Concordance of an Artificial Intelligence Model (ChatGPT 4.0) with Physician Decisions in Smoking Cessation Clinics: A Comparative Evaluation
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Participants and Sampling
- Patients aged 18 years or older,
- First-time applicants to the smoking cessation clinic,
- Initiated on pharmacological or behavioral therapy following evaluation.
- Pregnant or lactating individuals,
- Patients under 18 years of age,
- Records with missing or inaccurate clinical information,
- Patients not eligible for smoking cessation treatment.
2.3. Data Collection
2.4. Clinical Decision Reference Standards
- The Turkish Ministry of Health Smoking Cessation Guideline [6],
- The WHO Clinical Treatment Guideline for Tobacco Cessation in Adults [7],
- The Consensus Report on Diagnosis and Treatment of Smoking Cessation by the Tobacco Control Working Group of the Turkish Thoracic Society [8],
- The National Institute for Health and Care Excellence Guideline: Tobacco—Preventing Uptake, Promoting Quitting, and Treating Dependence [9],
- Local clinic protocols developed in accordance with national reimbursement policies and medication availability.
2.5. AI Evaluation Protocol
- Standard clinical intake forms used at the clinic (also used in data collection for sociodemographic and dependence-related variables),
- Indications and contraindications for pharmacological treatments derived from national and international clinical algorithms,
- Contextual limitations at the time of the study included:
- ○
- Cytisine is available free of charge,
- ○
- Varenicline is not available in the country,
- ○
- Nicotine patches are available in 16 h forms of 25 mg, 15 mg, and 10 mg,
- ○
- Behavioral therapy can be used as monotherapy.
2.6. Prompting Protocol
‘Based on the structured patient information I will provide, recommend the most appropriate smoking cessation treatment. When making your decisions, rely on the uploaded national and international clinical guidelines as well as treatment indications and contraindications. Take into account the following contextual constraints: cytisine is available free of charge; varenicline is not available in this country; nicotine patches are available in 16-h formulations (25/15/10 mg); behavioral therapy can be used as monotherapy. For each case: (1) Primary treatment recommendation, (2) Possible alternative treatments, and (3) Detailed justification for why these treatments were or were not preferred.’
2.7. Information Quality and Concordance Assessment
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization. |
| AI | Artificial Intelligence. |
References
- World Health Organization (WHO). Tobacco. Available online: https://www.who.int/health-topics/tobacco#tab=tab_1 (accessed on 26 July 2025).
- Cohen, G.; Bellanca, C.M.; Bernardini, R.; Rose, J.E.; Polosa, R. Personalized and adaptive interventions for smoking cessation: Emerging trends and determinants of efficacy. iScience 2024, 27, 111090. [Google Scholar] [CrossRef] [PubMed]
- Rane, N.; Choudhary, S.; Rane, J. Explainable Artificial Intelligence (XAI) in healthcare: Interpretable Models for Clinical Decision Support. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Eum, Y.H.; Kim, H.J.; Bak, S.; Lee, S.H.; Kim, J.; Park, S.H.; Hwang, S.E.; Oh, B. Factors related to the success of smoking cessation: A retrospective cohort study in Korea. Tob. Induc. Dis. 2022, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- OpenAI GPT-4 Technical Report. 27 March 2023. Available online: https://cdn.openai.com/papers/gpt-4.pdf (accessed on 26 July 2025).
- Tobacco Control Strategic Document and Action Plan 2018–2023. Available online: https://havanikoru.saglik.gov.tr/havanikoru/dosya/eylem_plani_ve_strateji_tutun_HD.pdf (accessed on 20 July 2025).
- World Health Organization Clinical Treatment Guideline for Tobacco Cessation in Adults. Available online: https://www.who.int/publications/i/item/9789240096431 (accessed on 20 July 2025).
- Turkish Thoracic Society. Smoking Cessation Diagnosis and Treatment Consensus Report. Tobacco Control Working Group. 2014. Available online: https://toraks.org.tr/site/sf/books/pre_migration/ef712e27e221af17ab3b44ca23fe11aa49b62032270561dce9e62214188110ac.pdf (accessed on 21 July 2025).
- NICE Guideline NG 209. Tobacco: Preventing Uptake, Promoting Quitting and Treating Dependence. Available online: https://www.nice.org.uk/guidance/ng209 (accessed on 20 July 2025).
- Riley, H.; Ainani, N.; Turk, A.; Headley, S.; Szalai, H.; Stefan, M.; Lindenauer, P.K.; Pack, Q.R. Smoking cessation after hospitalization for myocardial infarction or cardiac surgery: Assessing patient interest, confidence, and physician prescribing practices. Clin. Cardiol. 2019, 42, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.H.; Davies, N.M.; Taylor, A.E.; Taylor, G.M.J.; Gunnell, D.; Martin, R.M.; Douglas, I. Risk of neuropsychiatric and cardiovascular adverse events following treatment with varenicline and nicotine replacement therapy in the UK Clinical Practice Research Datalink: A case–cross-over study. Addiction 2021, 116, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Liverpool HIV Interactions. Available online: https://www.hiv-druginteractions.org/interactions/93358 (accessed on 30 June 2025).
- Tutka, P.; Vinnikov, D.; Courtney, R.J.; Benowitz, N.L. Cytisine for nicotine addiction treatment: A review of pharmacology, therapeutics and an update of clinical trial evidence for smoking cessation. Addiction 2019, 114, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Report on the Global Tobacco Epidemic 2023: Protect People from Tobacco Smoke; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240077164 (accessed on 19 July 2025).
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Howe, C.; Glover, M.; McRobbie, H.; Barnes, J.; Nosa, V.; Parag, V.; Bassett, B.; Bullen, C. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 2014, 371, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, B.; Cai, Z.; Seery, S.; Gonzalez, M.J.; Ali, N.M.; Ren, R.; Qiao, Y.; Xue, P.; Jiang, Y. Acceptance of clinical artificial intelligence among physicians and medical students: A systematic review with cross-sectional survey. Front. Med. 2022, 9, 990604. [Google Scholar] [CrossRef] [PubMed]
- Mosch, L.; Fürstenau, D.; Brandt, J.; Wagnitz, J.; Al Klopfenstein, S.; Poncette, A.S.; Balzer, F. The medical profession transformed by artificial intelligence: Qualitative study. Digit. Health 2022, 8, 20552076221143903. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Joseph, A.P. Artificial intelligence in mental healthcare: Transformative potential vs. the necessity of human interaction. Front. Psychol. 2024, 15, 1378904. [Google Scholar] [CrossRef] [PubMed]
- Vırıt, A.; Öter, A. Kardiyovasküler Hastalıkların Derin Öğrenme Algoritmaları İle Tanısı [Diagnosis of cardiovascular diseases using deep learning algorithms]. Gazi Univ. J. Sci. Part C Des. Technol. 2024, 12, 902–912. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Han, M.; Xue, Y.; Wei, W.; Li, L.-J.; Li, F.-F. Thoracic Disease Identification and Localization with Limited Supervision. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 8290–8299. [Google Scholar] [CrossRef]
- Enamorado-Díaz, E.; Morales-Trujillo, L.; García-García, J.A.; Marcos, A.T.; Navarro-Pando, J.; Escalona-Cuaresma, M.J. A novel machine learning-based proposal for early prediction of endometriosis disease. Expert Syst. Appl. 2025, 271, 126621. [Google Scholar] [CrossRef]
- Bendotti, H.; Lawler, S.; Chan, G.C.K.; Gartner, C.; Ireland, D.; Marshall, H.M. Conversational artificial intelligence interventions to support smoking cessation: A systematic review and meta-analysis. Digit. Health 2023, 9, 20552076231211634. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Li, L.; Xin, Z.; Xiang, D.; Huang, J.; Zhou, H.; Shi, F.; Zhu, W.; Cai, J.; Peng, T.; et al. A literature review of artificial intelligence (AI) for medical image segmentation: From AI and explainable AI to trustworthy AI. Quant. Imaging Med. Surg. 2024, 14, 9620–9652. [Google Scholar] [CrossRef]
- Jiang, F.; Jiang, Y.; Zhi, H.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; et al. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef]
- Gerke, S.; Minssen, T.; Cohen, I.G. Ethical and legal challenges of artificial intelligence-driven healthcare. Nat. Mach. Intell. 2020, 2, 333–335. [Google Scholar] [CrossRef]
- Benjamens, S.; Dhunnoo, P.; Meskó, B. The state of artificial intelligence-based FDA-approved medical devices and algorithms: An online database. npj Digit. Med. 2020, 3, 118. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan. 2021. Available online: https://www.fda.gov/media/145022/download (accessed on 20 July 2025).
- European Commission. Proposal for a Regulation on Artificial Intelligence. 2021. Available online: https://digital-strategy.ec.europa.eu/en/library/proposal-regulation-laying-down-harmonised-rules-artificial-intelligence (accessed on 20 July 2025).
- Morley, J.; Machado, C.C.; Burr, C.; Cowls, J.; Joshi, I.; Taddeo, M.; Floridi, L. Ethics of AI in health care: A mapping review. Soc. Sci. Med. 2020, 260, 113172. [Google Scholar] [CrossRef] [PubMed]
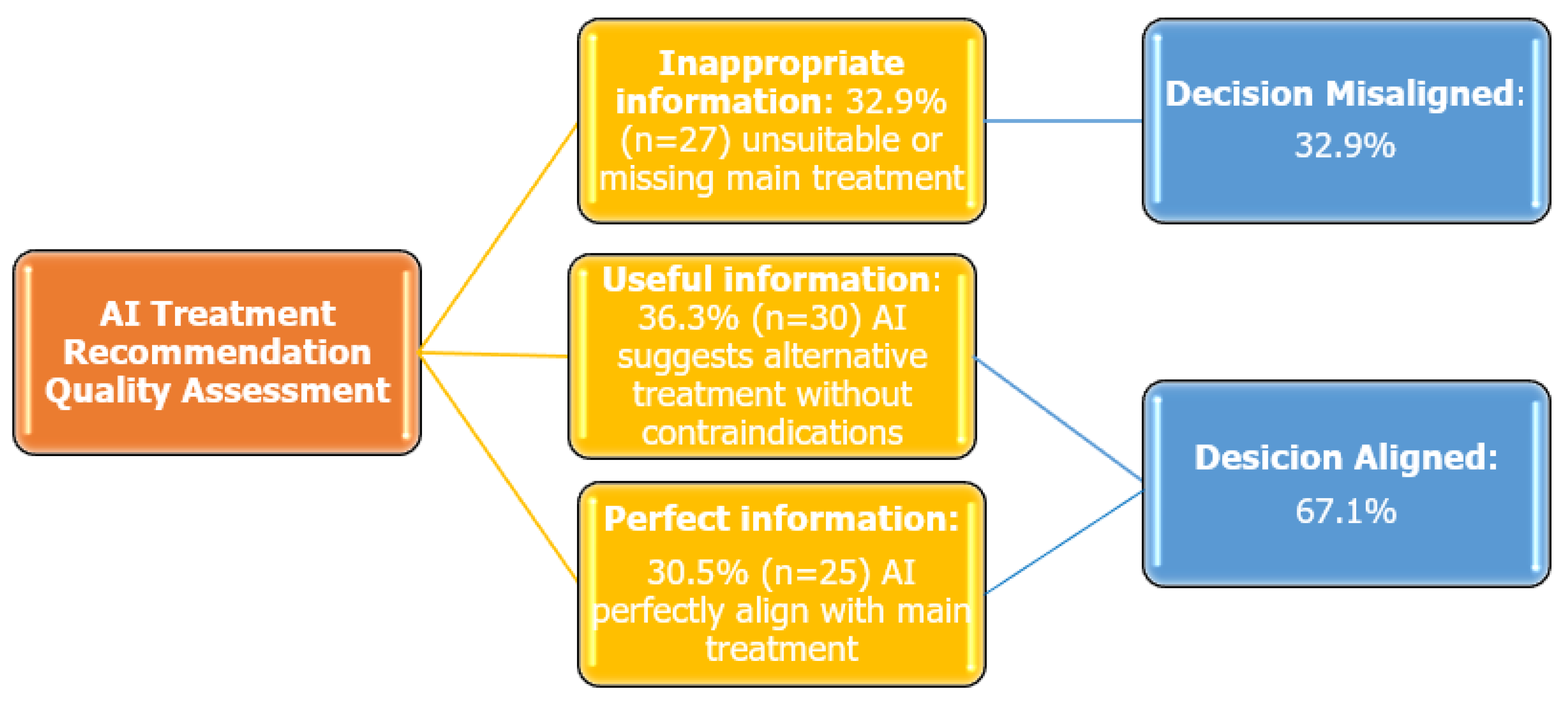
| Inappropriate Information 1 | Useful Information 2 | Perfect Information 3 | p-Value | Post Hoc Comparison | ||
|---|---|---|---|---|---|---|
| Age * (mean ± SD, min–max) | 40.40 ± 14.15 (20–69) | 39.20 ± 12.69 (19–63) | 42.88 ± 11.82 (19–61) | 0.572 | ||
| FNBT * (mean ± SD, min–max) | 5.92 ± 2.01 (1–9) | 6.16 ± 2.29 (1–10) | 6.44 ± 2.58 (1–10) | 0.724 | ||
| Pack-years * (mean ± SD, min–max) | 19.18 ± 11.24 (2–42) | 23.13 ± 14.39 (5–50) | 24.52 ± 12.99 (3–50) | 0.306 | ||
| Daily cigarette consumption ^ (mean ± SD, min–max) | 21.81 ± 12.67 (6–60) | 25.26 ± 13.43 (3–80) | 23.60 ± 11.14 (4–40) | 0.452 | ||
| Gender n(%) | Female | 11 (40.7) | 11 (36.7) | 11 (44.0) | 0.857 | |
| Male | 16 (59.3) | 19 (63.3) | 14 (56.0) | |||
| Employment status n(%) | Employed | 17 (63.0) | 25 (83.3) | 16 (64.0) | 0.162 | |
| Unemployed | 10 (37.0) | 5 (16.7) | 9 (36.0) | |||
| Chronic disease n(%) | None | 5 (18,5) | 16 (53.3) | 8 (32.0) | 0.021 | 1 > 2 ª |
| Present | 22 (81.5) | 14 (46.7) | 17 (68.0) | |||
| Previous surgery n(%) | None | 18 (66.7) | 16 (53.3) | 10 (40.0) | 0.156 | |
| Present | 9 (33.3) | 14 (46.7) | 15 (60.0) | |||
| Regular medication n(%) | None | 6 (22.2) | 17 (56.7) | 11 (44.0) | 0.030 | - |
| Present | 21 (77.8) | 13 (43.3) | 14 (56.0) | |||
| ECG n(%) | Nsr | 18 (66.7) | 29 (96.7) | 21 (84.0) | 0.011 | 2 > 1 ª |
| Other | 9 (33.3) | 1 (3.3) | 4 (16.0) | |||
| Blood pressure n(%) | Regulated | 26 (96.3) | 29 (96.7) | 24 (96.0) | 0.991 | |
| Other | 1 (3.7) | 1 (3.3) | 1 (4.0) | |||
| Mood n(%) | Euthymic | 23 (85.2) | 30 (100) | 21 (84.0) | 0.077 | |
| Other | 4 (14.8) | 0 | 4 (16.0) | |||
| Total | 27 (100) | 30 (100) | 25 (100) | |||
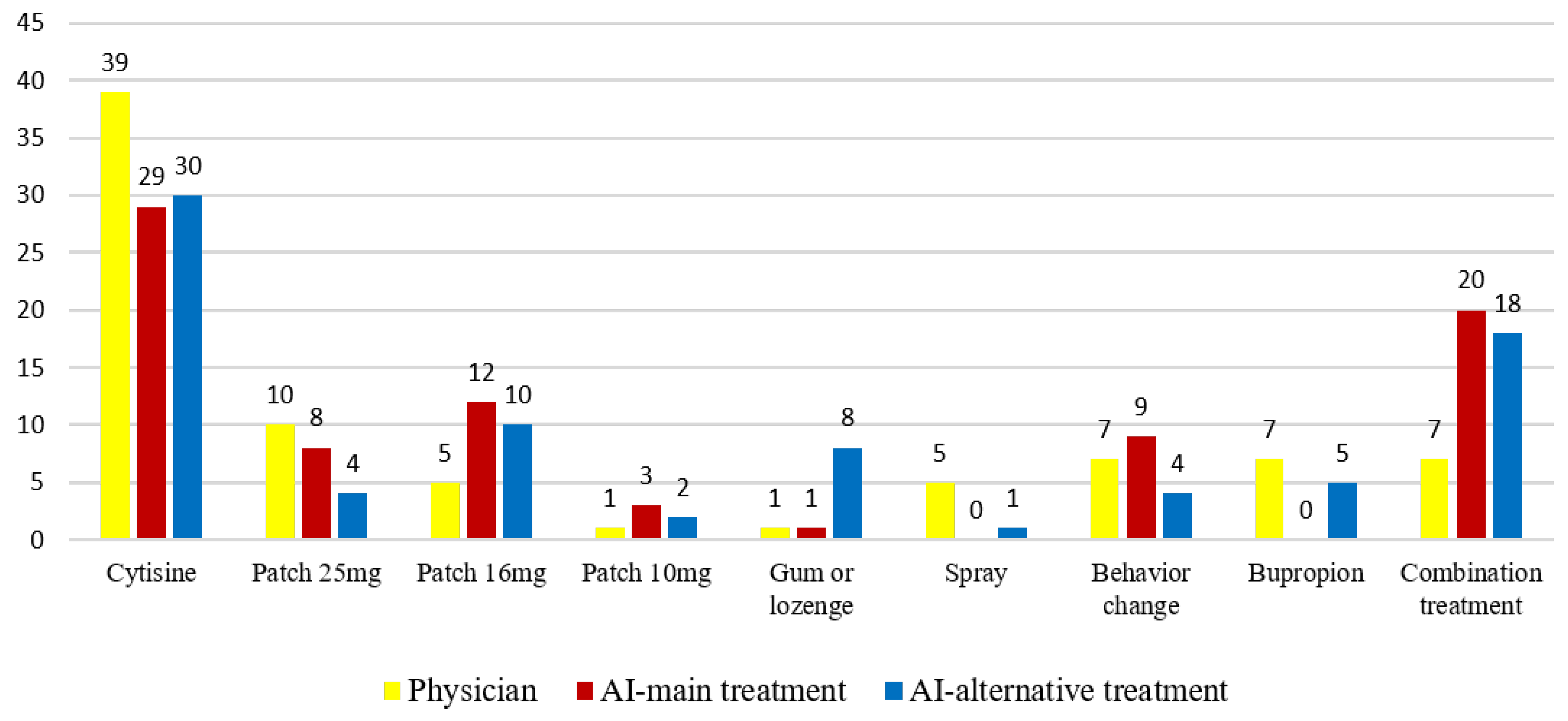
| Patient Information | AI Treatment Recommendation | Physician Treatment | Physician Comment | Decision Concordance | |
|---|---|---|---|---|---|
| Case 1 | 24-year-old female 7 pack-years, 20 cigarettes/day. FNDT: 5 Known Medical Conditions (KMC): Subclinical hypothyroidism Current Medications (CM): None Surgical History (SH): None Previous Quit Attempts (PQA): Two previous trials with nicotine patches, maximum abstinence duration: 1 week. Psychological Status (PS): Euthymic, no suicidal ideation EKG: Normal sinus rhythm (NSR), Blood Pressure (BP):125/85 mmHg Smoking Triggers (ST): Stress, family-related issues, post-meal cravings Quit Motivation (QM): Health-related reasons. Alcohol Use (AU): None | Primary Treatment (PT): Cytisine
| Cytisine | Treatment plan and justifications align completely with the physician’s decision. | Perfect information |
| Case 44 | 54-year-old female 42 pack-years, 15 cigarettes/day FNDT: 7 KMC: Brain neoplasm, Epilepsy, Chronic obstructive pulmonary disease CM: Formoterol Tiotropium Levetiracetam SH: Right upper lung lobectomy (2023) PQA: None PS: Euthymic ECG: NSR, BP: 120/70 mmHg ST: Stress QM: Health concerns AU: None | PT: Nicotine Patch (15 mg, 16 h release):
| Nicotine patch 15 mg and gum or lozenge | Treatment plan and justifications align completely with the physician’s decision. | Perfect information |
| Case 54 | 54-year-old female 15 pack-years, 4–8 cigarettes/day FNDT: 3 KMC: Premenopause CM: None SH: None PQA: None PS: Dysthymic disorder, no suicidal ideation ECG: NSR, BP: 115/75 mmHg ST: Sadness, post-meal cravings, stress QM: Financial reasons AU: Occasionally. | PT: Behavioral Management (Non-Pharmacological Approach)
| Nicotine spray | The physician’s treatment was suggested as an alternative by AI, and there were no errors in the AI’s recommendation | Useful Information |
| Case 45 | 31-year-old male 11.5 pack-years, 30 cigarettes/day FNDT: 8 KMC: Aortic and tricuspid valve abnormalities, Acne CM: Isotretinoin SH: None PQA: Tried 6 times, successfully quit for 6 months with bupropion PS: Euthymic ECG: NSR, BP: 120/80 mmHg ST: Social environment, alcohol consumption QM: Health reasons AU: 2–3 beers per week | PT: Nicotine Patch (25 mg, 16 h release):
| Bupropion | The patient, who had previously experienced a successful outcome with bupropion, was restarted on bupropion treatment after consulting cardiology | Useful Information |
| Case 65 | 31-year-old male 20 pack-years, 30 cigarettes/day FNDT: 7 KMC: None CM: None SH: None PQA: Tried 5 times, but never lasted more than 3 days PS: Euthymic ECG: NSR, BP: 110/70 mmHg ST: Stress, coffee, post-meal cravings, social and alcohol-related situations QM: Health concerns AU: Occasionally (2 times/month, 3–4 whiskey servings). | PT: Nicotine Patch (25 mg):
| Cytisine | The physician’s treatment was suggested as an alternative by AI, and there were no errors in the AI’s recommendation. | Useful Information |
| Case 16 | 69-year-old male. 22 pack-years, 10 cigarettes/day FNDT: 1 KMC: Benign Prostatic Hyperplasia, Anxiety Disorder CM: Tamsulosin Sertraline SH: None PQA: None PS: Euthymic ECG: Left axis deviation, first-degree AV block BP: 110/70 mmHg. ST: Tea and coffee consumption QT: Health-related reasons AU: None | PT: Cytisine:
| Behavior change | Pharmacological treatment was not required for low dependence levels. The use of cytisine is not recommended in this age group due to limited clinical evidence. Drug interactions between cytisine and antidepressants are not well-documented. | Inappropriate Information |
| Case 23 | 54-year-old male 20 pack-years, 10 cigarettes/day FNDT: 6 KMC: Recent myocardial infarction (MI) (last week) with two stents placed Mitral regurgitation Tricuspid regurgitation CM: Acetylsalicylic acid, Atorvastatin, Ticagrelor, Metoprolol, Perindopril SH: None PQA: None PS: Euthymic ECG: Signs of prior MI, atrial enlargement BP: 125/85 mmHg ST: Coffee, stress, anger, morning craving QM: Health-related reasons. AU: None | PT: Nicotine Patch (15 mg):
| Behavior change | The 15 mg nicotine patch was deemed inappropriate for the patient with a recent extensive anterior myocardial infarction (MI) history [10,11]. | Inappropriate Information |
| Case 51 | 28-year-old male 10 pack-years, 20 cigarettes/day. FNDT: 4 KMC: HIV+. CM: Bictegravir/Emtricitabine/Tenofovir Alafenamide. SH: None PQA: None PS: Euthymic ECG: NSR BP: 100/80 mmHg ST: Stress QM: Health and financial reasons AU: Social drinker | PT: Nicotine Patch (10–15 mg):
| Bupropion | The patient did not want to use NRT. The interaction of cytisine with active ingredients is unknown. There are no known interactions between bupropion and the active ingredients [12]. | Inappropriate Information |
| Case 4 | 45-year-old female 28 pack-years, 20 cigarette/day. FNDT: 8 KMC: Hypertension, Insomnia, Major Depressive Disorder, Generalized Anxiety Disorder CM: Ramipril, Aripiprazole, Fluoxetine, Quetiapine SH: None PQA: Attempted quitting independently and with varenicline, but did not use it consistently and relapsed within 10 days. PS: Euthymic, no suicidal ideation ECG: NSR BP: 110/70 mmHg ST: Post-meal cravings, coffee consumption QM: Health-related reasons AU: None | PT: Cytisine:
| Nicotine patch 25 mg | There is insufficient data on the safety of cytisine use in patients with mental illnesses [13]. There is limited data on drug interactions involving cytisine. | Inappropriate Information |
| Case 20 | 28-year-old female 28 pack-years, 20 cigarette/day. FNDT: 8 KMC: Multiple Sclerosis, Familial Mediterranean Fever, Acne CM: Isotretinoin, Glatiramer Acetate, Colchicine SH: None PQA: Tried at least four times using nicotine gum, patch, spray, and varenicline, but relapsed within 15 days each time. PS: Euthymic ECG: NSR, BP: 100/70 mmHg ST: Emotional changes, tea/coffee, social interactions QM: Health concerns due to MS AU: None | PT: Cytisine:
| Bupropion | Bupropion treatment was initiated following a neurology consultation. There is insufficient data on the use of cytisine in MS and FMF patients, as well as its potential drug interactions. | Inappropriate Information |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokseven Arda, Y.; Zeren Ozturk, G. Concordance of an Artificial Intelligence Model (ChatGPT 4.0) with Physician Decisions in Smoking Cessation Clinics: A Comparative Evaluation. Healthcare 2025, 13, 2283. https://doi.org/10.3390/healthcare13182283
Gokseven Arda Y, Zeren Ozturk G. Concordance of an Artificial Intelligence Model (ChatGPT 4.0) with Physician Decisions in Smoking Cessation Clinics: A Comparative Evaluation. Healthcare. 2025; 13(18):2283. https://doi.org/10.3390/healthcare13182283
Chicago/Turabian StyleGokseven Arda, Yagmur, and Guzin Zeren Ozturk. 2025. "Concordance of an Artificial Intelligence Model (ChatGPT 4.0) with Physician Decisions in Smoking Cessation Clinics: A Comparative Evaluation" Healthcare 13, no. 18: 2283. https://doi.org/10.3390/healthcare13182283
APA StyleGokseven Arda, Y., & Zeren Ozturk, G. (2025). Concordance of an Artificial Intelligence Model (ChatGPT 4.0) with Physician Decisions in Smoking Cessation Clinics: A Comparative Evaluation. Healthcare, 13(18), 2283. https://doi.org/10.3390/healthcare13182283






