Sedentary Duration and Systemic Health Burden: Nonlinear Associations with Muscle, Fat, and Vascular Phenotypes in a US Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
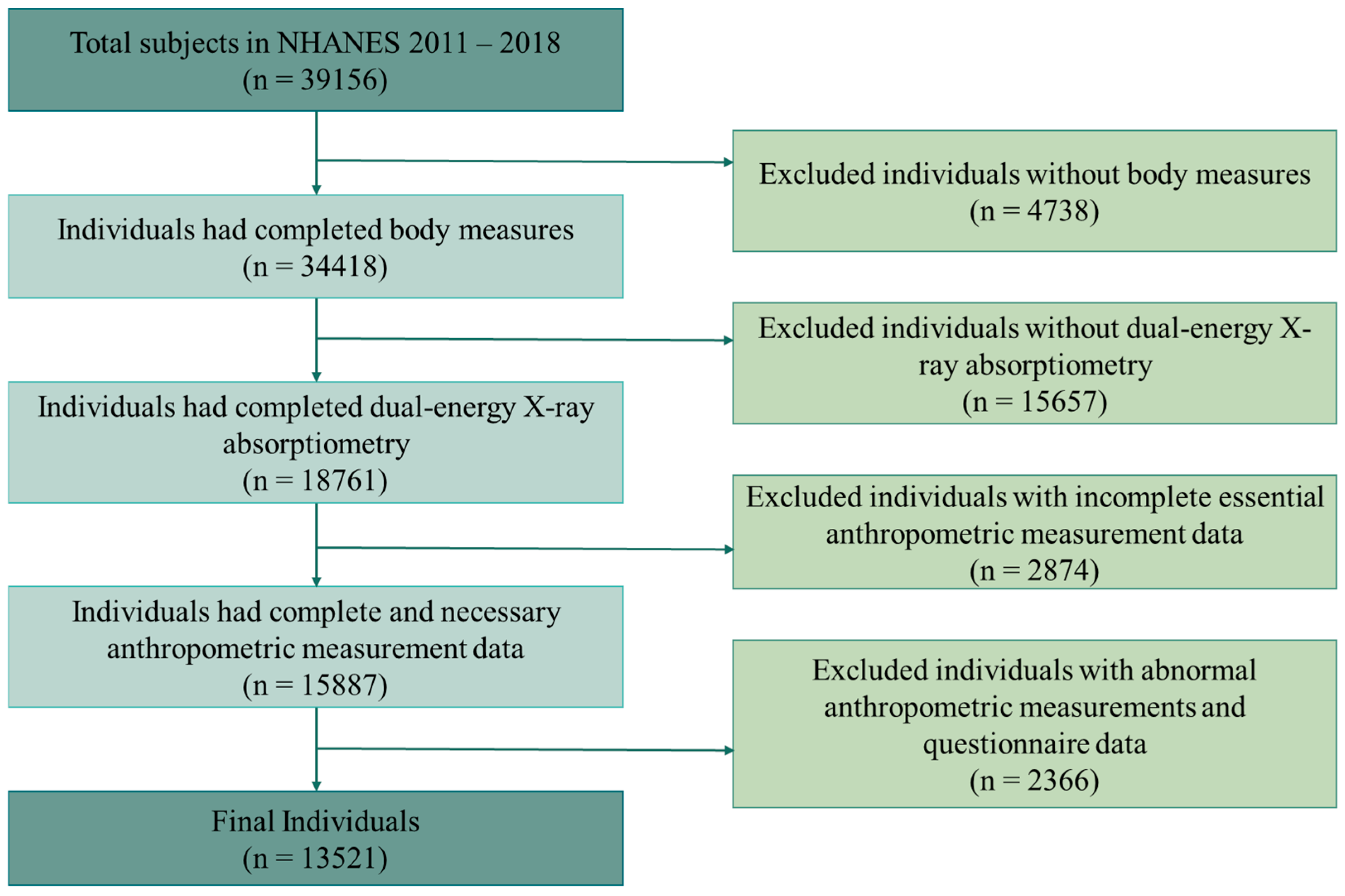
- Study Population and Inclusion Criteria
- Exposure and Outcome Variables
- Covariates
- Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
3.2. Association Between SD and Sarcopenia, Fat Distribution, and Hemodynamics
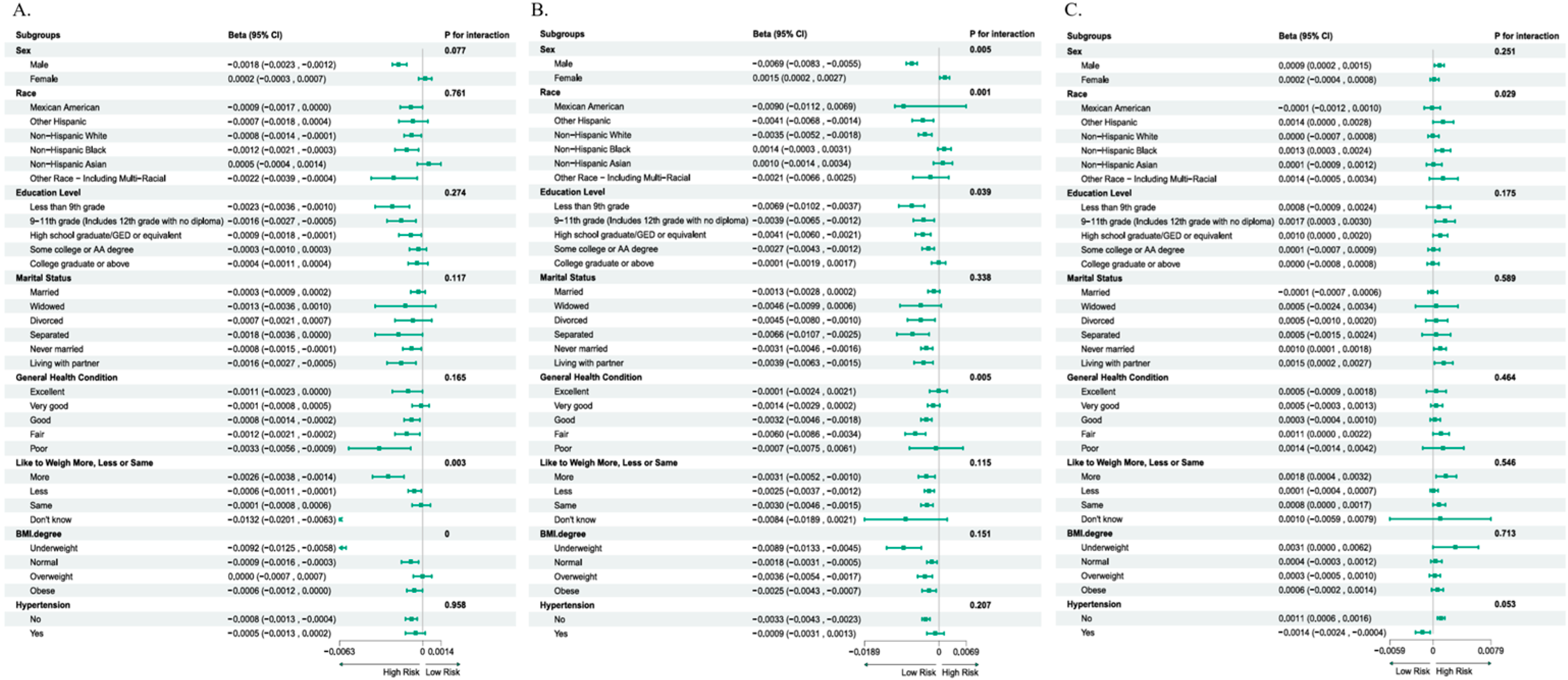
3.3. Stratified Analysis of the Associations
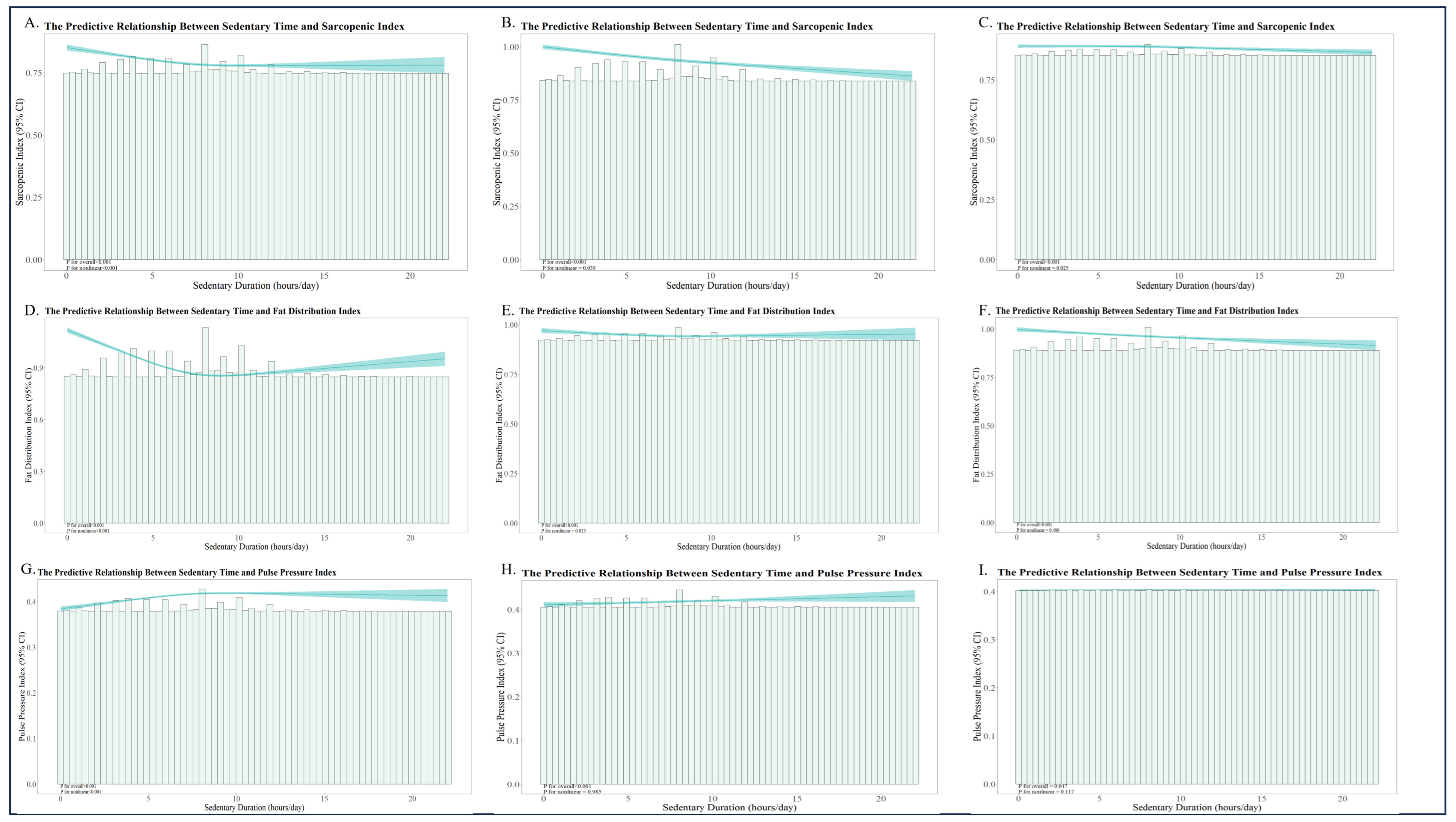
3.4. Nonlinear Relationships Between SD and Muscle–Fat–Flow Indicators
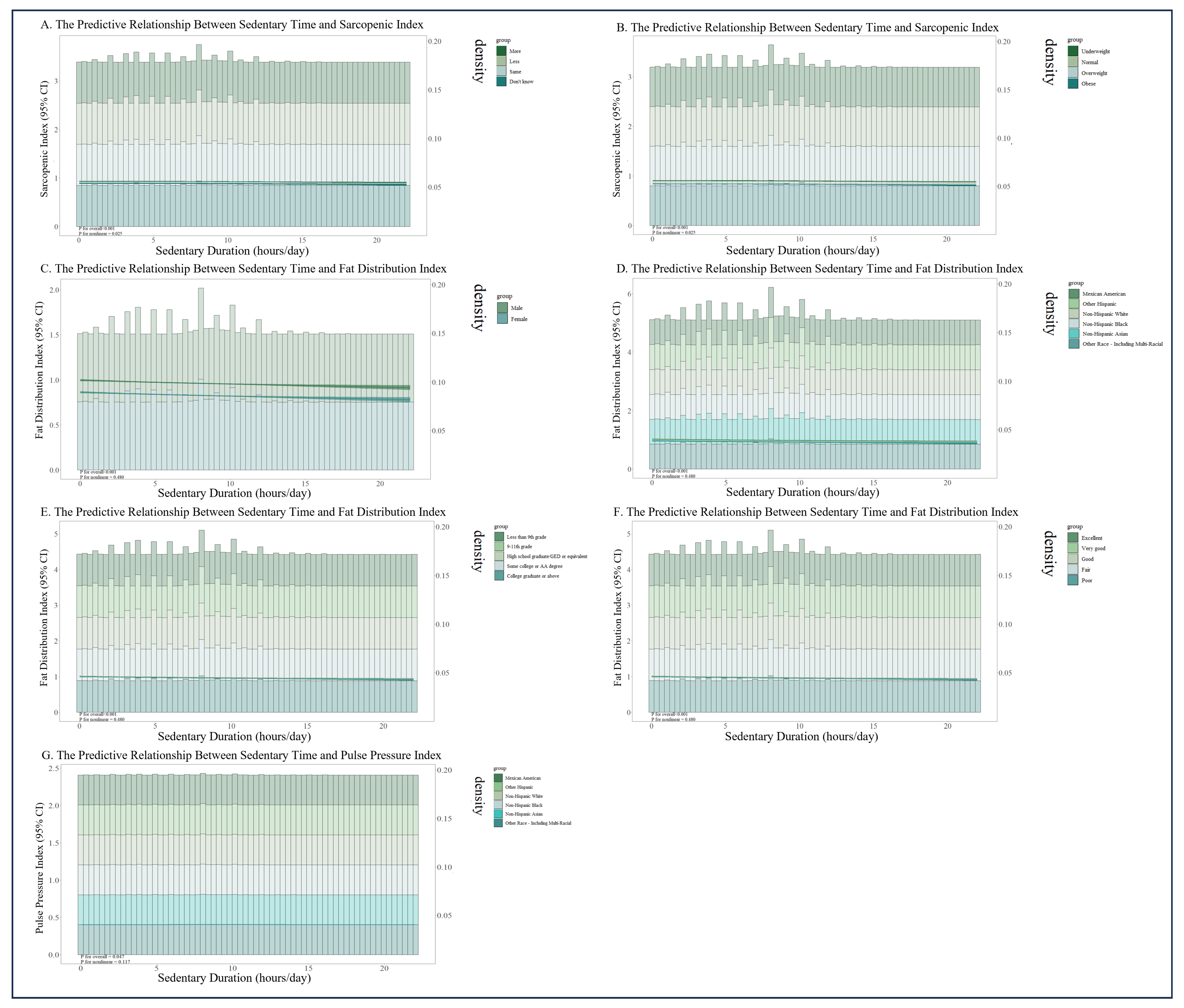
3.5. Stratified RCS Analysis in Positive Subgroups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NHANES | National Health and Nutrition Examination Survey |
| SD | Sedentary Duration |
| SI | Sarcopenic Index |
| FDI | Fat Distribution Index |
| PPI | Pulse Pressure Index |
| SB | Sedentary Behavior |
| NCHS | National Center for Health Statistics |
| CDC | Centers for Disease Control and Prevention |
| DXA | Dual-energy X-ray Absorptiometry |
| MEC | Mobile Examination Center |
| CAPI | Computer-Assisted Personal Interview |
| MIL | Maximum Inflation Level |
| BMI | Body Mass Index |
| RCS | Restricted Cubic Spline |
References
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; Altenburg, T.M.; et al. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project Process and Outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Oh, K.; Min, J.; Kang, S.; Min, K.-B. Association of Sedentary Lifestyle with Skeletal Muscle Strength and Mass in US Adolescents: Results from the National Health and Nutrition Examination Survey (2011–2014). J. Prev. Med. Public Health = Yebang Uihakhoe Chi 2025, 58, 278–288. [Google Scholar] [CrossRef]
- Bullock, V.; Griffiths, P.; Sherar, L.; Clemes, S. Sitting Time and Obesity in a Sample of Adults from Europe and the USA. Ann. Hum. Biol. 2017, 44, 230–236. [Google Scholar] [CrossRef]
- Bailey, D.; Hewson, D.; Champion, R.; Sayegh, S. Sitting Time and Risk of Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Padulo, J.; Filingeri, D.; Chamari, K.; Migliaccio, G.; Calcagno, G.; Bosco, G.; Annino, G.; Tihanyi, J.; Pizzolato, F. Acute Effects of Whole-Body Vibration on Running Gait in Marathon Runners. J. Sports Sci. 2014, 32, 1120–1126. [Google Scholar] [CrossRef]
- Paterson, C.; Fryer, S.; Stone, K.; Zieff, G.; Turner, L.; Stoner, L. The Effects of Acute Exposure to Prolonged Sitting, with and without Interruption, on Peripheral Blood Pressure among Adults: A Systematic Review and Meta-Analysis. Sports Med. 2021, 52, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.; Powell, K.; Jakicic, J.; Troiano, R.; Piercy, K. Sedentary Behavior and Health: Update from the 2018 Physical Activity Guidelines Advisory Committee. Med. Sci. Sports Exerc. 2019, 51, 1227–1241. [Google Scholar] [CrossRef]
- Falck, R.; Davis, J.; Liu-Ambrose, T. What Is the Association between Sedentary Behaviour and Cognitive Function? A Systematic Review. Br. J. Sports Med. 2016, 51, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.; Blair, S. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Grao-Cruces, A.; Sánchez-Oliva, D.; Padilla-Moledo, C.; Izquierdo-Gómez, R.; Cabanas-Sánchez, V.; Castro-Piñero, J. Changes in the School and Non-School Sedentary Time in Youth: The UP&DOWN Longitudinal Study. J. Sports Sci. 2020, 38, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Yonemoto, K.; Honda, T.; Kishimoto, H.; Yoshida, D.; Hata, J.; Mukai, N.; Shibata, M.; Hirakawa, Y.; Ninomiya, T.; Kumagai, S. Longitudinal Changes of Physical Activity and Sedentary Time in the Middle-Aged and Older Japanese Population: The Hisayama Study. J. Phys. Act. Health 2019, 16, 165–171. [Google Scholar] [CrossRef]
- Suorsa, K.; Pulakka, A.; Leskinen, T.; Pentti, J.; Vahtera, J.; Stenholm, S. Changes in Prolonged Sedentary Behaviour across the Transition to Retirement. Occup. Environ. Med. 2020, 78, 409–412. [Google Scholar] [CrossRef]
- van Ekris, E.; Wijndaele, K.; Altenburg, T.M.; Atkin, A.J.; Twisk, J.; Andersen, L.B.; Janz, K.F.; Froberg, K.; Northstone, K.; Page, A.S.; et al. Tracking of Total Sedentary Time and Sedentary Patterns in Youth: A Pooled Analysis Using the International Children’s Accelerometry Database (ICAD). Int. J. Behav. Nutr. Phys. Act. 2020, 17, 65. [Google Scholar] [CrossRef]
- Carter, S.; Hartman, Y.; Holder, S.; Thijssen, D.; Hopkins, N. Sedentary Behavior and Cardiovascular Disease Risk: Mediating Mechanisms. Exerc. Sport Sci. Rev. 2017, 45, 80–86. [Google Scholar] [CrossRef]
- Marshall, S.; Ramirez, E. Reducing Sedentary Behavior. Am. J. Lifestyle Med. 2011, 5, 518–530. [Google Scholar] [CrossRef]
- Hamilton, M.; Hamilton, D.; Zderic, T. Role of Low Energy Expenditure and Sitting in Obesity, Metabolic Syndrome, Type 2 Diabetes, and Cardiovascular Disease. Diabetes 2007, 56, 2655–2667. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.; Matthews, C.; Dashti, S.; Doherty, A.; Bergouignan, A.; Van Roekel, E.; Dunstan, D.; Wareham, N.; Yates, T.; Wijndaele, K.; et al. Sedentary Behavior and Chronic Disease: Mechanisms and Future Directions. J. Phys. Act. Health 2019, 17, 52–61. [Google Scholar] [CrossRef]
- Evans, W. Skeletal Muscle Loss: Cachexia, Sarcopenia, and Inactivity. Am. J. Clin. Nutr. 2010, 91, 1123–1127. [Google Scholar] [CrossRef]
- Hogenbirk, R.; Van Der Plas, W.; Hentzen, J.; Van Wijk, L.; Wijma, A.; Buis, C.; Viddeleer, A.; De Bock, G.; Van Der Schans, C.; Van Dam, G.; et al. Postoperative Muscle Loss, Protein Intake, Physical Activity and Outcome Associations. Br. J. Surg. 2022, 110, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.; Corriere, M.; Ferrucci, L. Age-Related and Disease-Related Muscle Loss: The Effect of Diabetes, Obesity, and Other Diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Argiles, J.; Campos, N.; López-Pedrosa, J.; Rueda, R.; Rodríguez-Mañas, L. Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease. J. Am. Med. Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef]
- Stefan, N. Causes, Consequences, and Treatment of Metabolically Unhealthy Fat Distribution. Lancet Diabetes Endocrinol. 2020, 8, 616–627. [Google Scholar] [CrossRef]
- Agarwal, N.; St. John, J.; Van Iterson, E.H.; Laffin, L.J. Association of Pulse Pressure with Death, Myocardial Infarction, and Stroke among Cardiovascular Outcome Trial Participants. Am. J. Prev. Cardiol. 2024, 17, 100623. [Google Scholar] [CrossRef]
- Said, M.A.; Eppinga, R.N.; Lipsic, E.; Verweij, N.; van der Harst, P. Relationship of Arterial Stiffness Index and Pulse Pressure with Cardiovascular Disease and Mortality. J. Am. Heart Assoc. 2018, 7, e007621. [Google Scholar] [CrossRef]
- Ramos, J.; Ramos, M.; Dalleck, L.; Borrani, F.; Walker, K.; Fassett, R.; Sharman, J.; Coombes, J. Fitness Is Independently Associated with Central Hemodynamics in Metabolic Syndrome. Med. Sci. Sports Exerc. 2016, 48, 1539–1547. [Google Scholar] [CrossRef]
- Moore, C.; Cao, R. The Hemo-Neural Hypothesis: On the Role of Blood Flow in Information Processing. J. Neurophysiol. 2008, 99, 2035–2047. [Google Scholar] [CrossRef]
- Yamazaki, H.; Tauchi, S.; Machann, J.; Haueise, T.; Yamamoto, Y.; Dohke, M.; Hanawa, N.; Kodama, Y.; Katanuma, A.; Stefan, N.; et al. Fat Distribution Patterns and Future Type 2 Diabetes. Diabetes 2022, 71, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Vecchio, G.; Weber, C.; Kämmer, D.; Bell, A.; Maffulli, N. Management of Transient Bone Osteoporosis: A Systematic Review. Br. Med. Bull. 2023, 147, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Durrleman, S.; Simon, R. Flexible Regression Models with Cubic Splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Wang, M.; Song, Y.; Baker, J.; Fekete, G.; Gu, Y. Sitting to Standing Postural Changes: Energy Expenditure and a Possible Mechanism to Alleviate Sedentary Behavior. Physiol. Int. 2018, 105, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.; Alfaraidhy, M.; Alhajri, N.; Rohant, N.; Kumar, M.; Malouf, C.A.; Bahrainy, S.; Kwak, M.J.; Batchelor, W.; Forman, D.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.B.; Frazee, E.N.; Kukrálová, L.; Sarvottam, K.; Herasevich, V.; Young, P.M.; Kashyap, R.; Lieske, J.C. Evaluating Muscle Mass by Using Markers of Kidney Function: Development of the Sarcopenia Index. Crit. Care Med. 2017, 45, e23–e29. [Google Scholar] [CrossRef]
- Shungin, D.; Winkler, T.; Croteau-Chonka, D.; Ferreira, T.; Locke, A.; Mägi, R.; Strawbridge, R.; Pers, T.; Fischer, K.; Justice, A.; et al. New Genetic Loci Link Adipose and Insulin Biology to Body Fat Distribution. Nature 2014, 518, 187–196. [Google Scholar] [CrossRef]
- Contaldo, F.; Di Biase, G.; Panico, S.; Trevisan, M.; Farinaro, E.; Mancini, M. Body Fat Distribution and Cardiovascular Risk in Middle-Aged People in Southern Italy. Atherosclerosis 1986, 61, 169–172. [Google Scholar] [CrossRef]
- Yang, P.-Y.; Li, Y.-C. Pulse Pressure Index (Pulse Pressure/Systolic Pressure) May Be Better than Pulse Pressure for Assessment of Cardiovascular Outcomes. Med. Hypotheses 2009, 72, 729–731. [Google Scholar] [CrossRef]
- Baker, J.S.; Quach, B.; Jiao, J.; Liang, W.; Gao, Y. Body Composition Matters When Designing and Prescribing HIIT Protocols to Individuals for Health Promotion. Phys. Act. Health 2020, 4, 158–161. [Google Scholar] [CrossRef]
- Ding, W.; Liu, J.T.; Zhao, X.Y.; Zhang, L.; Jia, L.N. A17462 Association of DXA-Derived Indices of Fat Distribution with Cardiometabolic Risk in Chinese Children and Adolescents. J. Hypertens. 2018, 36, e341–e342. [Google Scholar] [CrossRef]
- Karadavut, S.; Keleşoğlu, Ş.; Elçik, D. Relationship between the Progression of Coronary Artery Disease and Pulse Pressure Index: A Cross-Sectional Work. Angiology 2022, 74, 687–692. [Google Scholar] [CrossRef]
- Guo, B.; Liu, X.; Si, Q.; Zhang, D.; Li, M.; Li, X.; Zhao, Y.; Hu, F.; Zhang, M.; Liu, Y.; et al. Associations of CBC-Derived Inflammatory Indicators with Sarcopenia and Mortality in Adults: Evidence from Nhanes 1999~2006. BMC Geriatr. 2024, 24, 432. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic Obesity: Prevalence and Association with Metabolic Syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Guo, B.; Gong, J.; Tang, Y.; Shang, J.; Cheng, Y.; Xu, H. Sex- and Age-Specific Percentiles of Body Composition Indices for Chinese Adults Using Dual-Energy X-Ray Absorptiometry. Eur. J. Nutr. 2016, 56, 2393–2406. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Park, J.; Seo, Y.-G. Relationship between Arm-to-Leg and Limbs-to-Trunk Body Composition Ratio and Cardiovascular Disease Risk Factors. Sci. Rep. 2021, 11, 17414. [Google Scholar] [CrossRef]
- Gavi, S.; Feiner, J.; Melendez, M.; Mynarcik, D.; Gelato, M.; Mcnurlan, M. Limb Fat to Trunk Fat Ratio in Elderly Persons Is a Strong Determinant of Insulin Resistance and Adiponectin Levels. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 997–1001. [Google Scholar] [CrossRef]
- Van Genderen, M.; Bartels, S.; Lima, A.; Bezemer, R.; Ince, C.; Bakker, J.; Van Bommel, J. Peripheral Perfusion Index as an Early Predictor for Central Hypovolemia in Awake Healthy Volunteers. Anesth. Analg. 2013, 116, 351–356. [Google Scholar] [CrossRef]
- Scafoglieri, A.; Clarys, J. Dual Energy X-ray Absorptiometry: Gold Standard for Muscle Mass? J. Cachexia Sarcopenia Muscle 2018, 9, 786–787. [Google Scholar] [CrossRef]
- Erlandson, M.; Lorbergs, A.; Mathur, S.; Cheung, A. Muscle Analysis Using pQCT, DXA and MRI. Eur. J. Radiol. 2016, 85, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Saugel, B.; Dueck, R.; Wagner, J. Measurement of Blood Pressure. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 309–322. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; González-Gálvez, N.; López-Vivancos, A.; Espeso-García, A.; Martínez-Aranda, L.M.; Gea-García, G.M.; Orquín-Castrillón, F.J.; Carbonell-Baeza, A.; Jiménez-García, J.D.; Velázquez-Díaz, D.; et al. Sarcopenia, Diet, Physical Activity and Obesity in European Middle-Aged and Older Adults: The LifeAge Study. Nutrients 2021, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.; Folsom, A.; Prineas, R.; Potter, J.; Gapstur, S. The Association of Body Fat Distribution with Lifestyle and Reproductive Factors in a Population Study of Postmenopausal Women. Int. J. Obes. 1990, 14, 583–591. [Google Scholar] [CrossRef]
- McEniery, C.M.; Yasmin, N.; McDonnell, B.; Munnery, M.; Wallace, S.; Rowe, C.; Cockcroft, J.; Wilkinson, I. Central Pressure: Variability and Impact of Cardiovascular Risk Factors: The Anglo-Cardiff Collaborative Trial II. Hypertension 2008, 51, 1476–1482. [Google Scholar] [CrossRef]
- Thorp, A.; Owen, N.; Neuhaus, M.; Dunstan, D. Sedentary Behaviors and Subsequent Health Outcomes in Adults a Systematic Review of Longitudinal Studies, 1996–2011. Am. J. Prev. Med. 2011, 41, 207–215. [Google Scholar] [CrossRef]
- Vandenbroucke, J.; Von Elm, E.; Altman, D.; Gøtzsche, P.; Mulrow, C.; Pocock, S.; Poole, C.; Schlesselman, J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2007, 12, 1500–1524. [Google Scholar] [CrossRef]
- Elm, E.; Altman, D.; Egger, M.; Pocock, S.; Gøtzsche, P.; Vandenbroucke, J. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Sullivan, L.; Dukes, K.; Losina, E. An Introduction to Hierarchical Linear Modelling. Stat. Med. 1999, 18, 855–888. [Google Scholar] [CrossRef]
- Lee, Y.; Nelder, J. Hierarchical Generalised Linear Models: A Synthesis of Generalised Linear Models, Random-Effect Models and Structured Dispersions. Biometrika 2001, 88, 987–1006. [Google Scholar] [CrossRef]
- Desquilbet, L.; Mariotti, F. Dose-Response Analyses Using Restricted Cubic Spline Functions in Public Health Research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Buse, A. Cubic Splines as a Special Case of Restricted Least Squares. J. Am. Stat. Assoc. 1977, 72, 64–68. [Google Scholar] [CrossRef]
- Quartagno, M.; Carpenter, J.R.; Goldstein, H. Multiple Imputation with Survey Weights: A Multilevel Approach. J. Surv. Stat. Methodol. 2020, 8, 965–989. [Google Scholar] [CrossRef]
- Quartagno, M.; Grund, S.; Carpenter, J. Jomo: A Flexible Package for Two-Level Joint Modelling Multiple Imputation. R J. 2019, 11, 205–228. [Google Scholar] [CrossRef]
- Muntner, P.; Hardy, S.T.; Fine, L.J.; Jaeger, B.C.; Wozniak, G.; Levitan, E.B.; Colantonio, L.D. Trends in Blood Pressure Control among US Adults with Hypertension, 1999-2000 to 2017-2018. JAMA 2020, 324, 1190–1200. [Google Scholar] [CrossRef]
- Harrell, F. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Schnyder, S.; Handschin, C. Skeletal Muscle as an Endocrine Organ: PGC-1α, Myokines and Exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef]
- Liao, J.; Cao, C.; Hur, J.; Cohen, J.; Chen, W.; Zong, X.; Colditz, G.; Yang, L.; Stamatakis, E.; Cao, Y. Association of Sedentary Patterns with Body Fat Distribution among US Children and Adolescents: A Population-Based Study. Int. J. Obes. 2021, 45, 2048–2057. [Google Scholar] [CrossRef]
- Liu, T.; Nie, X.; Wu, Z.; Zhang, Y.; Feng, G.; Cai, S.; Lv, Y.; Peng, X. Can Statistic Adjustment of OR Minimize the Potential Confounding Bias for Meta-Analysis of Case-Control Study? A Secondary Data Analysis. BMC Med. Res. Methodol. 2017, 17, 179. [Google Scholar] [CrossRef]
- Seth, N.; Seal, A.; Ruchin, P.; McGirr, J. The Accuracy of Self-Perception of Obesity in a Rural Australian Population: A Cross-Sectional Study. J. Prim. Care Community Health 2022, 13, 21501319221115256. [Google Scholar] [CrossRef]
- Moyon, L.; Gonthier, C.; Brun, L.; Cabagno, G.; Somat, A.; Foll, D.L. Global Self-Esteem and Degree of Overweight/Obesity: Are They Linked in the Adult Population? A Systematic Review and Meta-Analysis. Psychol. Health 2024, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Brosnan, B.; Chu, A.; Skeaff, C.; Rehrer, N.; Perry, T.; Peddie, M. Sedentary Behavior and Body Weight and Composition in Adults: A Systematic Review and Meta-Analysis of Prospective Studies. Sports Med. 2018, 48, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.L.; Oliveira Werneck, A.; Rodrigues da Silva, D.; Kovalskys, I.; Gómez, G.; Rigotti, A.; Yadira Cortés Sanabria, L.; García, M.C.; Pareja, R.G.; Herrera-Cuenca, M.; et al. Socio-Demographic Correlates of Total and Domain-Specific Sedentary Behavior in Latin America: A Population-Based Study. Int. J. Environ. Res. Public Health 2020, 17, 5587. [Google Scholar] [CrossRef]
- Matthews, C.; Carlson, S.; Saint-Maurice, P.; Patel, S.; Salerno, E.; Loftfield, E.; Troiano, R.; Fulton, J.; Sampson, J.; Tribby, C.; et al. Sedentary Behavior in U.S. Adults: Fall 2019. Med. Sci. Sports Exerc. 2021, 53, 2512–2519. [Google Scholar] [CrossRef]
- Martins, L.C.G.; Lopes, M.V.d.O.; Diniz, C.M.; Guedes, N.G. The Factors Related to a Sedentary Lifestyle: A Meta-Analysis Review. J. Adv. Nurs. 2021, 77, 1188–1205. [Google Scholar] [CrossRef] [PubMed]
- Zamora, A.N.; Patel, M.L.; King, A.C. Abstract P528: Associations between Sleep Duration and Sedentary Behavior among Racial/Ethnic Minorities: Analysis of NHANES 2011-2018. Circulation 2024, 149, AP528. [Google Scholar] [CrossRef]
- Mattingly, J.L.; Petrov, M.E. Abstract P306: Racial/Ethnic Differences in Self-Reported Sedentary Time Are Related to Differential Risk for Prevalent Hypertension: NHANES 2011-2016. Circulation 2020, 141, AP306. [Google Scholar] [CrossRef]
- Latouche, C.; Jowett, J.; Carey, A.; Bertovic, D.; Owen, N.; Dunstan, D.; Kingwell, B. Effects of Breaking up Prolonged Sitting on Skeletal Muscle Gene Expression. J. Appl. Physiol. 2013, 114, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Schinkel-Ivy, A.; Nairn, B.; Drake, J. Investigation of Trunk Muscle Co-Contraction and Its Association with Low Back Pain Development during Prolonged Sitting. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2013, 23, 778–786. [Google Scholar] [CrossRef]
- Čular, D.; Miletić, A.; Babić, M. The Prevalence of Constituent Year Effect in Youth Olympic Games: Implications for Talent Identification and Development in Basketball. Acta Kinesiol. 2024, 18, 4–8. [Google Scholar] [CrossRef]
- Russo, L.; D’Eramo, U.; Padulo, J.; Foti, C.; Schiffer, R.; Scoppa, F. Day-Time Effect on Postural Stability in Young Sportsmen. Muscle Ligaments Tendons J. 2019, 5, 38. [Google Scholar] [CrossRef]
- Washif, J.; Farooq, A.; Krug, I.; Pyne, D.; Verhagen, E.; Taylor, L.; Wong, D.; Mujika, I.; Cortis, C.; Haddad, M.; et al. Training during the COVID-19 Lockdown: Knowledge, Beliefs, and Practices of 12,526 Athletes from 142 Countries and Six Continents. Sports Med. 2021, 52, 933–948. [Google Scholar] [CrossRef]

| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95%CI | p-Value | Beta | 95%CI | p-Value | Beta | 95%CI | p-Value | |
| SI | −0.004 | −0.005, −0.002 | <0.001 | −0.006 | −0.007, −0.005 | <0.001 | −0.001 | −0.001, 0.000 | 0.004 |
| FDI | −0.009 | −0.012, −0.007 | <0.001 | 0.000 | −0.002, 0.002 | 0.800 | −0.003 | −0.005, −0.002 | <0.001 |
| PPI | 0.001 | 0.000, 0.002 | 0.014 | 0.000 | 0.000, 0.001 | 0.200 | 0.000 | 0.000, 0.000 | 0.200 |
| Different Subgroups | Category | n | β | 95% CI | SE | t-Value | Power |
|---|---|---|---|---|---|---|---|
| SI—Like to weigh more, less, or same | More | 1884 | −0.0026 | −0.0038~−0.0014 | 0.000612 | −4.25 | 98.9% |
| Less | 7217 | −0.0006 | −0.0011~−0.0001 | 0.000255 | −2.35 | 65.2% | |
| Same | 4189 | −0.0001 | −0.0008~0.0006 | 0.000357 | −0.28 | 5.9% | |
| Don’t know | 231 | −0.0132 | −0.0201~−0.0063 | 0.003520 | −3.75 | 96.2% | |
| SI—BMI degree | Underweight | 1132 | −0.0092 | −0.0125~−0.0058 | 0.00138 | −6.67 | >99% |
| Normal weight | 4896 | −0.0009 | −0.0016~−0.0003 | 0.00033 | −2.73 | 89% | |
| Overweight | 3648 | 0.0000 | −0.0007~0.0007 | 0.00036 | 0 | 5% | |
| Obese | 3846 | −0.0006 | −0.0012~0.0000 | 0.00031 | −1.94 | 62% | |
| FDI—Sex | Male | 6801 | −0.0069 | −0.0083~−0.0055 | 0.00092 | −7.50 | >99% |
| Female | 6720 | 0.0015 | 0.0002~0.0027 | 0.00077 | 1.95 | 62% | |
| FDI—Race | Mexican American | 2330 | −0.0090 | −0.0112~−0.0069 | 0.00058 | −15.52 | >99% |
| Other Hispanic | 1434 | −0.0041 | −0.0068~−0.0014 | 0.00119 | −3.44 | 99% | |
| Non-Hispanic White | 4357 | −0.0035 | −0.0052~−0.0018 | 0.00069 | −5.07 | >99% | |
| Non-Hispanic Black | 2838 | 0.0014 | −0.0003~0.0031 | 0.00089 | 1.57 | 42% | |
| Non-Hispanic Asian | 1869 | 0.0010 | −0.0014~0.0034 | 0.00122 | 0.82 | 18% | |
| Other Race—including multi-racial | 693 | −0.0021 | −0.0066~0.0025 | 0.00230 | −0.91 | 20% | |
| FDI—Education level | Less than 9th grade | 1070 | −0.0069 | −0.0102~−0.0037 | 0.00133 | −5.19 | >99% |
| 9–11th grade (including 12th grade with no diploma) | 1701 | −0.0039 | −0.0065~−0.0012 | 0.00143 | −2.73 | 89% | |
| High school graduate/GEO or equivalent | 2860 | −0.0041 | −0.0003~0.0031 | 0.00164 | −0.25 | 6% | |
| Some college or AA degree | 4235 | −0.0027 | −0.0043~−0.0012 | 0.00056 | −4.82 | >99% | |
| College graduate or above | 3655 | −0.0001 | −0.0019~0.0017 | 0.00097 | −0.10 | 5% | |
| FDI—General health condition | Excellent | 1604 | −0.0001 | −0.0024~0.0021 | 0.00118 | −0.08 | 5% |
| Very good | 4152 | −0.0014 | −0.0029~0.0002 | 0.00088 | −1.59 | 42% | |
| Good | 5455 | −0.0032 | −0.0046~0.0018 | 0.00072 | −4.44 | >99% | |
| Fair | 1951 | −0.0060 | −0.0086~−0.0034 | 0.00133 | −4.50 | >99% | |
| Poor | 359 | −0.0007 | −0.0075~0.0061 | 0.00350 | −0.20 | 6% | |
| PPI—Race | Mexican American | 2330 | −0.0001 | −0.0012~0.0010 | 0.00056 | −0.18 | 6% |
| Other Hispanic | 1434 | 0.0014 | 0.0000~0.0028 | 0.00071 | 1.97 | 63% | |
| Non-Hispanic White | 4357 | 0.0000 | −0.0007~0.0008 | 0.00038 | 0.00 | 5% | |
| Non-Hispanic Black | 2838 | 0.0013 | 0.0003~0.0024 | 0.00055 | 2.36 | 76% | |
| Non-Hispanic Asian | 1869 | 0.0001 | −0.0009~0.0012 | 0.00054 | 0.19 | 6% | |
| Other Race—including multi-racial | 693 | 0.0014 | −0.0005~0.0034 | 0.00101 | 1.39 | 33% |
| Model | Effect Size | 95%CI | p for Likelihood Ratio Test |
|---|---|---|---|
| SD-SI—Model 1 | <0.001 | ||
| Standard Linear Regression | −0.005 | −0.006, −0.004 | |
| Two-piecewise Linear Regression | |||
| Inflection Point of ST | |||
| <9.467 | −0.01 | −0.011, −0.008 | |
| >9.467 | 0.01 | 0.006, 0.013 | |
| SD-SI—Model 2 | <0.001 | ||
| Standard Linear Regression | −0.007 | −0.008, −0.006 | |
| Two-piecewise Linear Regression | |||
| Inflection Point of ST | |||
| <10.517 | −0.01 | −0.011, −0.009 | |
| >10.517 | 0.011 | 0.008, 0.014 | |
| SD-SI—Model 3 | <0.001 | ||
| Standard Linear Regression | −0.001 | −0.001, 0 | |
| Two-piecewise Linear Regression | |||
| Inflection Point of ST | |||
| <10.733 | −0.001 | −0.002, −0.001 | |
| >10.733 | 0.002 | 0, 0.004 | |
| SD-FDI—Model 1 | <0.001 | ||
| Standard Linear Regression | −0.005 | −0.006, −0.004 | |
| Two-piecewise Linear Regression | |||
| Inflection Point of ST | |||
| <9.467 | −0.01 | −0.011, −0.008 | |
| >9.467 | 0.01 | 0.006, 0.013 | |
| SD-FDI—Model 2 | <0.001 | ||
| Standard Linear Regression | −0.007 | −0.008, −0.006 | |
| Two-piecewise Linear Regression | |||
| Inflection Point of ST | |||
| <10.517 | −0.01 | −0.011, −0.009 | |
| >10.517 | 0.011 | 0.008, 0.014 | |
| SD-PPI—Model 1 | <0.001 | ||
| Standard Linear Regression | −0.005 | −0.006, −0.004 | |
| Two-piecewise Linear Regression | |||
| Inflection Point of ST | |||
| <9.467 | −0.01 | −0.011, −0.008 | |
| >9.467 | 0.01 | 0.006, 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Song, Y.; Sun, D.; Lu, Z.; Chen, H.; Cen, X.; Janićijević, D.; Radak, Z.; Gao, Z.; Baker, J.S.; et al. Sedentary Duration and Systemic Health Burden: Nonlinear Associations with Muscle, Fat, and Vascular Phenotypes in a US Population-Based Study. Healthcare 2025, 13, 2309. https://doi.org/10.3390/healthcare13182309
Hu C, Song Y, Sun D, Lu Z, Chen H, Cen X, Janićijević D, Radak Z, Gao Z, Baker JS, et al. Sedentary Duration and Systemic Health Burden: Nonlinear Associations with Muscle, Fat, and Vascular Phenotypes in a US Population-Based Study. Healthcare. 2025; 13(18):2309. https://doi.org/10.3390/healthcare13182309
Chicago/Turabian StyleHu, Chen, Yang Song, Dong Sun, Zhenghui Lu, Hairong Chen, Xuanzhen Cen, Danica Janićijević, Zsolt Radak, Zixiang Gao, Julien Steven Baker, and et al. 2025. "Sedentary Duration and Systemic Health Burden: Nonlinear Associations with Muscle, Fat, and Vascular Phenotypes in a US Population-Based Study" Healthcare 13, no. 18: 2309. https://doi.org/10.3390/healthcare13182309
APA StyleHu, C., Song, Y., Sun, D., Lu, Z., Chen, H., Cen, X., Janićijević, D., Radak, Z., Gao, Z., Baker, J. S., & Gu, Y. (2025). Sedentary Duration and Systemic Health Burden: Nonlinear Associations with Muscle, Fat, and Vascular Phenotypes in a US Population-Based Study. Healthcare, 13(18), 2309. https://doi.org/10.3390/healthcare13182309













