The Economic Impact of Premarital Screening (PMS) of Sickle Cell Anemia on the Saudi Health System: A Cost Analysis Study
Abstract
1. Introduction
2. Materials and Methods
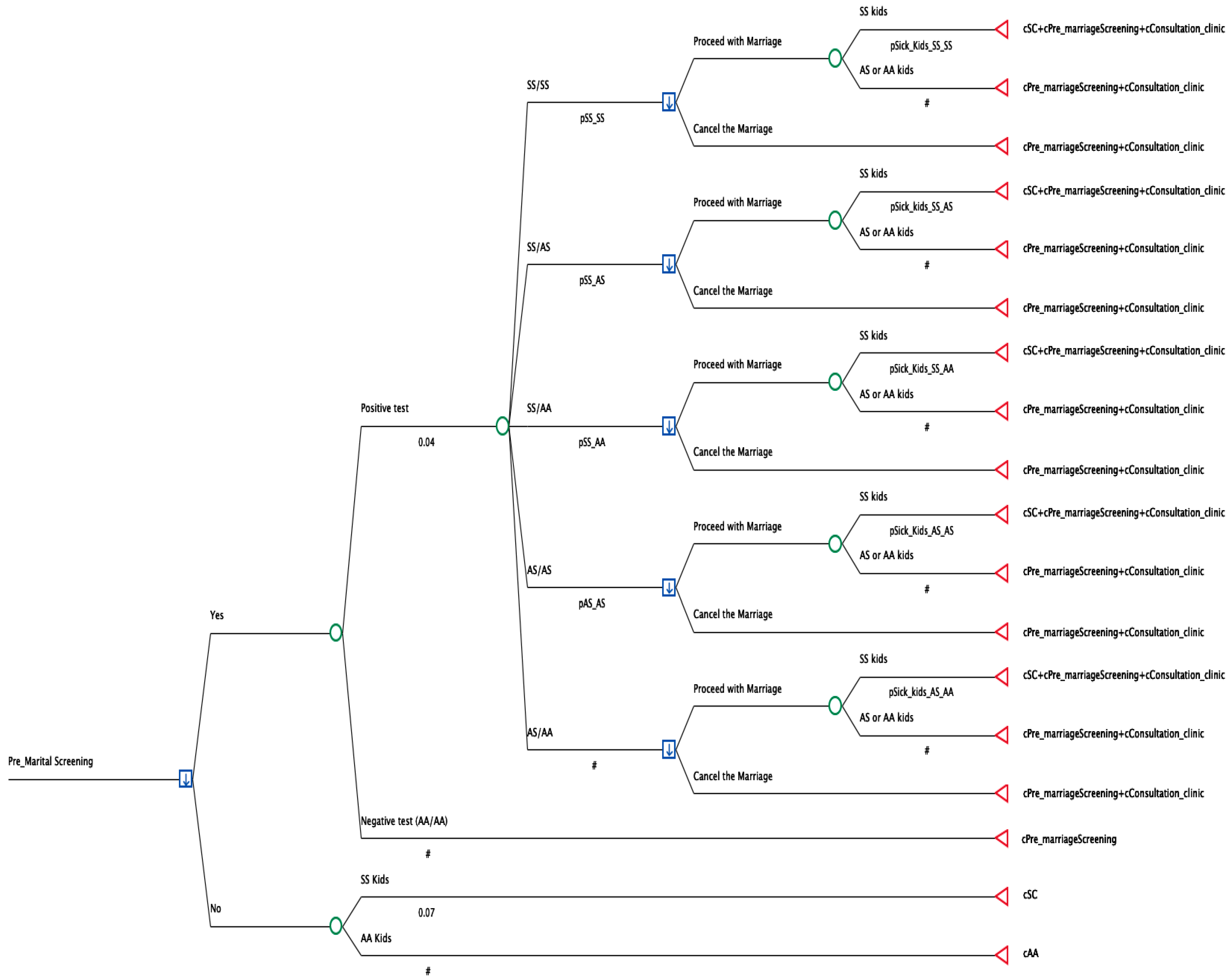
2.1. Model Development
- −
- Sickle cell disease and Sickle cell disease (SS/SS);
- −
- Sickle cell disease and Sickle cell trait (SS/AS);
- −
- Sickle cell disease and Healthy hemoglobin (SS/AA);
- −
- Sickle cell trait and Healthy hemoglobin (AS/AA);
- −
- Sickle cell trait and Sickle cell trait (AS/AS).
2.2. Variables/Input
2.3. Sensitivity Analysis
3. Results
3.1. Base Results
3.2. Sensitivity Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Health. Premarital Screening. Available online: https://www.moh.gov.sa/en/HealthAwareness/Beforemarriage/Pages/default.aspx (accessed on 23 August 2025).
- Petrou, M. Genetic Counselling. In Prevention of Thalassaemias and Other Haemoglobin Disorders: Volume 1: Principles [Internet], 2nd ed.; Thalassaemia International Federation: Nicosia, Cyprus, 2013. [Google Scholar]
- Alswaidi, F.M.; O’brien, S.J. Premarital Screening Programmes for Haemoglobinopathies, HIV and Hepatitis Viruses: Review and Factors Affecting Their Success. J. Med. Screen 2009, 16, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Executive Board, 118. Thalassaemia and Other Haemoglobinopathies: Report by the Secretariat, 2006, Executive Board 118th Session, Provisional Agenda Item 5.2. Available online: https://iris.who.int/handle/10665/21519 (accessed on 3 July 2025).
- Jastaniah, W. Epidemiology of Sickle Cell Disease in Saudi Arabia. Ann. Saudi Med. 2011, 31, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Owaidah, T.M.; Saeedi, M.Y. Marked Regional Variations in the Prevalence of Sickle Cell Disease and β-Thalassemia in Saudi Arabia: Findings from the Premarital Screening and Genetic Counseling Program. J. Epidemiol. Glob. Health 2011, 1, 61–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aljabry, M.; Sulimani, S.; Alotaibi, G.; Aljabri, H.; Alomary, S.; Aljabri, O.; Sallam, M.; Alsultan, A. Prevalence and Regional sDistribution of Beta-Hemoglobin Variants in Saudi Arabia: Insights from the National Premarital Screening Program. J. Epidemiol. Glob. Health 2024, 14, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Memish, Z.A.; Saeedi, M.Y. Six-Year Outcome of the National Premarital Screening and Genetic Counseling Program for Sickle Cell Disease and β-Thalassemia in Saudi Arabia. Ann. Saudi Med. 2011, 31, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, E.S.; Farhat, G.N.; Assiri, A.M.; Memish, Z.; Ahmed, E.M.; Saeedi, M.Y.; Al-Dossary, M.F.; Bashawri, H. Distribution of Hemoglobinopathy Disorders in Saudi Arabia Based on Data from the Premarital Screening and Genetic Counseling Program, 2011–2015. J. Epidemiol. Glob. Health 2018, 7 (Suppl. S1), S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Alfadhel, M. Prevention of Hemoglobinopathies in Saudi Arabia: Efficacy of National Premarital Screening and the Feasibility of Preimplantation Genetic Diagnosis. J. Biochem. Clin. Genet. 2020, 3, 94–99. [Google Scholar] [CrossRef]
- Alotaibi, M.M. Sickle Cell Disease in Saudi Arabia: A Challenge or Not. J. Epidemiol. Glob. Health 2017, 7, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Gosadi, I.M. National Screening Programs in Saudi Arabia: Overview, Outcomes, and Effectiveness. J. Infect. Public Health 2019, 12, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Premarital Screening—Accredited Premarital Screening Centers. Available online: https://www.moh.gov.sa/en/HealthAwareness/Beforemarriage/Pages/002.aspx (accessed on 26 August 2025).
- Abu-Shaheen, A.; Heena, H.; Nofal, A.; Abdelmoety, D.A.; Almatary, A.; Alsheef, M.; AlFayyad, I. Epidemiology of Thalassemia in Gulf Cooperation Council Countries: A Systematic Review. BioMed Res. Int. 2020, 2020, 1509501. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Xiao, H.; Garg, M.; Shah, C.; Wilkie, D.J.; Iii, A.M. The Economic Burden of Sickle Cell Disease in the United States. Value Health 2018, 21, S108. [Google Scholar] [CrossRef]
- Baldwin, Z.; Jiao, B.; Basu, A.; Roth, J.; Bender, M.A.; Elsisi, Z.; Johnson, K.M.; Cousin, E.; Ramsey, S.D.; Devine, B. Medical and Non-Medical Costs of Sickle Cell Disease and Treatments from a US Perspective: A Systematic Review and Landscape Analysis. PharmacoEconomics Open 2022, 6, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Bhor, M.; Xie, L.; Paulose, J.; Yuce, H. Medical Resource Use and Costs of Treating Sickle Cell-Related Vaso-Occlusive Crisis Episodes: A Retrospective Claims Study. J. Health Econ. Outcomes Res. 2020, 7, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Holdford, D.; Vendetti, N.; Sop, D.M.; Johnson, S.; Smith, W.R. Indirect Economic Burden of Sickle Cell Disease. Value Health 2021, 24, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Shdaifat, E.; Abu-Sneineh, F.; Alsaleh, N.; Ibrahim, A. Economic Burden of Sickle Cell Disease in Saudi Arabia. Value Health Reg. Issues 2025, 45, 101038. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. MOH News. MOH Obtains First Place in Institutional Excellence in Spending Efficiency. Available online: https://www.moh.gov.sa/en/Pages/Default.aspx (accessed on 26 August 2025).
- AlHamdan, N.A.; AlMazrou, Y.Y.; AlSwaidi, F.M.; Choudhry, A.J. Premarital Screening for Thalassemia and Sickle Cell Disease in Saudi Arabia. Genet. Med. 2007, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- AlRuthia, Y. The Direct Medical Costs of Sickle Cell Disease in Saudi Arabia: Insights from a Single Center Study. Healthcare 2025, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- The United Nations Development Programme (UNDP). The Cost of Health Services Delivered at Primary Care Facilities in SAUDI ARABIA. 2019. Available online: www.undp.org/sites/g/files/zskgke326/files/2024-02/ksa_phc_costing_report.pdf (accessed on 26 August 2025).
- Sheehan, V.A.; Gordeuk, V.R.; Kutlar, A. Disorders of Hemoglobin Structure: Sickle Cell Anemia and Related Abnormalities. In Williams Hematology, 10e; Kaushansky, K., Prchal, J.T., Burns, L.J., Lichtman, M.A., Levi, M., Linch, D.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2021. [Google Scholar]
- Asraf, N.O.; Aljibreen, S.A.; Alotaibi, M.B.; Hadadi, M.H.; Ahmed, H.A.F.; Batwie, A.A.; Alghamdi, W.M.; Al-Jaffer, Y.H.; Alhai, S.M.; Alshehab, K.A.; et al. Prevalence of Sickle-Cell Disease in Saudi Arabia: A Systematic Review. Uttar Pradesh J. Zool. 2022, 46, 210–223. [Google Scholar] [CrossRef]
| NAME | DESCRIPTION | Value [Reference] |
|---|---|---|
| cConsultation_clinic | Cost of consultation clinic for partner with positive test | USD 200 * |
| cPre_marriageScreening | Cost of pre-marriage screening test | USD 300 * |
| cSC | Cost of treating sickle cell case | USD 26,626 [19,22] |
| cAA | Average healthcare expenditure per capita | USD 68.60 [23] |
| pPostitveTest | Probability of having positive premarital test (Diseased or trait) | 0.045 [7,21] |
| pSS_AA | Probability of SS/AA couple | 0.025 [8] |
| pSS_SS | Probability of SS/SS couple | 0.025 [8] |
| pSS_AS | Probability of SS/AS couple | 0.025 [8] |
| pSick_Kids_SS_AA | Probability of having sickle cell kid from SS/AA parents | 0 [24] |
| pSick_Kids_SS_SS | Probability of having sickle cell kid from SS/SS parents | 1 [24] |
| pSick_kids_SS_AS | Probability of having sickle cell kid from SS/AS parents | 0.5 [24] |
| pSick_kids_AS_AA | Probability of having sickle cell kid from AS/AA parents | 0 [24] |
| pSick_kids_AS_AS | Probability of having sickle cell kid from AS/AS parents | 0.25 [24] |
| pNegative test | Probability of having a negative test | 0.953 [7,21] |
| pProceeding_with_marraige | Probability for couple with positive test to proceed with marriage | 0.77 [8,21] |
| Healthcare Perspective | Value | Reference |
|---|---|---|
| Sample population | 300,000 | [9] |
| Costs of managing sickle cell disease | USD 26,626 | [22] |
| SS/SS genotype marriage canceled | USD 8,137,800 | calculated |
| SS/AS genotype marriage canceled | USD 2,071,950 | calculated |
| AS/AS genotype marriage canceled | USD 536,700 | calculated |
| Total costs saving | USD 10,746,450 |
| Strategy | EV * | Cost |
|---|---|---|
| Screening | USD 308 | USD 10,746,450 |
| No screening | USD 1928 | USD 40,488,000 |
| Incremental difference | USD 1620 | USD 29,741,550 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, A.F.; Almalki, R.A.; Alsheikh, M.Y.; Omran, G.O.; Althobaiti, H.A.; AlQurashi, W.S. The Economic Impact of Premarital Screening (PMS) of Sickle Cell Anemia on the Saudi Health System: A Cost Analysis Study. Healthcare 2025, 13, 2243. https://doi.org/10.3390/healthcare13172243
Alotaibi AF, Almalki RA, Alsheikh MY, Omran GO, Althobaiti HA, AlQurashi WS. The Economic Impact of Premarital Screening (PMS) of Sickle Cell Anemia on the Saudi Health System: A Cost Analysis Study. Healthcare. 2025; 13(17):2243. https://doi.org/10.3390/healthcare13172243
Chicago/Turabian StyleAlotaibi, Amal F., Rami A. Almalki, Mona Y. Alsheikh, Ghufran O. Omran, Hana A. Althobaiti, and Wejdan S. AlQurashi. 2025. "The Economic Impact of Premarital Screening (PMS) of Sickle Cell Anemia on the Saudi Health System: A Cost Analysis Study" Healthcare 13, no. 17: 2243. https://doi.org/10.3390/healthcare13172243
APA StyleAlotaibi, A. F., Almalki, R. A., Alsheikh, M. Y., Omran, G. O., Althobaiti, H. A., & AlQurashi, W. S. (2025). The Economic Impact of Premarital Screening (PMS) of Sickle Cell Anemia on the Saudi Health System: A Cost Analysis Study. Healthcare, 13(17), 2243. https://doi.org/10.3390/healthcare13172243






