Quantification of Thoracic Volume and Spinal Length of Pediatric Scoliosis Patients on Chest MRI Using a 3D U-Net Segmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. 3D U-Net
2.3. Three-Dimensional Spinal Length Quantification
2.4. Validation Metrics
- Dice similarity coefficient (DSC): two times the overlap between the predicted segmentation and the ground truth segmentation over the total area of the predicted and ground truth segmentation.
- Hausdorff’s distance (HD): the Euclidean distances between the prediction and ground truth boundaries. Additionally, the 95th percentile of the Hausdorff distance (95HD) is considered, which disregards large outliers.
- Precision or positive predictive value: defined as the ratio of true positives to the total number of positive predictions.
- Recall or true positive rate: the ratio of correctly predicted pixels corresponding to volume to the total number of ground truth volume pixels.
3. Results
3.1. Quantitative Analysis
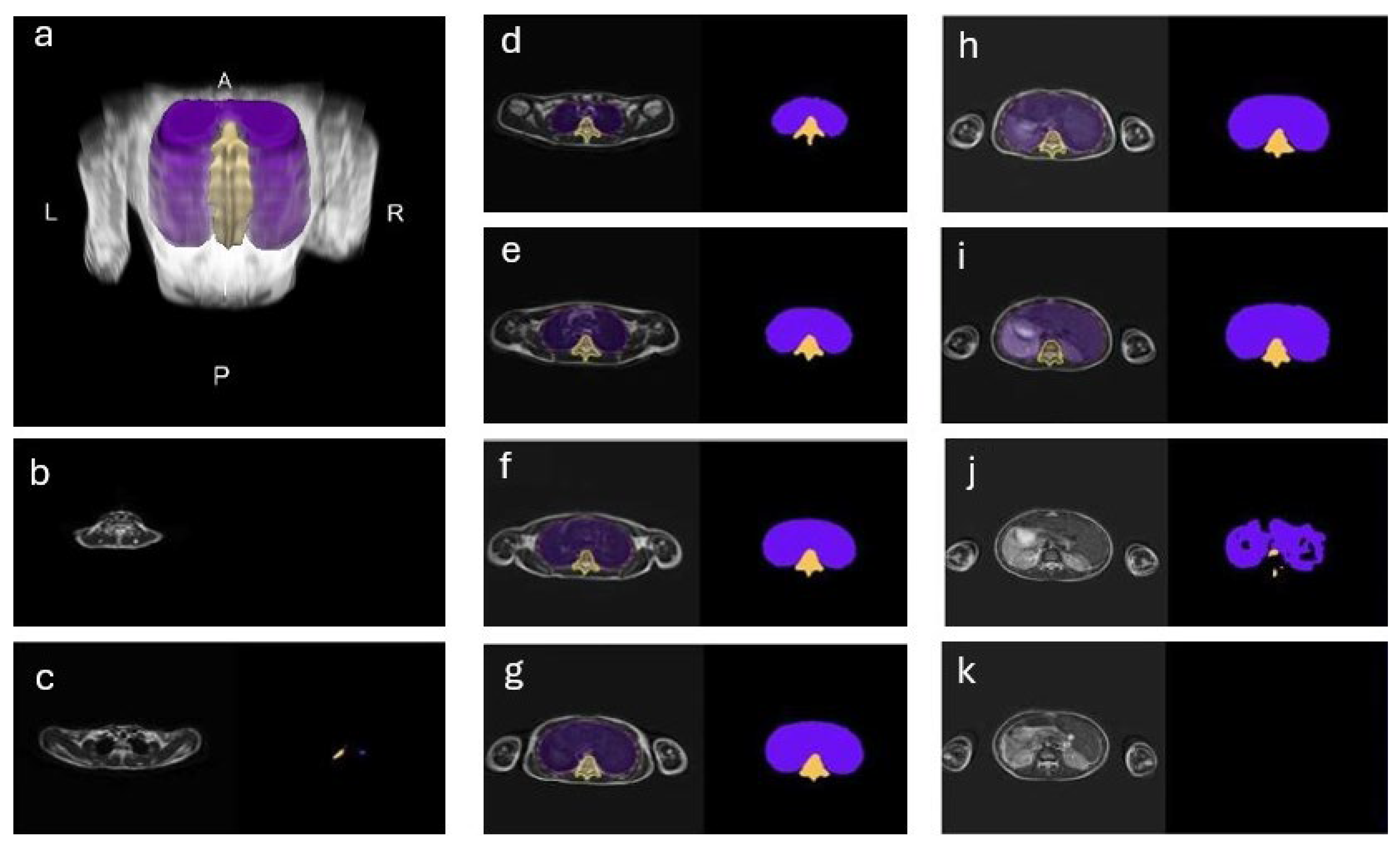
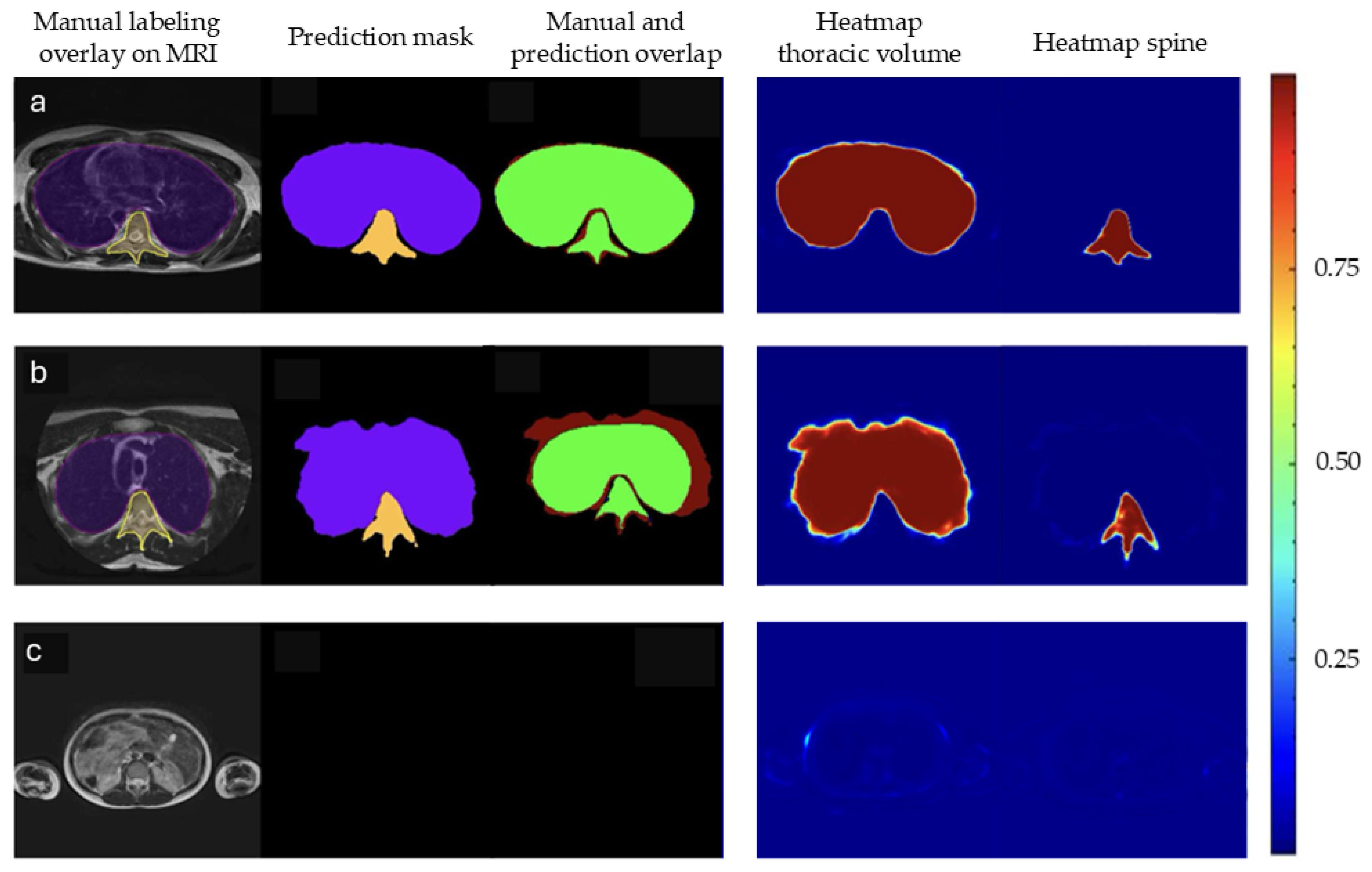
3.2. Qualitative Analysis
4. Discussion
5. Conclusions
- -
- A 3D U-net CNN using MRI images to quantify thoracic volume and spinal length could successfully be created.
- -
- Our CNN showed reasonably high accuracy, but an overestimation of volumes and length.
- -
- This proof of concept needs further training on bigger datasets before use in clinics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Adolescent Idiopathic Scoliosis |
| CNN | Convolutional Neural Network |
| GT | Ground truth |
| DSC | Dice’s similarity coefficient |
| HD | Hausdorff’s distance |
| 3D | Three dimensional |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| T2w | T2-weighted |
| COM | Center of mass |
| 95HD | 95th percentile of the Hausdorff distance |
| ROI | Region of interest |
| ALARA | As low as reasonably achievable |
| AI | Artificial Intelligence |
References
- Weinstein, S.L. The Natural History of Adolescent Idiopathic Scoliosis. J. Pediatr. Orthop. 2019, 39, S44–S46. [Google Scholar] [CrossRef]
- Cheng, J.C.; Castelein, R.M.; Chu, W.C.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.B.; Gurnett, C.A.; Luk, K.D.; Moreau, A.; Newton, P.O.; et al. Adolescent Idiopathic Scoliosis. Nat. Rev. Dis. Primers 2015, 1, 15030. [Google Scholar] [CrossRef]
- Schlösser, T.P.C.; Colo, D.; Castelein, R.M. Etiology and Pathogenesis of Adolescent Idiopathic Scoliosis. Semin. Spine Surg. 2015, 27, 2–8. [Google Scholar] [CrossRef]
- Farrell, J.; Garrido, E.; Vavruch, L.; Schlösser, T.P.C. Thoracic Morphology and Bronchial Narrowing Are Related to Pulmonary Function in Adolescent Idiopathic Scoliosis. J. Bone Jt. Surg. 2021, 103, 2014–2023. [Google Scholar] [CrossRef]
- Kan, M.M.P.; Negrini, S.; Di Felice, F.; Cheung, J.P.Y.; Donzelli, S.; Zaina, F.; Samartzis, D.; Cheung, E.T.C.; Wong, A.Y.L. Is Impaired Lung Function Related to Spinal Deformities in Patients with Adolescent Idiopathic Scoliosis? A Systematic Review and Meta-Analysis-SOSORT 2019 Award Paper. Eur. Spine J. 2023, 32, 118–139. [Google Scholar] [CrossRef]
- Ng, S.-Y.; Bettany-Saltikov, J. Imaging in the Diagnosis and Monitoring of Children with Idiopathic Scoliosis. Open Orthop. J. 2017, 11, 1500–1520. [Google Scholar] [CrossRef]
- Farrell, J.; Garrido, E. Predicting Preoperative Pulmonary Function in Patients with Thoracic Adolescent Idiopathic Scoliosis from Spinal and Thoracic Radiographic Parameters. Eur. Spine J. 2021, 30, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Pietton, R.; Bouloussa, H.; Langlais, T.; Taytard, J.; Beydon, N.; Skalli, W.; Vergari, C.; Vialle, R. Estimating Pulmonary Function after Surgery for Adolescent Idiopathic Scoliosis Using Biplanar Radiographs of the Chest with 3D Reconstruction. Bone Jt. J. 2022, 104-B, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Simony, A.; Hansen, E.J.; Christensen, S.B.; Carreon, L.Y.; Andersen, M.O. Incidence of Cancer in Adolescent Idiopathic Scoliosis Patients Treated 25 Years Previously. Eur. Spine J. 2016, 25, 3366–3370. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Elliston, C.; Hall, E.; Berdon, W. Estimated Risks of Radiation-Induced Fatal Cancer from Pediatric CT. AJR Am. J. Roentgenol. 2001, 176, 289–296. [Google Scholar] [CrossRef]
- Striano, B.M.; Crawford, A.M.; Verhofste, B.P.; Hresko, A.M.; Hedequist, D.J.; Schoenfeld, A.J.; Simpson, A.K. Intraoperative Navigation Increases the Projected Lifetime Cancer Risk in Patients Undergoing Surgery for Adolescent Idiopathic Scoliosis. Spine J. 2024, 24, 1087–1094. [Google Scholar] [CrossRef]
- Duke, A.; Marchese, R.; Komatsu, D.E.; Barsi, J. Radiation in Adolescent Idiopathic Scoliosis Management: Estimated Cumulative Pre-Operative, Intra-Operative, and Post-Operative Exposure. Orthop. Res. Rev. 2022, 14, 487–493. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Chu, P.W.; Azman Firdaus, H.; Stewart, C.; Malekhedayat, M.; Alber, S.; Bolch, W.E.; Mahendra, M.; Berrington de González, A.; Miglioretti, D.L. Projected Lifetime Cancer Risks From Current Computed Tomography Imaging. JAMA Intern. Med. 2025, 185, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.-J.; Wan, Y.; Mak, K.-C.; Ma, C.-J.; Wang, H.-Q. Cancer and Mortality Risks of Patients with Scoliosis from Radiation Exposure: A Systematic Review and Meta-Analysis. Eur. Spine J. 2020, 29, 3123–3134. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Sir Craft, A.W.; et al. Radiation Exposure from CT Scans in Childhood and Subsequent Risk of Leukaemia and Brain Tumours: A Retrospective Cohort Study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Miglioretti, D.L.; Johnson, E.; Williams, A.; Greenlee, R.T.; Weinmann, S.; Solberg, L.I.; Feigelson, H.S.; Roblin, D.; Flynn, M.J.; Vanneman, N.; et al. The Use of Computed Tomography in Pediatrics and the Associated Radiation Exposure and Estimated Cancer Risk. JAMA Pediatr. 2013, 167, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Journy, N.M.Y.; Lee, C.; Harbron, R.W.; McHugh, K.; Pearce, M.S.; Berrington de González, A. Projected Cancer Risks Potentially Related to Past, Current, and Future Practices in Paediatric CT in the United Kingdom, 1990–2020. Br. J. Cancer 2017, 116, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Andrew, J.; DivyaVarshini, M.; Barjo, P.; Tigga, I. Spine Magnetic Resonance Image Segmentation Using Deep Learning Techniques. In Proceedings of the 2020 6th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 6–7 March 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 945–950. [Google Scholar]
- Azad, R.; Aghdam, E.K.; Rauland, A.; Jia, Y.; Avval, A.H.; Bozorgpour, A.; Karimijafarbigloo, S.; Cohen, J.P.; Adeli, E.; Merhof, D. Medical Image Segmentation Review: The Success of U-Net. IEEE Trans. Pattern Anal. Mach. Intell. 2024, 46, 10076–10095. [Google Scholar] [CrossRef]
- Singh, S.P.; Wang, L.; Gupta, S.; Goli, H.; Padmanabhan, P.; Gulyás, B. 3D Deep Learning on Medical Images: A Review. Sensors 2020, 20, 5097. [Google Scholar] [CrossRef]
- Siddique, N.; Paheding, S.; Elkin, C.P.; Devabhaktuni, V. U-Net and Its Variants for Medical Image Segmentation: A Review of Theory and Applications. IEEE Access 2021, 9, 82031–82057. [Google Scholar] [CrossRef]
- Lafranca, P.P.G.; Stempels, H.W.; de Reuver, S.; Houben, M.L.; Kok, J.; Kruyt, M.C.; Castelein, R.M.; Seevinck, P.R.; van der Velden, T.; Shcherbakova, Y.M.; et al. EARLYBIRD: Catching the Earliest Changes of the Bone and Intervertebral Discs in Children at Increased Risk for Scoliosis Development with MRI—Study Protocol of a Prospective Observational Cohort Study. BMJ Open 2025, 15, e098929. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Soto-Rey, I.; Kramer, F. Towards a Guideline for Evaluation Metrics in Medical Image Segmentation. BMC Res. Notes 2022, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, M.; Yu, K.; Zhang, Y.; Yu, L. Lumbar Spine Segmentation Method Based on Deep Learning. J. Appl. Clin. Med. Phys. 2023, 24, e13996. [Google Scholar] [CrossRef]
- Suri, A.; Jones, B.C.; Ng, G.; Anabaraonye, N.; Beyrer, P.; Domi, A.; Choi, G.; Tang, S.; Terry, A.; Leichner, T.; et al. A Deep Learning System for Automated, Multi-Modality 2D Segmentation of Vertebral Bodies and Intervertebral Discs. Bone 2021, 149, 115972. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.U.; Dikaios, N.; Dastgir, A.; Ali, G.; Hamid, M.; Hajjej, F. An Automated Deep Learning Approach for Spine Segmentation and Vertebrae Recognition Using Computed Tomography Images. Diagnostics 2023, 13, 2658. [Google Scholar] [CrossRef]
- Swarup, I.; Silberman, J.; Blanco, J.; Widmann, R. Incidence of Intraspinal and Extraspinal MRI Abnormalities in Patients With Adolescent Idiopathic Scoliosis. Spine Deform. 2019, 7, 47–52. [Google Scholar] [CrossRef]
- Morbée, L.; Chen, M.; Herregods, N.; Pullens, P.; Jans, L.B.O. MRI-Based Synthetic CT of the Lumbar Spine: Geometric Measurements for Surgery Planning in Comparison with CT. Eur. J. Radiol. 2021, 144, 109999. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Klukowska, A.M.; Schröder, M.L. Pedicle Screw Revision in Robot-Guided, Navigated, and Freehand Thoracolumbar Instrumentation: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 116, 433–443.e8. [Google Scholar] [CrossRef]
- van der Kolk, B.Y.M.; Slotman, D.J.; Nijholt, I.M.; van Osch, J.A.C.; Snoeijink, T.J.; Podlogar, M.; van Hasselt, B.A.A.M.; Boelhouwers, H.J.; van Stralen, M.; Seevinck, P.R.; et al. Bone Visualization of the Cervical Spine with Deep Learning-Based Synthetic CT Compared to Conventional CT: A Single-Center Noninferiority Study on Image Quality. Eur. J. Radiol. 2022, 154, 110414. [Google Scholar] [CrossRef]
- Shcherbakova, Y.M.; Lafranca, P.P.G.; Foppen, W.; van der Velden, T.A.; Nievelstein, R.A.J.; Castelein, R.M.; Ito, K.; Seevinck, P.R.; Schlosser, T.P.C. A Multipurpose, Adolescent Idiopathic Scoliosis-Specific, Short MRI Protocol: A Feasibility Study in Volunteers. Eur. J. Radiol. 2024, 177, 111542. [Google Scholar] [CrossRef]
- Costa, L.; Lafranca, P.; Van Der Velden, T.; Castelein, R.’; Schlosser, T.; Seevinck, P.; Van Stralen, M. 95 Radiation-Free Assessment of the 3D Morphology of the Adolescent Scoliotic Spine: A Feasibility Study on Synthetic CT. Column Cord 2025, 5. Available online: https://columnandcord.scholasticahq.com/api/v1/articles/132118-radiation-free-assessment-of-the-3d-morphology-of-the-adolescent-scoliotic-spine-a-feasibility-study-on-synthetic-ct.pdf?utm_source=chatgpt.com (accessed on 30 July 2025).
- Lafranca, P.P.G.; Rommelspacher, Y.; Walter, S.G.; Muijs, S.P.J.; van der Velden, T.A.; Shcherbakova, Y.M.; Castelein, R.M.; Ito, K.; Seevinck, P.R.; Schlösser, T.P.C. The Safety and Accuracy of Radiation-Free Spinal Navigation Using a Short, Scoliosis-Specific BoneMRI-Protocol, Compared to CT. Eur. Spine J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.R.; Bernstein, E.F. The Anatomic Basis for Respiratory Variation in Median Arcuate Ligament Compression of the Celiac Artery. Surgery 1973, 73, 381–385. [Google Scholar] [PubMed]
| Participant | Volume [L] | DSC | HD [mm] | HD95 [mm] | Precision | Recall |
|---|---|---|---|---|---|---|
| 1 | 10.10 | 0.89 | 54.38 | 27.19 | 0.83 | 0.96 |
| 2 | 6.03 | 0.94 | 27.19 | 1.77 | 0.95 | 1.00 |
| 3 | 2.42 | 0.87 | 179.41 | 25.50 | 0.78 | 1.00 |
| 4 | 2.68 | 0.91 | 25.72 | 3.33 | 0.93 | 1.00 |
| 5 | 3.02 | 0.94 | 25.50 | 2.36 | 0.94 | 1.00 |
| 6 | 4.93 | 0.94 | 25.50 | 1.67 | 0.96 | 1.00 |
| 7 | 2.42 | 0.90 | 25.55 | 25.50 | 0.89 | 1.00 |
| Mean [range] | 4.51 [2.42–10.10] | 0.91 [0.87–0.94] | 51.89 [25.50–179.41] | 12.47 [1.67–27.19] | 0.90 [0.78–0.96] | 0.99 [0.96–1.00] |
| Participant | Length [cm] | DSC | HD [mm] | HD95 [mm] | Precision | Recall |
|---|---|---|---|---|---|---|
| 1 | 30.6 | 0.79 | 55.43 | 27.56 | 0.68 | 0.96 |
| 2 | 28.4 | 0.85 | 27.25 | 4.51 | 0.74 | 1.00 |
| 3 | 22.8 | 0.83 | 26.04 | 4.71 | 0.71 | 0.99 |
| 4 | 17.6 | 0.87 | 8.33 | 3.33 | 0.77 | 1.00 |
| 5 | 19.6 | 0.84 | 13.44 | 4.71 | 0.72 | 1.00 |
| 6 | 21.5 | 0.86 | 25.77 | 25.50 | 0.76 | 1.00 |
| 7 | 20.2 | 0.88 | 25.61 | 3.33 | 0.78 | 1.00 |
| Mean [range] | 23.0 [17.6–30.6] | 0.85 [0.79–0.88] | 25.98 [8.33–55.43] | 10.523 [3.33–27.56] | 0.74 [0.68–0.78] | 0.99 [0.96–1.00] |
| Participant | Manual Volume [L] | Model Volume [L] | % Difference |
|---|---|---|---|
| 1 | 8.79 | 10.10 | +14.9 |
| 2 | 5.77 | 6.03 | +4.5 |
| 3 | 1.87 | 2.42 | +29.4 |
| 4 | 2.49 | 2.68 | +7.1 |
| 5 | 2.83 | 3.02 | +6.7 |
| 6 | 4.76 | 4.93 | +3.6 |
| 7 | 2.16 | 2.42 | +12.0 |
| Mean [range] | 4.10 [1.87–8.79] | 4.51 [2.42–10.10] | 11.0 [+3.6–+29.4] |
| Cohen’s d = 1.01; 95% CI: [0.08, 1.95] |
| Participant | Manual Volume [L] | Model Volume [L] | % Difference |
|---|---|---|---|
| 1 | 24.6 | 30.6 | +24.4 |
| 2 | 25.0 | 28.4 | +13.6 |
| 3 | 19.1 | 22.8 | +19.4 |
| 4 | 17.4 | 17.6 | +1.1 |
| 5 | 20.0 | 19.6 | −2.0 |
| 6 | 20.6 | 21.5 | +4.4 |
| 7 | 16.7 | 20.2 | +20.1 |
| Mean [range] | 20.5 [16.7–25.0] | 23.0 [17.6–30.6] | 11.6 [−2.0–+24.4] |
| Cohen’s d = 1.07; 95% CI: [0.12, 2.03] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buijs, R.E.; Cornelissen, D.M.; Devetzis, D.; Lafranca, P.P.G.; Le, D.; Zhang, J.; Veta, M.; Vincken, K.L.; Schlösser, T.P.C. Quantification of Thoracic Volume and Spinal Length of Pediatric Scoliosis Patients on Chest MRI Using a 3D U-Net Segmentation. Healthcare 2025, 13, 2327. https://doi.org/10.3390/healthcare13182327
Buijs RE, Cornelissen DM, Devetzis D, Lafranca PPG, Le D, Zhang J, Veta M, Vincken KL, Schlösser TPC. Quantification of Thoracic Volume and Spinal Length of Pediatric Scoliosis Patients on Chest MRI Using a 3D U-Net Segmentation. Healthcare. 2025; 13(18):2327. https://doi.org/10.3390/healthcare13182327
Chicago/Turabian StyleBuijs, Romy E., Dingina M. Cornelissen, Dimo Devetzis, Peter P. G. Lafranca, Daniel Le, Jiaxin Zhang, Mitko Veta, Koen L. Vincken, and Tom P. C. Schlösser. 2025. "Quantification of Thoracic Volume and Spinal Length of Pediatric Scoliosis Patients on Chest MRI Using a 3D U-Net Segmentation" Healthcare 13, no. 18: 2327. https://doi.org/10.3390/healthcare13182327
APA StyleBuijs, R. E., Cornelissen, D. M., Devetzis, D., Lafranca, P. P. G., Le, D., Zhang, J., Veta, M., Vincken, K. L., & Schlösser, T. P. C. (2025). Quantification of Thoracic Volume and Spinal Length of Pediatric Scoliosis Patients on Chest MRI Using a 3D U-Net Segmentation. Healthcare, 13(18), 2327. https://doi.org/10.3390/healthcare13182327






