruction: Enhanced Vessel Identification
Abstract
1. Introduction
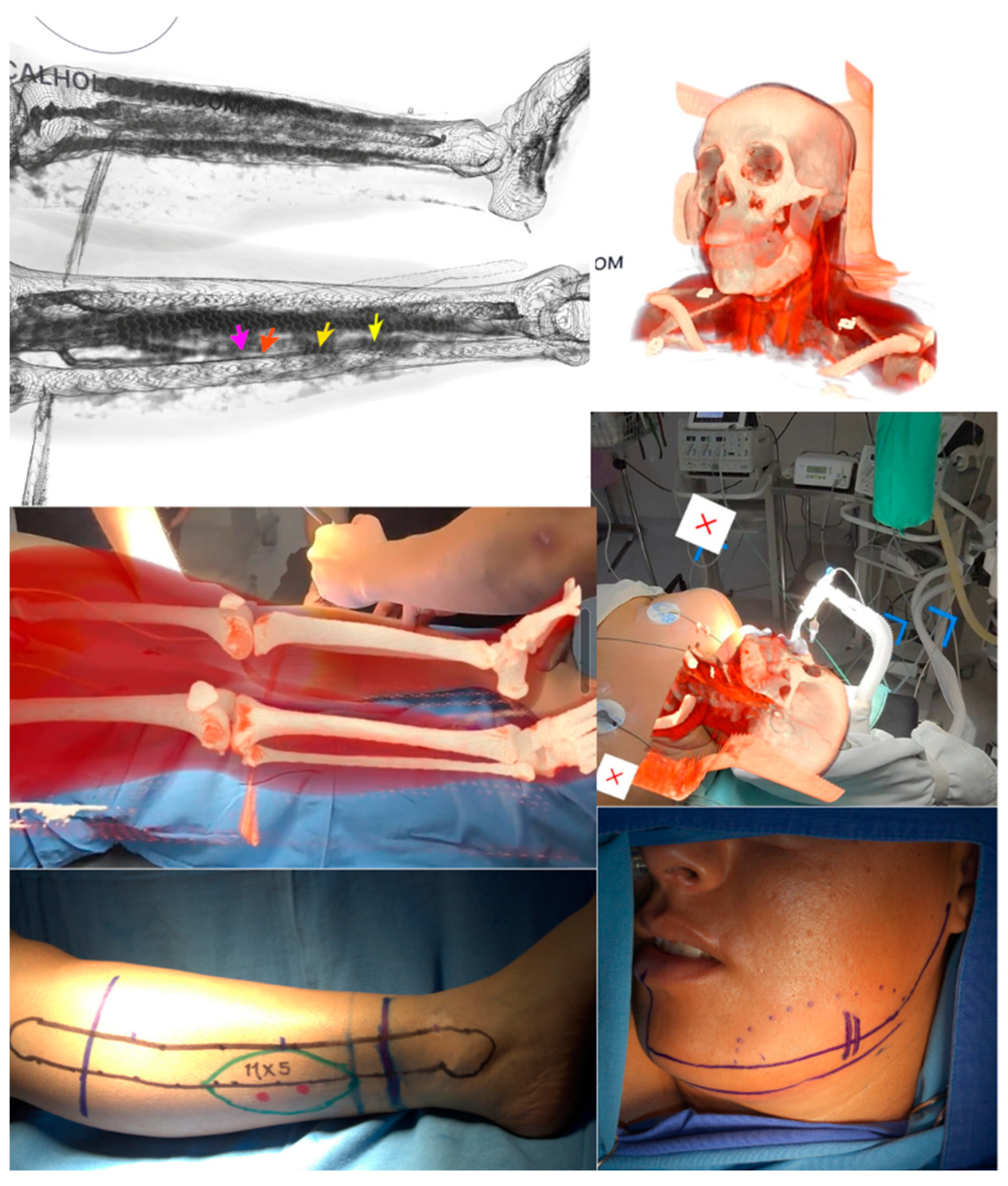
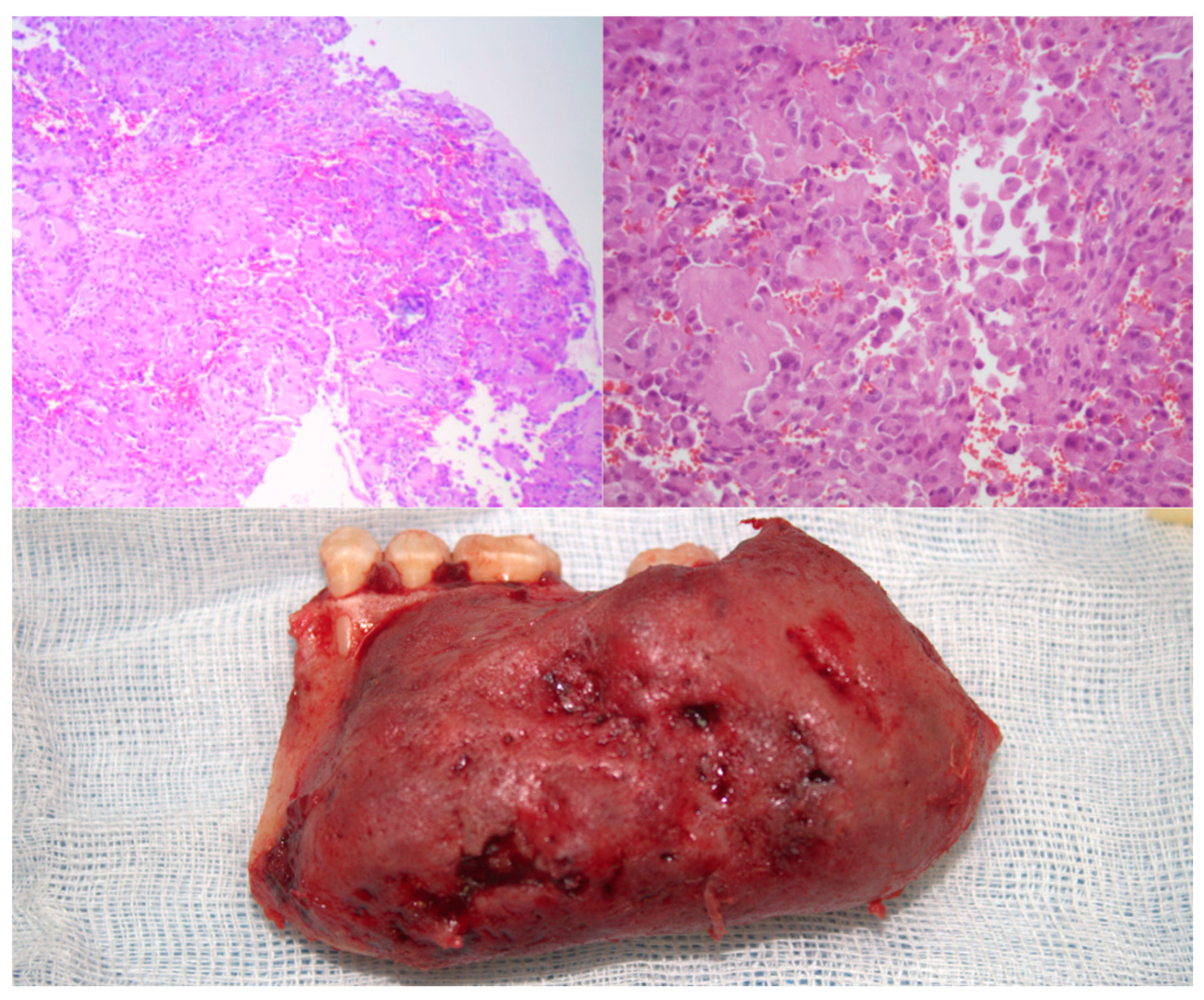
2. Case Presentation
2.1. Holographic Navigation Workflow
2.2. Surgical Technique
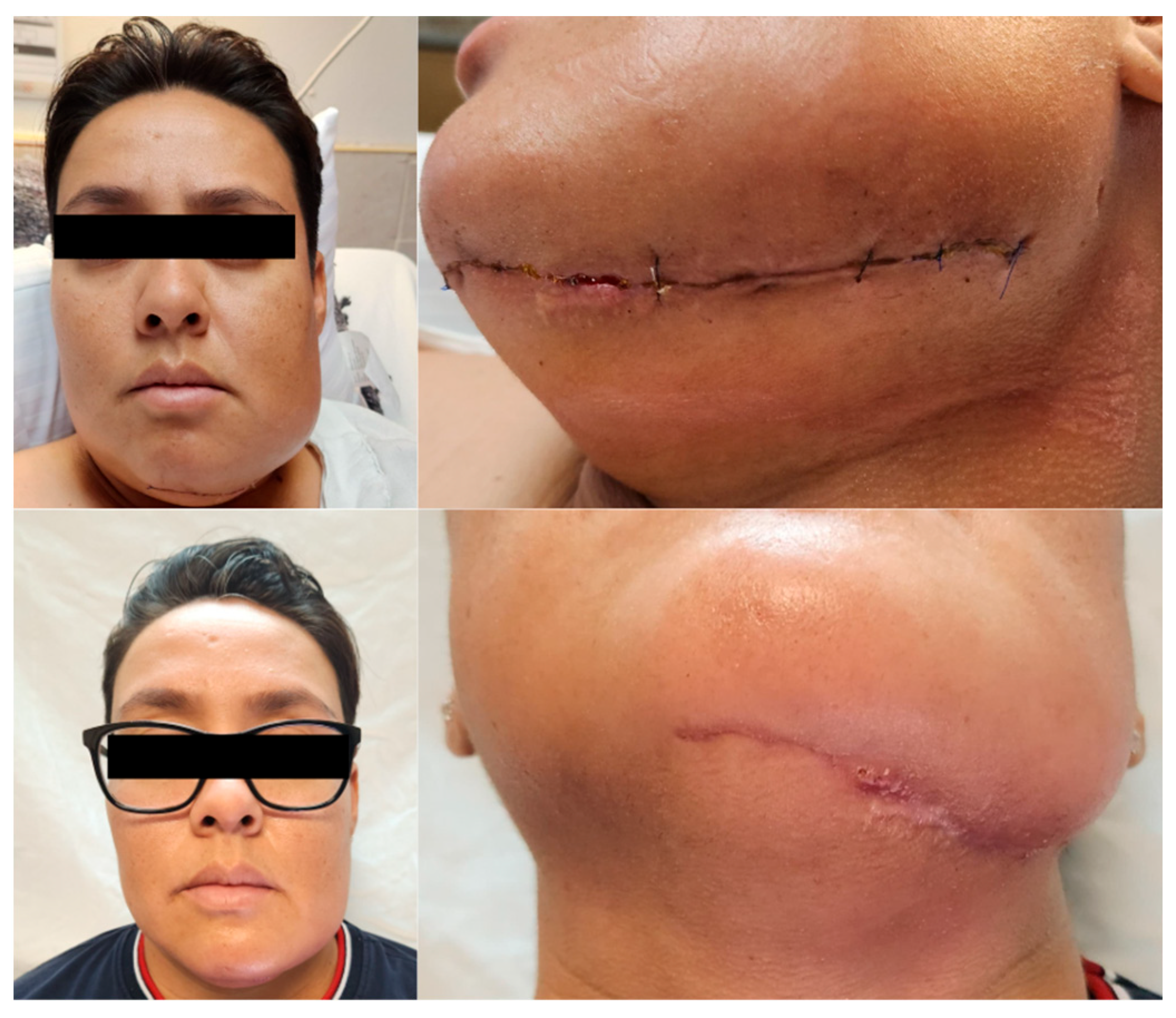
2.3. Postoperative Course
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suster, D.; Mackinnon, A.C.; Jarzembowski, J.A.; Carrera, G.; Suster, S.; Klein, M.J. Epithelioid osteoblastoma. Clinicopathologic and immunohistochemical study of 17 cases. Hum. Pathol. 2022, 125, 68–78. [Google Scholar] [CrossRef]
- Caruso, D.P.; Fernandes, R.P.; Silva, M.M.; Bunnell, A. A benign tumor of substantial size: Mandibular epithelioid osteoblastoma in a socioeconomically challenged patient. J. Craniomaxillofac. Surg. 2025, 53, 44–51. [Google Scholar] [CrossRef]
- Mesfin, A.; Boriani, S.; Gambarotti, M.; Bandiera, S.; Gasbarrini, A. Can osteoblastoma evolve to malignancy? A challenge in the decision-making process of a benign spine tumor. World Neurosurg. 2020, 136, 150–156. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Yacoub, B.; Barakat, A.; Dergham, M.Y.; Maroun, G.; Haddad, H.; Haidar, M.B. Case report of epithelioid osteoblastoma of the mandible: Findings on positron emission tomography/computed tomography and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, e16–e20. [Google Scholar] [CrossRef]
- Pereira, T.S.F.; de Andrade, B.A.B.; Romañach, M.J.; Pereira, N.B.; Gomes, C.C.; Mariz, B.A.L.A.; Fonseca, F.P. Clinicopathologic study of 6 cases of epithelioid osteoblastoma of the jaws with immunoexpression analysis of FOS and FOSB. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 191–199. [Google Scholar] [CrossRef]
- Tamura, M.; Hoshimoto, Y.; Seta, S.; Nakanishi, Y.; Sasaki, M.; Aoki, T.; Ota, Y. A case of extremely rare mandibular osteoblastoma successfully treated with functional reconstruction. J. Oral Maxillofac. Surg. Med. Pathol. 2024, 36, 89–94. [Google Scholar] [CrossRef]
- Harazono, Y.; Yoshitake, H.; Fukawa, Y.; Ikeda, T.; Yoda, T. Osteoblastoma in the mandible of an older adult patient without FOS gene rearrangement: A case report and literature review. J. Oral Maxillofac. Surg. Med. Pathol. 2024, 36, 773–777. [Google Scholar] [CrossRef]
- Britt, J.D.; Murphey, M.D.; Castle, J.T. Epithelioid Osteoblastoma. Head Neck Pathol. 2012, 6, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Fliss, E.; Yanko, R.; Bracha, G.; Teman, R.; Amir, A.; Horowitz, G.; Muhanna, N.; Fliss, D.M.; Gur, E.; Zaretski, A. The Evolution of the Free Fibula Flap for Head and Neck Reconstruction: 21 Years of Experience with 128 Flaps. J. Reconstr. Microsurg. 2020, 37, 372–379. [Google Scholar] [CrossRef]
- Sacco, R.; Sacco, N.; Hamid, U.; Ali, S.H.; Singh, M.; Blythe, J.S.J. Microsurgical Reconstruction of the Jaws Using Vascularised Free Flap Technique in Patients with Medication-Related Osteonecrosis: A Systematic Review. BioMed Res. Int. 2018, 2018, 9858921. [Google Scholar] [CrossRef]
- Suh, J.M.; Chung, C.H.; Chang, Y.J. Head and neck reconstruction using free flaps: A 30-year medical record review. Arch. Craniofac. Surg. 2021, 22, 38–44. [Google Scholar] [CrossRef]
- Barr, M.L.; Haveles, C.S.; Rezzadeh, K.S.; Nolan, I.T.; Castro, R.; Lee, J.C.; Steinbacher, D.; Pfaff, M.J. Virtual Surgical Planning for Mandibular Reconstruction With the Fibula Free Flap: A Systematic Review and Meta-analysis. Ann. Plast. Surg. 2019, 84, 117–122. [Google Scholar] [CrossRef]
- Knitschke, M.; Sonnabend, S.; Bäcker, C.; Schmermund, D.; Böttger, S.; Howaldt, H.-P.; Attia, S. Partial and Total Flap Failure after Fibula Free Flap in Head and Neck Reconstructive Surgery: Retrospective Analysis of 180 Flaps over 19 Years. Cancers 2021, 13, 865. [Google Scholar] [CrossRef]
- Sandoval, M.L.; Rosen, E.B.; Allen, R.J.; Nelson, J.A.; Matros, E.; Gelblum, D.Y. Immediate dental implants in fibula free flaps to reconstruct the mandible: A pilot study of the short-term effects on radiotherapy for patients with head and neck cancer. Clin. Implant Dent. Relat. Res. 2020, 22, 91–95. [Google Scholar] [CrossRef]
- Lin, D.T.; Coppit, G.L.; Burkey, B.B.; Netterville, J.L. Use of the ulnar forearm flap in head and neck reconstruction. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 1455–1459. [Google Scholar]
- Wang, K.-Y.; Liu, W.-C.; Chen, L.-W.; Chen, C.-F.; Yang, K.-C.; Chen, H.-C. Osteomyocutaneous Free Fibula Flap Prevents Osteoradionecrosis and Osteomyelitis in Head and Neck Cancer Reconstruction. J. Reconstr. Microsurg. 2021, 37, 524–529. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, J.T.M.; Abbink, J.H.; van Es, R.J.J.; Rosenberg, A.J.W.P.; Koole, R.; Van Cann, E.M. Early and late complications in the reconstructed mandible with free fibula flaps. J. Surg. Oncol. 2018, 117, 773–780. [Google Scholar] [CrossRef]
- Kim, H.S.; Chung, C.H.; Chang, Y.J. Free-flap reconstruction in recurrent head and neck cancer: A retrospective review of 124 cases. Arch. Craniofac. Surg. 2020, 21, 27–34. [Google Scholar] [CrossRef]
- Gsaxner, C.; Pepe, A.; Li, J.; Ibrahimpasic, U.; Wallner, J.; Schmalstieg, D.; Egger, J. Augmented Reality for Head and Neck Carcinoma Imaging: Description and Feasibility of an Instant Calibration, Markerless Approach. Comput. Methods Programs Biomed. 2021, 200, 105854. [Google Scholar] [CrossRef]
- Profeta, A.C.; Schilling, C.; McGurk, M. Augmented reality visualization in head and neck surgery: An overview of recent findings in sentinel node biopsy and future perspectives. Br. J. Oral Maxillofac. Surg. 2016, 54, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Tel, A.; Raccampo, L.; Vinayahalingam, S.; Troise, S.; Abbate, V.; Dell’Aversana Orabona, G.; Sembronio, S.; Robiony, M. Complex Craniofacial Cases through Augmented Reality Guidance in Surgical Oncology: A Technical Report. Diagnostics 2024, 14, 1108. [Google Scholar] [CrossRef] [PubMed]
- Cercenelli, L.; Babini, F.; Badiali, G.; Battaglia, S.; Tarsitano, A.; Marchetti, C.; Marcelli, E. Augmented Reality to Assist Skin Paddle Harvesting in Osteomyocutaneous Fibular Flap Reconstructive Surgery: A Pilot Evaluation on a 3D-Printed Leg Phantom. Front. Oncol. 2022, 11, 804748. [Google Scholar] [CrossRef]
- Tong, G.; Xu, J.; Pfister, M.; Atoum, J.; Prasad, K.; Miller, A.; Topf, M.; Wu, J.Y. Development of an augmented reality guidance system for head and neck cancer resection. Healthc. Technol. Lett. 2024, 11, 93–100. [Google Scholar] [CrossRef]
- Modabber, A.; Ayoub, N.; Redick, T.; Gesenhues, J.; Kniha, K.; Möhlhenrich, S.C.; Raith, S.; Abel, D.; Hölzle, F.; Winnand, P. Comparison of augmented reality and cutting guide technology in assisted harvesting of iliac crest grafts—A cadaver study. Ann. Anat. 2022, 239, 151834. [Google Scholar] [CrossRef]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Wasserthal, J.; Breit, H.-C.; Meyer, M.T.; Pradella, M.; Hinck, D.; Sauter, A.W.; Heye, T.; Boll, D.T.; Cyriac, J.; Yang, S.; et al. TotalSegmentator: Robust Segmentation of 104 Anatomic Structures in CT Images. Radiol. Artif. Intell. 2023, 5, e230024. [Google Scholar] [CrossRef]
- Pereira, N.; Kufeke, M.; Parada, L.; Troncoso, E.; Bahamondes, J.; Sanchez, L.; Roa, R. Augmented Reality Microsurgical Planning with a Smartphone (ARM-PS): A dissection route map in your pocket. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 759–762. [Google Scholar] [CrossRef]
- Tel, A.; Zeppieri, M.; Robiony, M.; Sembronio, S.; Vinayahalingam, S.; Pontoriero, A.; Pergolizzi, S.; Angileri, F.F.; Spadea, L.; Ius, T. Exploring Deep Cervical Compartments in Head and Neck Surgical Oncology through Augmented Reality Vision: A Proof of Concept. J. Clin. Med. 2023, 12, 6650. [Google Scholar] [CrossRef]
- Ochandiano, S.; García-Mato, D.; Gonzalez-Alvarez, A.; Moreta-Martinez, R.; Tousidonis, M.; Navarro-Cuellar, C.; Navarro-Cuellar, I.; Salmerón, J.I.; Pascau, J. Computer-Assisted Dental Implant Placement Following Free Flap Reconstruction: Virtual Planning, CAD/CAM Templates, Dynamic Navigation and Augmented Reality. Front. Oncol. 2022, 11, 754943. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Miller, A.; Sharif, K.; Colazo, J.M.; Ye, W.; Necker, F.; Baik, F.; Lewis, J.S., Jr.; Rosenthal, E.; Topf, M.C. Augmented-Reality Surgery to Guide Head and Neck Cancer Re-resection: A Feasibility and Accuracy Study. Ann. Surg. Oncol. 2023, 30, 4994–5000. [Google Scholar] [CrossRef]
- Heredero, S.; Gómez, V.; Sanjuan-Sanjuan, A. The Role of Virtual Reality, Augmented Reality and Mixed Reality in Modernizing Free Flap Reconstruction Techniques. Oral Maxillofac. Surg. Clin. N. Am. 2025, 37, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Winter, R.; Tuca, A.C.; Michelitsch, B.; Schenkenfelder, B.; Hartmann, R.; Giretzlehner, M.; Reishofer, G.; Kamolz, L.-P.; Lumenta, D.B. Workflow Assessment of an Augmented Reality Application for Planning of Perforator Flaps in Plastic Reconstructive Surgery: Game or Game Changer? Digit. Health 2023, 9, 20552076231173554. [Google Scholar] [CrossRef] [PubMed]
- Sunnysinh, D.; Dandagi, S.; Sikkerimath, B.C.; Jose, A.; Bora, S.P. Analysis of Variation in Anatomy of Lower Limb Vasculature and Implications for Free Fibula Flap by Color Doppler Imaging. J. Maxillofac. Oral Surg. 2022, 21, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, W.; Yu, M.; Wu, J.; Wu, S.; Zhou, L.; Chen, Q. Comparison between Mixed Reality with Artificial Algorithms and Ultrasound in Localization of Anterior Thigh Flap Perforators: A Prospective Randomized Controlled Study. BMC Med. 2025, 23, 374. [Google Scholar] [CrossRef]
- Phan, R.; Chae, M.P.; Hunter-Smith, D.J.; Rozen, W.M. Advances in Perforator Imaging through Holographic CTA and Augmented Reality: A Systematic Review. Australas. J. Plast. Surg. 2022, 5, 32–38. [Google Scholar] [CrossRef]
- Lacey, H.; Khoong, Y.M.; Dheansa, B. Augmented Reality for Perforator Mapping: A Systematic Review and MetaAnalysis. J. Plast. Reconstr. Aesthet. Surg. 2025, 104, 170–180. [Google Scholar] [CrossRef]
- Kaplan, N.; Marques, M.; Scharf, I.; Yang, K.; Alkureishi, L.; Purnell, C.; Patel, P.; Zhao, L. Virtual Reality and Augmented Reality in Plastic and Craniomaxillofacial Surgery: A Scoping Review. Bioengineering 2023, 10, 480. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendón Mejía, N.A.; Gómez Arámbula, H.; Baeza Ramos, J.H.; Villa Martínez, Y.; Hernández Ávila, F.; Quiñonez Pérez, M.; Caraveo Aguilar, C.; Mariñelarena Hernández, R.; Reyes Montero, C.; Ramírez Espinoza, C.; et al. Implementation of Medicalholodeck® for Augmented Reality Surgical Navigation in Microsurgical Mandibular Reconstruction: Enhanced Vessel Identification. Healthcare 2025, 13, 2406. https://doi.org/10.3390/healthcare13192406
Rendón Mejía NA, Gómez Arámbula H, Baeza Ramos JH, Villa Martínez Y, Hernández Ávila F, Quiñonez Pérez M, Caraveo Aguilar C, Mariñelarena Hernández R, Reyes Montero C, Ramírez Espinoza C, et al. Implementation of Medicalholodeck® for Augmented Reality Surgical Navigation in Microsurgical Mandibular Reconstruction: Enhanced Vessel Identification. Healthcare. 2025; 13(19):2406. https://doi.org/10.3390/healthcare13192406
Chicago/Turabian StyleRendón Mejía, Norman Alejandro, Hansel Gómez Arámbula, José Humberto Baeza Ramos, Yidam Villa Martínez, Francisco Hernández Ávila, Mónica Quiñonez Pérez, Carolina Caraveo Aguilar, Rogelio Mariñelarena Hernández, Claudio Reyes Montero, Claudio Ramírez Espinoza, and et al. 2025. "Implementation of Medicalholodeck® for Augmented Reality Surgical Navigation in Microsurgical Mandibular Reconstruction: Enhanced Vessel Identification" Healthcare 13, no. 19: 2406. https://doi.org/10.3390/healthcare13192406
APA StyleRendón Mejía, N. A., Gómez Arámbula, H., Baeza Ramos, J. H., Villa Martínez, Y., Hernández Ávila, F., Quiñonez Pérez, M., Caraveo Aguilar, C., Mariñelarena Hernández, R., Reyes Montero, C., Ramírez Espinoza, C., & Reyes Carrillo, A. I. (2025). Implementation of Medicalholodeck® for Augmented Reality Surgical Navigation in Microsurgical Mandibular Reconstruction: Enhanced Vessel Identification. Healthcare, 13(19), 2406. https://doi.org/10.3390/healthcare13192406







