Abstract
Background: Total hip (THR) and knee replacement (TKR) effectively treat end-stage osteoarthritis, but many patients continue to experience functional limitations and reduced quality of life (QoL) after rehabilitation. The aim of this pilot study was to assess the changes in terms of QoL in people with THR and TKR after a specifically designed PA intervention. The secondary aim was to evaluate changes in physical function through strength and mobility tests. Methods: Eighteen participants (mean age 65.8 ± 7.1 years) were enrolled at the Rizzoli Orthopedic Institute and were randomly assigned to an intervention group (IG), which completed a six-month supervised PA program (6 months after surgery), or a control group (CG), which received standard care. Assessments were conducted at four time points: before surgery, after rehabilitation, and at 9- and 12-month post-surgery. Repeated measures ANOVA was used to assess within- and between-group differences over time, with post hoc pairwise comparisons conducted using independent t-tests with Sidak correction. The level of statistical significance was set at p < 0.05 for all analyses. Results: Both groups showed significant improvements in QoL over time, with greater gains in physical functioning observed in the IG. Lower limb strength increased more in the IG; however, the differences between groups were not statistically significant. The Timed Up and Go and 30-Second Chair Stand Test improved in both groups. No adverse events were reported. Conclusions: These findings support the feasibility and potential benefits of adapted PA programs after rehabilitation. Although no significant differences emerged between groups, clinically relevant improvements were observed in the IG. Larger studies are warranted to confirm these results and explore long-term outcomes across multiple domains.
1. Introduction
Osteoarthritis (OA) is a common joint disorder, and its incidence is rising as the world’s population ages [1]. Clinical symptoms of OA include pain, brief morning stiffness, and crepitus during joint mobility, all of which significantly reduce daily quality of life (QoL) [2,3]. Moreover, OA can result in a variety of personal, social, and economic consequences, including decreased productivity, early retirement from the workforce, loss of independence, the need for assistance, and increased use of health services [4,5,6].
Nowadays, the preferred treatment for end stage OA is total joint replacement since the surgical treatment can alleviate the pain [7,8,9] and thereby improve QoL. The multidimensional concept of QoL includes personal health (physical, mental, and spiritual), relationships, education status and social status, work environment, a sense of security and safety, and autonomy. These elements, especially physical and mental [10], are negatively influenced by OA, which most commonly affects the hip and knee [4], often leading to total hip and knee replacement (THR and TKR), the most frequently performed surgical procedures [11,12,13,14]. However, such surgical procedures alone may not fully restore the natural joint function [15,16] and patients report dissatisfaction rates of about 10% for THR and 15–30% for TKR [17].
According to the Organization for Economic Cooperation and Development (OECD), there were 193.9 THR and 137.0 TKR cases per 100,000 people in Italy [18]. These numbers are relatively consistent with OECD country averages for 2019 (174.1 THR and 136.7 TKR per 100,000 people [18]). There is consensus that people with THR and TKR can experience several benefits from being physically active, including a reduced risk of falls, increased bone density, improved prosthetic fixation, and decreased risk of prosthetic loosening [19,20]. According to the World Health Organization (WHO), adults aged 65 and older should aim for at least 150 min per week of moderate-intensity aerobic activity (or 75 min per week of vigorous activity), with strength training on two or more days per week [21]. Moreover, increased physical activity (PA) is strongly associated with improved quality of life (QoL) across age groups and populations [22]. Despite the advantages of postoperative PA program following TKR and THR [23] and the proposal of various PA interventions for people with total joint replacement, the existing heterogeneity in study designs and outcome measures, make difficult to draw definitive conclusions or establish universal protocols [24] concerning the type, setting, frequency, intensity, and duration of therapy. Moreover, the relationship between activity levels and implant failure in TKR remains unclear [25]. Randomized controlled trials (RCTs) are fundamental to assess the effectiveness of PA interventions and to provide evidence and clinical insights for individuals with THR and TKR. While several RCTs have been conducted among this population to improve strength, physical function, clinical outcomes [26,27,28,29,30], many of them have primarily focused on pre surgery [27,30] or early rehabilitation phases [26,28,29,30] with limited emphasis on the post rehabilitation period, which is critical for long-term recovery.
Although early rehabilitation phase following surgery plays a vital role in restoring joint function, overall outcome, and joint mobility [29,31], the post-rehabilitation period plays a key role in maintaining gains, preventing relapse into inactivity, and promoting an active lifestyle. However, the type and duration of the rehabilitation may vary significantly, influenced by national guidelines (if available) and clinic and therapist protocols. Unfortunately, a post rehabilitation exercise program often receives insufficient attention, despite their potential to promote an active lifestyle and enhance physical benefits.
Consequently, individuals may return to inactivity after completing the early rehabilitation. Therefore, supervised and adapted exercise programs beyond the early rehabilitation phase are essential to consolidate functional gains, enhance fitness, and support long-term joint health, while considering individual limitations to minimize the risk of injury.
The aim of this pilot study was to assess the changes in terms of QoL in people with THR and TKR after a specifically designed PA intervention. The secondary aim was to evaluate changes in physical function through strength and mobility tests. We hypothesize that participants with THR or TKR who engage in a specifically designed post-rehabilitation PA intervention will demonstrate a statistically significant and clinically meaningful improvement in QoL compared to those receiving standard care.
2. Materials and Methods
The “Physical ActIvity after hip and knee Replacement” (PAIR) project, funded within the Erasmus Plus Sport program (Grant Agreement 613008-EPP-1-2019-1-IT-SPO-SCP), conceived this pilot study. The study received approval from the Local Ethics Committee (Comitato Etico Indipendente di Area Vasta Emilia Centro, CE-AVEC) of the Emilia-Romagna Region (reference number AVEC: 1005/2020/Sper/IOR) and is registered on ClinicalTrial.Gov (NCT04761367). A detailed description of the methods is provided in a previous protocol study [32]. In brief, this single-blind randomized controlled pilot trial, with researchers performing the assessments blinded to group allocation, was designed to evaluate the effectiveness of a tailored exercise program in individuals following THR and TKR.
2.1. Recruitment and Randomization Process
The total number of patients with THR and TKR identified to conduct the large-scale RCT was 80, according to a priori power analysis calculated on the study’s primary outcome [32]. This pilot study reports preliminary results from a cohort of 18 patients with a mean age of 65.8 ± 7.1. Patients were recruited during their pre-surgery medical check-up, where they signed the informed consent form. Each patient underwent either TKR (n = 4) or THR (n = 14). Six months after surgical treatment and subsequent to the standard rehabilitation, participants were randomly assigned to one of two groups: Intervention Group (IG) and Control Group (CG). Randomization was performed using block randomization to ensure balanced group sizes throughout the study. The CG consisted of 11 participants (10 with THR and 1 with TKR), while the IG included 7 participants (4 with THR and 3 with TKR). The IG followed a 6-month exercise program specifically designed for people with knee and hip arthroplasty and received educational sessions on the importance of an active lifestyle. In contrast, the CG received the standard care recommended for such patients by surgeons and healthcare professionals. Standard care was coordinated among the orthopedic surgeon, physiatrist, and physical therapist, and adapted individually based on patient condition. The program included two daily sessions of gradually increasing difficulty and simple exercises that patients were instructed to perform independently several times per day. Discharge typically occurred 4–5 days after surgery. Patients were advised to continue the exercises learned during hospitalization at home and, depending on joint condition, to continue specific rehabilitation at a dedicated center.
Both groups were assessed at four time points (Figure 1):
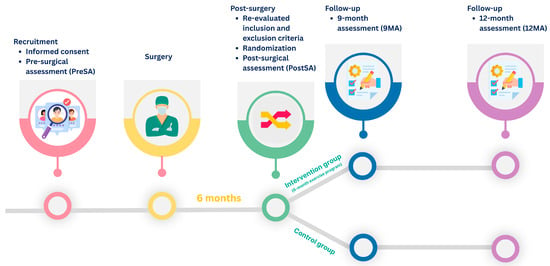
Figure 1.
Study timeline and assessment schedule.
- (1)
- within 2-week before surgery, corresponding to the pre-surgical assessment (PreSA);
- (2)
- six months after surgery (PostSA);
- (3)
- three months after the exercise program, corresponding to nine months after surgery (9MA);
- (4)
- six months after the exercise program, corresponding to 12 months after surgery (12MA).
Patients were asked to participate in the study during their PreSA and the patients signed the informed consent form. The recruitment procedure was carried out by the medical personnel of the Clinical Units Chirurgia Ortopedica Ricostruttiva Tecniche Innovative and Clinica Ortopedica e Traumatologica II of the Istituto Ortopedico Rizzoli of Bologna.
2.2. Inclusion and Exclusion Criteria
The inclusion and exclusion criteria were assessed two different times: during the PreSA and PostSA, which correspond to the end of the rehabilitation phase. The inclusion criteria were: signed informed consent, age between 50 and 80 years, advanced unilateral osteoarthritis requiring primary THR or TKR, and the American Society of Anesthesiologists classification between 1 and 2 levels. The exclusion criteria were: (1) Unable/unwilling to sign the informed consent form of the study and/or willing to comply with the study requests; (2) Poor knowledge of Italian language which prevents understanding of the content of the consent form and/or of instructions for assessment and/or training; (3) Severe functional limitations of other lower extremity joints besides that for which surgery is planned; (4) Impairment of communicative and/or sensory functions so severe to make impossible understanding or executing trainer’s instructions (dementia, aphasia, blindness, deafness); (5) Heart failure (NYHA 2 class > 2); (6) Unstable angina; (7) Pulmonary disease requiring oxygen therapy; (8) Symptomatic peripheral arteriopathy; (9) Recent myocardial infarction or hospital admission for any other reason in the previous six months; (10) Symptomatic orthostatic hypotension; (11). Hypertension in poor pharmacologic control (diastolic > 95 mmHg, systolic > 160 mmHg); (12) Relevant neurological condition impairing motor or cognitive function; (13) Any other condition that the medical doctor considers contraindicating the participation in an exercise program of moderate intensity; (14) Severe depression.
During PostSA, two additional exclusion criteria were checked: (1) Functional performance: Able to stand and walk > 500 m independently; (2) Pain: Visual Analogue Scale (VAS) score ≥ 4 during rest.
2.3. Exercise Program for Intervention Group
Two distinct, tailored exercise programs were developed for individuals with THR and TKR. These programs were designed in accordance with the WHO guidelines on PA for older and the clinical recommendations of orthopedic surgeons regarding PA after joint replacement [19]. All activities were gym-based and supervised by a qualified exercise specialist experienced in working with post-surgical populations. The content and progression of the programs were based on the findings of systematic reviews and grounded in clinical reasoning by experts in exercise specialist, rehabilitation medicine, and physical therapy. The primary goals of both programs were to enhance postural control, proprioception, muscular strength, joint range of motion, and cardiovascular endurance. To ensure safety, the programs excluded movements that could compromise prosthesis integrity, such as kneeling for individuals after TKR and excessive hip adduction or flexion for individuals after THR, consistent with current post-operative precautions [8,19].
Each exercise session followed a specific structure comprising three phases: initiation (warm-up), conditioning (balance and strength exercises), and cooling down and stretching. Table 1 provide a single representative example of an exercise session.

Table 1.
Representative example of an exercise session for individuals with total hip replacement and total knee replacement.
2.4. Outcomes
The primary outcome of this study was health-related QoL, assessed using the 36-Item Short Form Survey (SF-36) [33], which evaluates eight health domains: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain, and general health, along with a health change score [34]. The secondary outcomes included a combination of patient-reported outcome measures (PROMs), objective physical assessments, and functional tests.
PROMs comprised: The VAS for pain intensity [35,36], the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) for evaluating pain, stiffness, and physical function in patients with hip and knee OA [37], the Hip disability and Osteoarthritis Outcome Score (HOOS) [38] and the Knee injury and Osteoarthritis Outcome Score (KOOS) [39], which are joint-specific tools assessing symptoms, pain, function, and QoL related to hip and knee arthroplasty, respectively.
Additionally, objective strength measures were obtained using a handheld dynamometer [40], focusing on hip flexion, extension, abduction, and adduction of both the operated and non-operated limbs, as well as knee flexion [41].
Functional performance was assessed using three validated tests: Timed Up and Go (TUG) test, which measures basic functional mobility and fall risk [42], 30-Second Chair Stand Test (30sCST), which evaluates lower-body strength [43], and Single Stance Test, which assesses static balance and postural control.
Finally, the adherence to the exercise program of the IG was recorded during each session by the trainer.
Table 2 provides a summary of the instruments used, including their administration method and their main purpose.

Table 2.
Summary of the instruments used.
2.5. Statistics
All statistical analyses were conducted using Python (version 3.11.11) within the Spyder development environment. Descriptive statistics were reported as mean and standard deviation (SD) for normally distributed variables, and as median and interquartile range (IQR) for non-normally distributed variables.
When normality was confirmed, parametric tests were applied. The assumption of homogeneity of variances was assessed using Bartlett’s test, with a significance threshold of p < 0.01. If variances were unequal, Welch’s t-test was used; otherwise, the standard Student’s t-test was performed. The Mann–Whitney was used for non-parametric variables. The Chi-squared tests were used to assess differences in categorical variables (Surgery type and side, gender, educational level, occupation, and family status). Repeated measures ANOVA were performed to evaluate within-group and between-group differences across time points for each outcome variable. The assumption of sphericity was tested using Mauchly’s test; when violated, the Greenhouse–Geisser correction was applied. Pairwise post hoc comparisons were conducted using independent t-tests with Sidak correction for multiple comparisons. The level of statistical significance was set at p < 0.05 for all analyses.
3. Results
The comparison of the sample characteristics did not show differences between CG and IG (Table 3).

Table 3.
Sample characteristics.
3.1. Quality of Life
The analysis of the SF-36 results showed that both groups experienced improvements in different domains over the follow-up period (Table 4, Figure 2). The repeated measures ANOVA showed significant within-subject effects over time for physical functioning (p < 0.001, η2 = 0.53), pain (p = 0.001, η2 = 0.38), health change (p < 0.001, η2 = 0.53), and energy/fatigue (p = 0.028, η2 = 0.22), suggesting an overall improvement in QoL across both groups. A statistically significant interaction effect between group and assessment was found only for physical functioning (p = 0.009, η2 = 0.27), indicating that the improvement in this domain over time was more pronounced in the IG compared to the CG. No significant between-group differences were found in any SF-36 domains.

Table 4.
SF-36 domain scores across follow-up time points.
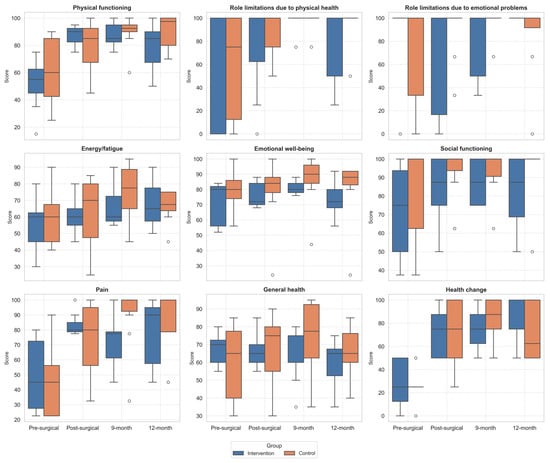
Figure 2.
Boxplot of changes in SF36 domains across follow-up assessments. Outliers, values outside the range of 1.5*IQR, are shown as circles.
The post hoc analysis showed that, in the IG, Physical functioning domain improved significantly from PreSA to PostSA (p = 0.024), and from PreSA to 9MA (p = 0.026). While Health changes improved significantly from PreSA to 9MA (p = 0.045) and PreSA to 12MA (p = 0.012).
In the CG, significant improvements were observed in the Pain domain, with differences between PreSA and 9MA (p = 0.005), in Health change domain with differences between PreSA and PostSA (p < 0.006), PreSA and 9MA (p < 0.001), and PreSA and 12MA (p = 0.026).
No statistically significant differences were found in the between-group comparisons at any of the four time points for any of the SF-36 domains.
3.2. Clinical Outcomes
The analysis of PROMs showed statistically significant within-subject effects over time for VAS (p = 0.005, η2 = 0.88) and significant interaction effects between group and time for both VAS (p = 0.017, η2 = 0.56) and WOMAC (p = 0.019, η2 = 0.27). A significant interaction effect was also found for the HOOS and KOOS composite score (p = 0.031, η2 = 0.27), while no significant differences were detected for any sub-score (Supplementary Material—Table S1).
Concerning the VAS, the improvements recorded over time were not statistically significant in the IG. On the contrary, in the CG, significant differences were observed between PreSA and PostSA (p = 0.001), PreSA and 3MA (p = 0.002), and PreSA and 6MA (p < 0.001). No significant between-group differences were found at any follow-up time point.
For WOMAC, no within-group or between-group comparisons were statistically significant.
Regarding HOOS and KOOS, no statistically significant differences were found in the total and their subscales. Descriptive values indicate that both groups followed similar trajectories over time for stiffness, pain, function, sport, and QoL domains.
3.3. Strength
The analysis of muscle strength (expressed in kilograms) on both the surgery and non-surgery sides revealed distinct time-related patterns within and between the IG and CG. The strength tests on the surgery leg side revealed statistically significant within-subject effects over time on hip flexion (p = 0.040, η2 = 0.42), extension (p = 0.002, η2 = 0.76), and adduction (p = 0.038, η2 = 0.56) and knee flexion (p < 0.001, η2 = 0.77) (Supplementary Material—Table S2).
In general, all the strength tests improved from PostSA to 9MA in the IG. Not all the improvements were maintained at 12MA (Figure 3). However, no statistically significant improvements emerged from the post hoc analysis in both groups.
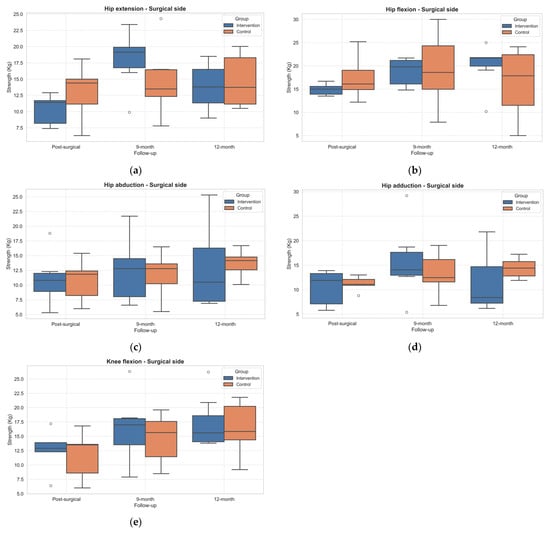
Figure 3.
Boxplot of changes in muscle strength across follow-up assessments in the operated limb. (a) Hip extension; (b) Hip flexion; (c) Hip abduction; (d) Hip adduction; (e) Knee flexion. Outliers, values outside the range of 1.5*IQR, are shown as circles.
3.4. Physical Function
Concerning the functional tests, statistically significant within-subject effects over time emerged in TUG (p = 0.001, η2 = 0.57) and 30sCST (p = 0.041, η2 = 0.29). The performance of both TUG (Figure 4) and 30sCST (Figure 5) improved in both groups over time. However, the post hoc did not show significant differences.
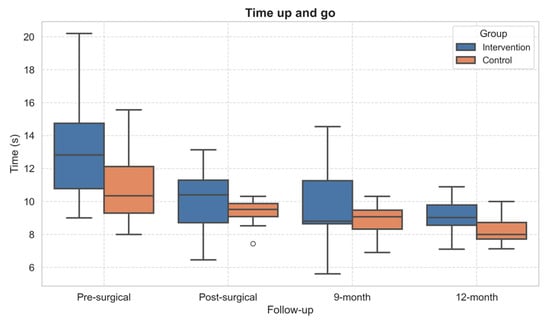
Figure 4.
Boxplot of Timed Up and Go test performance across follow-up assessments. Outliers, values outside the range of 1.5*IQR, are shown as circles.
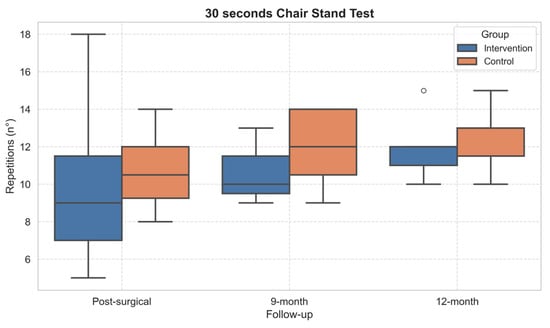
Figure 5.
Boxplot of 30-Second Chair Stand Test performance across follow-up assessments. Outliers, values outside the range of 1.5*IQR, are shown as circles.
Notably, the single stance test improved sensibly in the IG group with respect to the CG, both in the surgical side and non-surgical side (Figure 6). However, no statistically significant differences emerged.
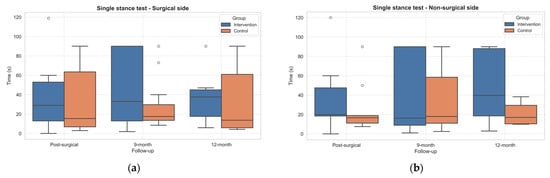
Figure 6.
Boxplots of Single Stance Test results (expressed in seconds) on the surgical (a) and non-surgical (b) sides in both groups across follow-up assessments. Outliers, values outside the range of 1.5*IQR, are shown as circles.
3.5. Adherence to the Intervention
The adherence to a total of 48 exercise sessions for the IG was considerate moderate (63.0 ± 16.1%) [44]. No adverse events were recorded during the intervention.
4. Discussion
This pilot RCT evaluated the effectiveness of a specifically adapted PA intervention on the QoL of individuals after THR and TKR. The main result of this study was that, in the IG, the QoL domains of physical functioning and health change improved significantly from PreSA to the 12 months after surgery. This findings aligns with previous studies suggesting that continued engagement in structured PA following standard rehabilitation contributes to enhanced functional recovery and QoL in joint replacement patients [23]. Notably, even the CG showed significant improvements in pain and health changes domains. While this likely reflects the natural trajectory of recovery post-arthroplasty [7], the more pronounced gains in physical functioning in the IG suggest that continuing exercise post-rehabilitation may provide additional value beyond usual care, particularly in domains related to physical capabilities and perceived health improvements. These results also confirm the importance of bridging the gap between rehabilitation discharge and full functional recovery, which is frequently underestimated in clinical practice [20,28]. However, even when statistical significance is observed, it should be acknowledged that the limited sample size may compromise the robustness and generalizability of the findings.
Nevertheless, no significant between-group differences were found in the overall SF-36 scores. Additionally, the non-significant changes observed in the emotional well-being and social functioning domains may suggest that, while PA influences physical health and perception of improvement, its impact on psychological and social domains require either longer follow-up or the integration of complementary psychosocial or behavioral interventions [45,46]. Enhancing these domains require targeted interventions addressing mental health, social support, and lifestyle factors, which were not included in our protocol.
In terms of clinical outcomes, the VAS score improved significantly over time in both groups, with the CG showing notable improvements. This might reflect the efficacy of surgical treatment and early rehabilitation in relieving pain [7]. In contrast, no differences were found in WOMAC, KOOS and HOOS scores. This may partially be explained by the fact that these scales were only administered after surgery, showing that the surgery has notable impact on PROMs improvements. Similar findings have been reported in previous studies assessing clinical outcomes after PA interventions [28]. However, the exercise programs appear to have had a limited impact on the PROMs assessed.
Interestingly, although not statistically significant, individuals in the IG showed clinically relevant improvements in strength after the PA intervention, especially in hip extension, flexion, abduction, and knee flexion in the operated leg. This trend suggests a potential positive impact of the intervention on muscle recovery [8,47]. The absence of significant between-group differences may reflect heterogeneity in patient adherence, baseline functional levels, or unmeasured confounders such as differences in rehabilitation intensity prior to randomization. Additionally, the strength of the CG remained stable or slightly declined over time, underscoring the need for targeted strength training to prevent deconditioning during long-term recovery. Increased muscle strength, especially in the quadriceps and hip abductors, is associated with improved functional outcomes such as walking speed, stair climbing, and daily activities as well as better PROMs and QoL measures, including reduced pain after both TKR and THR [48].
It is important to note that pre-surgical strength assessment was not performed in this study to avoid bias caused by pain-related arthrogenic muscle inhibition, a reflexive decrease in voluntary muscle activation which occurs in the presence of joint pain and swelling, and can significantly distort strength measurements before surgery [49]. However, previous studies reported an approximately 20% to 80% reduction in lower limb strength early after surgery [28,47,50,51], and may take several months or even years to recover. These persistent deficits are not only linked to functional impairment but may also increase the risk of prosthesis loosening and falls if not addressed through adequate interventions [8,47]. Therefore, post-rehabilitation programs should include structured strength training to fully restore pre-surgical functional levels and optimize implant longevity.
It is important to note that evaluating both surgical interventions (THR and TKR) together may have reduced the informative power of these findings. While both TKR and THR significantly improve pain and function, THR patients may report slightly better long-term pain relief and functional outcomes than TKR patients, especially at 5–8 years post surgery [11]. However, other research finds no significant difference in short-term (1 year) PROMs when using comprehensive measures [52].
Regarding functional performance, the TUG, the only test performed also before surgery, showed improvement across follow-up in both groups. This suggests that both standard care and the adapted PA intervention contributed to the recovery of basic daily activities. The observed improvements are consistent with previous studies indicating that TUG scores tend to improve significantly within the first 6–12 months after total joint replacement [26,42].
Moreover, both 30sCST and Single Stance performances improved during follow-ups. Notably, the Single Stance Tests improved more in the IG than the CG, although differences did not reach statistical significance. This finding suggests a potential benefit of the intervention in balance and postural control, which are often impaired after joint replacement due to proprioceptive deficits and changes in joint intracapsular components caused by surgery [53]. Enhancing balance performance is clinically important, as poor balance is a known predictor of fall risk in this population [42].
Overall, adherence to the PA-based intervention program was reported as moderate. This may have affected the clinical and functional outcomes assessed, as low adherence to PA programs consistently results in diminished benefits, including reduced improvements in physical function, metabolic health, and disease management. High adherence is necessary to accurately evaluate the true effect of the intervention [54]. Moreover, psychological factors, in both the cognitive and affective domains, may be important determinants of PA participation and should be considered when designing a PA-based intervention [55].
This study presents some limitations. First, the small sample size limits the statistical power and generalizability of the findings. Hence, the findings should be interpreted with caution and considered preliminary, serving to inform future research with larger cohorts. Second, the distribution of diagnoses was not balanced across the groups, as the CG included mostly participants with THR, while the IG included both THR and TKR. This imbalance may have influenced the results, given potential differences in recovery trajectories and functional outcomes between hip and knee replacements. Third, the rehabilitation phase after surgery was not monitored, and variability in rehabilitation practices may have influenced the observed outcomes. However, assessing patients after surgery and rehabilitation process created a new baseline that was useful to compare the improvements. Finally, the adherence to the intervention was measured only as a percentage of attendance. No additional tools, such as questionnaires or diaries, were used to capture factors influencing participation (e.g., motivation, health issues, or personal barriers). This may have limited our ability to fully understand the reasons behind adherence levels.
5. Conclusions
This pilot study supports the feasibility and potential benefits of an adapted PA program following standard rehabilitation in individuals undergoing THR and TKR. Continued exercise contributes to improvements in physical functioning, strength, and balance. Importantly, this study highlights the need for larger, adequately powered trials with long-term follow-up to confirm these findings and refine post-rehabilitation guidelines. Future studies with larger samples, longer follow-up, and additional psychological or behavioral support components could provide a more comprehensive understanding of the multi-dimensional benefits of adapted PA.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare13182333/s1, Table S1. Time and group comparison of clinical outcome measures. Table S2. Time and group comparison of muscle strength outcomes in hip and knee. Table S3. Time and group comparison of functional performance.
Author Contributions
Conceptualization, R.Z., E.P., G.B., D.D. and L.B.; Formal analysis, R.Z.; Investigation, E.P.; Methodology, R.Z., G.B. and L.B.; Resources, D.D.; Supervision, L.B.; Writing—original draft, R.Z. and E.P.; Writing—review and editing, M.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded within the PAIR project by Erasmus + Sport (grant agreement N. 613008-EPP-1-2019-1-IT-SPO-SCP).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Ethics Committee (Comitato Etico Indipendente di Area Vasta Emilia Centro, CE-AVEC) of the Emilia-Romagna Region (reference number AVEC: 1005/2020/Sper/IOR, the date of approval: 20 September 2020) and is registered on ClinicalTrial.Gov (NCT04761367).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors want to thank all the collaborators included in the PAIR study groups: Alessandro Mazzotta, Alina Iliescu, Andrea Fabio Manunta, Andreea Marin, Ani Dimitrova, Ann-Katrin Stensdotter, Cristiano Paggetti, Dante Dallari, Elena Tamburini, Francesco Benvenuti, Francesco Pegreffi, Havard Østerås, Ileana Ciobanu, Inge van den Akker-Scheek, Ivo Dimitrov, Jorunn Laegdheim Helbostad, Lora Yoncheva, Matei Teodorescu, Maya Tsvetanova, Mihai Berteanu, Monica Unsgaard-Tøndel, Natalya Shalamanova, Nicolay Todorov, Odd Magne Hals, Rumyana Shalamanova, Simona Geli, Umberto Cardinale, Yvet Mooiweer.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OA | Osteoarthritis |
| QoL | Quality of life |
| THR | Total hip replacement |
| TKR | Total knee replacement |
| OECD | Organization for Economic Cooperation and Development |
| PA | Physical activity |
| RCT | Randomized controlled trials |
| PAIR | Physical ActIvity after hip and knee Replacement |
| IG | Intervention group |
| CG | Control group |
| PreSA | Pre-surgical assessment |
| PostSA | Post-surgical assessment |
| 9MA | Nine months after surgery |
| 12MA | Twelve months after surgery |
| VAS | Visual Analogue Scale |
| SF-36 | 36-Item Short Form Survey |
| PROM | Patient-reported outcome measures |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
| HOOS | Hip disability and Osteoarthritis Outcome Score |
| KOOS | Knee injury and Osteoarthritis Outcome Score |
| TUG | Timed Up and Go |
| WHO | World Health Organization |
| 30sCST | 30-Second Chair Stand Test |
References
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Morales-Ivorra, I.; Romera-Baures, M.; Roman-Viñas, B.; Serra-Majem, L. Osteoarthritis and the Mediterranean Diet: A Systematic Review. Nutrients 2018, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.M. Impact of osteoarthritis on individuals and society: How much disability? Social consequences and health economic implications. Curr. Opin. Rheumatol. 2002, 14, 573–577. [Google Scholar] [CrossRef]
- Salmon, J.; Rat, A.; Sellam, J.; Michel, M.; Eschard, J.; Guillemin, F.; Jolly, D.; Fautrel, B. Economic impact of lower-limb osteoarthritis worldwide: A systematic review of cost-of-illness studies. Osteoarthr. Cartil. 2016, 24, 1500–1508. [Google Scholar] [CrossRef]
- Evans, J.T.; Walker, R.W.; Evans, J.P.; Blom, A.W.; Sayers, A.; Whitehouse, M.R. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet Lond. Engl. 2019, 393, 655–663. [Google Scholar] [CrossRef]
- Meira, E.P.; Zeni, J. Sports participation following total hip arthroplasty. Int. J. Sports Phys. Ther. 2014, 9, 839–850. [Google Scholar] [PubMed]
- Shao, Z.; Bi, S. Patient satisfaction after total hip arthroplasty: Influencing factors. Front. Surg. 2023, 9, 1043508. [Google Scholar] [CrossRef]
- Teoli, D.; Bhardwaj, A. Quality Of Life. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Green, A.; Walsh, A.; Al-Dadah, O. Comparison of clinical outcomes between total hip replacement and total knee replacement. World J. Orthop. 2023, 14, 853–867. [Google Scholar] [CrossRef]
- Lübbeke, A.; Silman, A.J.; Prieto-Alhambra, D.; Adler, A.I.; Barea, C.; Carr, A.J. The role of national registries in improving patient safety for hip and knee replacements. BMC Musculoskelet. Disord. 2017, 18, 414. [Google Scholar] [CrossRef]
- Maravic, M.; Landais, P. Usefulness of a national hospital database to evaluate the burden of primary joint replacement for coxarthrosis and gonarthrosis in patients aged over 40 years. Osteoarthr. Cartil. 2006, 14, 612–615. [Google Scholar] [CrossRef][Green Version]
- Piscitelli, P.; Iolascon, G.; Di Tanna, G.; Bizzi, E.; Chitano, G.; Argentiero, A.; Neglia, C.; Giolli, L.; Distante, A.; Gimigliano, R.; et al. Socioeconomic burden of total joint arthroplasty for symptomatic hip and knee osteoarthritis in the Italian population: A 5-year analysis based on hospitalization records. Arthritis Care Res. 2012, 64, 1320–1327. [Google Scholar] [CrossRef]
- Noble, P.C.; Gordon, M.J.; Weiss, J.M.; Reddix, R.N.; A Conditt, M.; Mathis, K.B. Does total knee replacement restore normal knee function? Clin Orthop. 2005, 431, 157–165. [Google Scholar] [CrossRef]
- Stief, F.; Schmidt, A.; van Drongelen, S.; Lenarz, K.; Froemel, D.; Tarhan, T.; Lutz, F.; Meurer, A. Abnormal loading of the hip and knee joints in unilateral hip osteoarthritis persists two years after total hip replacement. J. Orthop. Res. 2018, 36, 2167–2177. [Google Scholar] [CrossRef]
- Varacallo, M.; Chakravarty, R.; Denehy, K.; Star, A. Joint perception and patient perceived satisfaction after total hip and knee arthroplasty in the American population. J. Orthop. 2018, 15, 495–499. [Google Scholar] [CrossRef]
- OECD. Indicators. In Published Online 2021; OECD Publishing: Paris, France, 2021. [Google Scholar]
- Meester, S.B.; Wagenmakers, R.; Akker-Scheek, I.v.D.; Stevens, M.; Pérez-Prieto, D. Sport advice given by Dutch orthopaedic surgeons to patients after a total hip arthroplasty or total knee arthroplasty. PLoS ONE 2018, 13, e0202494. [Google Scholar] [CrossRef] [PubMed]
- Mooiweer, Y.; Stevens, M.; Akker-Scheek, I.v.D. Being active with a total hip or knee prosthesis: A systematic review into physical activity and sports recommendations and interventions to improve physical activity behavior. Eur. Rev. Aging Phys. Act. Off J. Eur. Group Res. Elder Phys. Act. 2022, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizations. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Puciato, D.; Borysiuk, Z.; Rozpara, M. Quality of life and physical activity in an older working-age population. Clin. Interv. Aging 2017, 12, 1627–1634. [Google Scholar] [CrossRef]
- Fortier, L.M.; Rockov, Z.A.; Chen, A.F.; Rajaee, S.S. Activity Recommendations After Total Hip and Total Knee Arthroplasty. J. Bone Jt. Surg. Am. 2021, 103, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Campi, S.; Vorini, F.; Zampogna, B.; Vasta, S.; Papalia, G.; Fossati, C.; Torre, G.; Denaro, V. The Role of Physical Activity and Rehabilitation Following Hip and Knee Arthroplasty in the Elderly. J. Clin. Med. 2020, 9, 1401. [Google Scholar] [CrossRef]
- Kornuijt, A.; Kuijer, P.; van Drumpt, R.; Siebelt, M.; Lenssen, A.; van der Weegen, W. A high physical activity level after total knee arthroplasty does not increase the risk of revision surgery during the first twelve years: A systematic review with meta-analysis and GRADE. Knee 2022, 39, 168–184. [Google Scholar] [CrossRef]
- Bade, M.J.; Struessel, T.; Dayton, M.; Foran, J.; Kim, R.H.; Miner, T.; Wolfe, P.; Kohrt, W.M.; Dennis, D.; Stevens-Lapsley, J.E. Early High-Intensity Versus Low-Intensity Rehabilitation After Total Knee Arthroplasty: A Randomized Controlled Trial. Arthritis Care Res. 2017, 69, 1360–1368. [Google Scholar] [CrossRef]
- Calatayud, J.; Casaña, J.; Ezzatvar, Y.; Jakobsen, M.D.; Sundstrup, E.; Andersen, L.L. High-intensity preoperative training improves physical and functional recovery in the early post-operative periods after total knee arthroplasty: A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Husby, V.S.; Foss, O.A.; Husby, O.S.; Winther, S.B. Randomized controlled trial of maximal strength training vs. standard rehabilitation following total knee arthroplasty. Eur. J. Phys. Rehabil. Med. 2018, 54, 371–379. [Google Scholar] [CrossRef]
- Karaborklu Argut, S.; Celik, D.; Kilicoglu, O.I. The Combination of Exercise and Manual Therapy Versus Exercise Alone in Total Knee Arthroplasty Rehabilitation: A Randomized Controlled Clinical Trial. PMR 2021, 13, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Łyp, M.; Kaczor, R.; Cabak, A.; Tederko, P.; Włostowska, E.; Stanisławska, I.; Szypuła, J.; Tomaszewski, W. A Water Rehabilitation Program in Patients with Hip Osteoarthritis Before and After Total Hip Replacement. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2635–2642. [Google Scholar] [CrossRef]
- Gstoettner, M.; Raschner, C.; Dirnberger, E.; Leimser, H.; Krismer, M. Preoperative proprioceptive training in patients with total knee arthroplasty. Knee 2011, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Zinno, R.; Pinelli, E.; PAIR Study Group; Benvenuti, F.; Bragonzoni, L. Evaluation of the Efficacy and Safety of an Exercise Program for Persons with Total Hip or Total Knee Replacement: Study Protocol for a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6732. [Google Scholar] [CrossRef]
- Ethgen, O.; Bruyère, O.; Richy, F.; Dardennes, C.; Reginster, J.Y. Health-Related Quality of Life in Total Hip and Total Knee Arthroplasty: A Qualitative and Systematic Review of the Literature. J. Bone Jt. Surg. 2004, 86, 963. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Alesi, D.; Di Paolo, S.; Bragonzoni, L.; Pizza, N.; Zaffagnini, S.; Zinno, R.; Muccioli, G.M.M. No kinematical difference between ultra-congruent and medial-congruent total knee arthroplasty when implanted with mechanical alignment: An in vivo dynamic RSA study. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 2975–2979. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Brockopp, D. Comparison of patients’ ratings and examination of nurses’ responses to pain intensity rating scales. Cancer Nurs. 1995, 18, 292. [Google Scholar] [CrossRef]
- Bellamy, N.; Wilson, C.; Hendrikz, J.; Whitehouse, S.L.; Patel, B.; Dennison, S.; Davis, T. Osteoarthritis Index delivered by mobile phone (m-WOMAC) is valid, reliable, and responsive. J. Clin. Epidemiol. 2011, 64, 182–190. [Google Scholar] [CrossRef]
- Klässbo, M.; Larsson, E.; Mannevik, E. Hip disability and osteoarthritis outcome score. An extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand. J. Rheumatol. 2003, 32, 46–51. [Google Scholar] [CrossRef]
- Insall, J.N.; Dorr, L.D.; Scott, R.D.; Norman, W. Rationale, of The Knee Society Clinical Rating System. Clin. Orthop. Relat. Res. 1989, 248, 13. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Adair, B.; Pua, Y.-H.; Williams, G.P.; McGaw, R.; A Clark, R. Assessment of Lower Limb Muscle Strength and Power Using Hand-Held and Fixed Dynamometry: A Reliability and Validity Study. PLoS ONE 2015, 10, e0140822. [Google Scholar] [CrossRef] [PubMed]
- Chopp-Hurley, J.N.; Wiebenga, E.G.; Gatti, A.A.; Maly, M.R. Investigating the Test–Retest Reliability and Validity of Hand-Held Dynamometry for Measuring Knee Strength in Older Women with Knee Osteoarthritis. Physiother. Can. 2019, 71, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Sawano, S.; Maegawa, S.; Ikezoe, T.; Ichihashi, N. Physical Activity Mediates the Relationship between Gait Function and Fall Incidence after Total Knee Arthroplasty. J. Knee Surg. 2020, 34, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Bruun, I.H.; Mogensen, C.B.; Nørgaard, B.; Schiøttz-Christensen, B.; Maribo, T. Validity and Responsiveness to Change of the 30-Second Chair-Stand Test in Older Adults Admitted to an Emergency Department. J. Geriatr. Phys. Ther. 2019, 42, 265–274. [Google Scholar] [CrossRef]
- Hawley-Hague, H.; Horne, M.; Skelton, D.A.; Todd, C. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open 2016, 6, e011560. [Google Scholar] [CrossRef]
- Meng, Y.; Deng, B.; Liang, X.; Li, J.; Li, L.; Ou, J.; Yu, S.; Tan, X.; Chen, Y.; Zhang, M. Effectiveness of self-efficacy-enhancing interventions on rehabilitation following total hip replacement: A randomized controlled trial with six-month follow-up. J. Orthop. Surg. 2022, 17, 225. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, Q.; Zhong, D.; Pan, Y.; He, T.; Hu, Y.; Wang, L. The Impact of Psychological Health on Patient Recovery After Arthroplasty. Front. Psychiatry 2022, 13, 817716. [Google Scholar] [CrossRef]
- Stevens-Lapsley, J.E.; Balter, J.E.; Wolfe, P.; Eckhoff, D.G.; Kohrt, W.M. Early Neuromuscular Electrical Stimulation to Improve Quadriceps Muscle Strength After Total Knee Arthroplasty: A Randomized Controlled Trial. Phys. Ther. 2012, 92, 210–226. [Google Scholar] [CrossRef]
- Winther, S.B.; Husby, V.S.; A Foss, O.; Wik, T.S.; Svenningsen, S.; Engdal, M.; Haugan, K.; Husby, O.S. Muscular strength after total hip arthroplasty. Acta Orthop. 2016, 87, 22–28. [Google Scholar] [CrossRef]
- Churchill, L.; Bade, M.J.; Koonce, R.C.; Stevens-Lapsley, J.E.; Bandholm, T. The past and future of peri-operative interventions to reduce arthrogenic quadriceps muscle inhibition after total knee arthroplasty: A narrative review. Osteoarthr. Cartil. Open 2024, 6, 100429. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.; Kristensen, M.T.; Bencke, J.; Husted, H.; Kehlet, H.; Bandholm, T. Loss of knee-extension strength is related to knee swelling after total knee arthroplasty. Arch. Phys. Med. Rehabil. 2010, 91, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Holm, B.; Thorborg, K.; Husted, H.; Kehlet, H.; Bandholm, T.; Hug, F. Surgery-induced changes and early recovery of hip-muscle strength, leg-press power, and functional performance after fast-track total hip arthroplasty: A prospective cohort study. PLoS ONE 2013, 8, e62109. [Google Scholar] [CrossRef]
- Berg, U.; W-Dahl, A.; Rolfson, O.; Nauclér, E.; Sundberg, M.; Nilsdotter, A. Influence of fast-track programs on patient-reported outcomes in total hip and knee replacement (THR/TKR) at Swedish hospitals 2011–2015: An observational study including 51,169 THR and 8,393 TKR operations. Acta Orthop. 2020, 91, 306–312. [Google Scholar] [CrossRef]
- de Lima, F.; Fernandes, D.A.; Melo, G.; Roesler, C.R.d.M.; Neves, F.d.S.; Neto, F.R. Effects of total hip arthroplasty for primary hip osteoarthritis on postural balance: A systematic review. Gait Posture 2019, 73, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Mikolaizak, A.S.; Taraldsen, K.; Boulton, E.; Gordt, K.; Maier, A.B.; Mellone, S.; Hawley-Hague, H.; Aminian, K.; Chiari, L.; Paraschiv-Ionescu, A.; et al. Impact of adherence to a lifestyle-integrated programme on physical function and behavioural complexity in young older adults at risk of functional decline: A multicentre RCT secondary analysis. BMJ Open 2022, 12, e054229. [Google Scholar] [CrossRef] [PubMed]
- Marini, S.; Zinno, R.; Belli, G.; Pinelli, E.; Bragonzoni, L.; Toselli, S.; Ekkekakis, P.; Monguzzi, G.A.; Murri, M.B.; Folesani, F.; et al. The Effect of Affective Exercise Experiences and Environmental Factors on Adherence to Outdoor Exercise Programs. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 31. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).