Association of Vitamin D Deficiency with Local Muscle–Fat Ratio in Geriatric Palliative Care Patients: An Ultrasonographic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Eligibility Criteria
2.3. Sample Size Calculation
2.4. Variables and Measurements
2.4.1. Demographic and Clinical Status
2.4.2. Anthropometric Measurement
2.4.3. Ultrasonographic Muscle–Fat Ratio
2.4.4. Vitamin D Measurement
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Group Comparisons
3.3. Multivariable Analysis
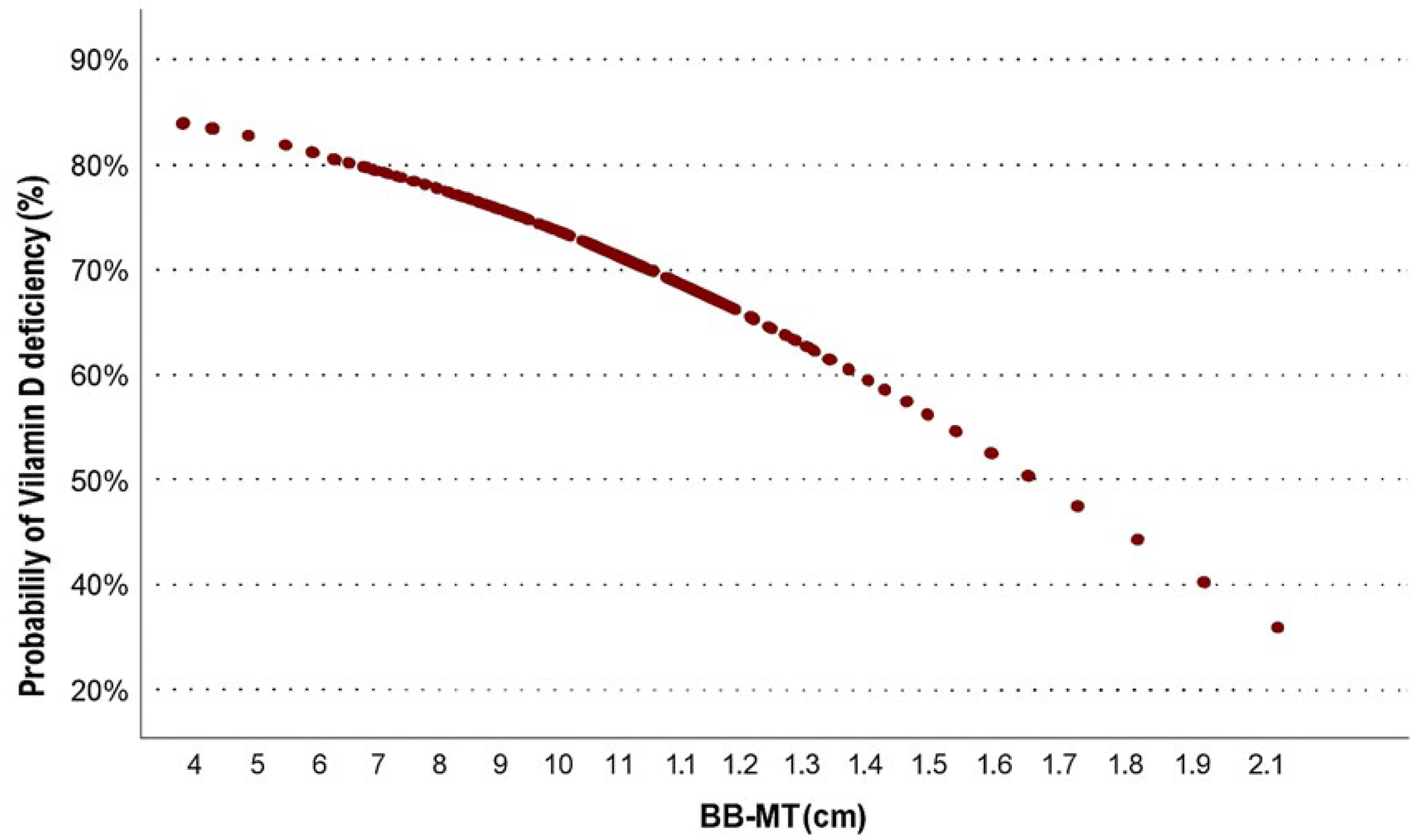
3.4. Probability Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | Activities of Daily Living |
| BB | Biceps brachii |
| BB-CSA | Biceps brachii cross-sectional area |
| BB-MT | Biceps brachii muscle thickness |
| BB-MT/SFT | Biceps muscle thickness to subcutaneous fat thickness ratio |
| BB-SFT | Biceps brachii subcutaneous fat thickness |
| BMI | Body mass index |
| CC | Calf circumference |
| CSA | Cross-sectional area |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 |
| ICC | Intraclass correlation coefficient |
| MUAC | Mid-upper calf circumference |
| RF | Rectus femoris |
| RF-CSA | Rectus femoris cross-sectional area |
| RF-MT | Rectus femoris muscle thickness |
| RF-MT/SFT | Rectus femoris muscle thickness to subcutaneous fat thickness ratio |
| RF-SFT | Rectus femoris subcutaneous fat thickness |
| SD | Standard deviation |
| SFT | Subcutaneous fat thickness |
| SPSS | Statistical package for social sciences |
| USG | Ultrasonography |
| VDR | Vitamin D receptors |
References
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2019, 10, 861. [Google Scholar] [CrossRef]
- Yu, P.C.; Hsu, C.C.; Lee, W.J.; Liang, C.K.; Chou, M.Y.; Lin, M.H.; Hsiao, F.Y.; Peng, L.N.; Chen, L.K. Muscle-to-fat ratio identifies functional impairments and cardiometabolic risk and predicts outcomes: Biomarkers of sarcopenic obesity. J. Cachexia Sarcopenia Muscle 2022, 13, 368–376. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: Overlap of clinical features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 213–219. [Google Scholar] [CrossRef]
- Wang, J.C.; Wu, W.T.; Chang, K.V.; Chen, L.R.; Chi, S.Y.; Kara, M.; Ozcakar, L. Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review. Life 2021, 12, 9. [Google Scholar] [CrossRef]
- Fried, A.M.; Coughlin, K.; Griffen, W.O. The sonographic fat/muscle ratio. Investig. Radiol. 1986, 21, 71–75. [Google Scholar] [CrossRef]
- Eglseer, D.; Traxler, M.; Schoufour, J.D.; Weijs, P.J.M.; Voortman, T.; Boirie, Y.; Cruz-Jentoft, A.J.; Reiter, L.; Bauer, S.; Consortium, S.-N. Nutritional and exercise interventions in individuals with sarcopenic obesity around retirement age: A systematic review and meta-analysis. Nutr. Rev. 2023, 81, 1077–1090. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- Agoncillo, M.; Yu, J.; Gunton, J.E. The Role of Vitamin D in Skeletal Muscle Repair and Regeneration in Animal Models and Humans: A Systematic Review. Nutrients 2023, 15, 4377. [Google Scholar] [CrossRef]
- Dogan, Y.; Kara, M.; Culha, M.A.; Ozcakar, L.; Kaymak, B. The relationship between vitamin D deficiency, body composition, and physical/cognitive functions. Arch. Osteoporos. 2022, 17, 66. [Google Scholar] [CrossRef]
- Granic, A.; Hill, T.R.; Davies, K.; Jagger, C.; Adamson, A.; Siervo, M.; Kirkwood, T.B.L.; Mathers, J.C.; Sayer, A.A. Vitamin D Status, Muscle Strength and Physical Performance Decline in Very Old Adults: A Prospective Study. Nutrients 2017, 9, 379. [Google Scholar] [CrossRef]
- Ladang, A.; Gendebien, A.-S.; Kovacs, S.; Demonceau, C.; Beaudart, C.; Peeters, S.; Alokail, M.S.; Al-Daghri, N.M.; Le Goff, C.; Reginster, J.-Y.; et al. Investigation of the Vitamin D Metabolite Ratio (VMR) as a Marker of Functional Vitamin D Deficiency: Findings from the SarcoPhAge Cohort. Nutrients 2024, 16, 3224. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Collins, S. Using middle upper arm circumference to assess severe adult malnutrition during famine. JAMA 1996, 276, 391–395. [Google Scholar] [CrossRef]
- Bonnefoy, M.; Jauffret, M.; Kostka, T.; Jusot, J.F. Usefulness of calf circumference measurement in assessing the nutritional state of hospitalized elderly people. Gerontology 2002, 48, 162–169. [Google Scholar] [CrossRef]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwee, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef]
- Galindo Martin, C.A.; Monares Zepeda, E.; Lescas Mendez, O.A. Bedside Ultrasound Measurement of Rectus Femoris: A Tutorial for the Nutrition Support Clinician. J. Nutr. Metab. 2017, 2017, 2767232. [Google Scholar] [CrossRef] [PubMed]
- Lambell, K.J.; Tierney, A.C.; Wang, J.C.; Nanjayya, V.; Forsyth, A.; Goh, G.S.; Vicendese, D.; Ridley, E.J.; Parry, S.M.; Mourtzakis, M.; et al. Comparison of Ultrasound-Derived Muscle Thickness with Computed Tomography Muscle Cross-Sectional Area on Admission to the Intensive Care Unit: A Pilot Cross-Sectional Study. J. Parenter. Enteral Nutr. 2021, 45, 136–145. [Google Scholar] [CrossRef]
- Rezaei, O.M.; Sharifi, F.; Moodi, M.; Zarban, A.; Tahergorabi, R.; Tahergorabi, Z. The Prevalence and Determinants of Vitamin D Status among Older Adults: Data from a Longitudinal Aging Study. Int. J. Prev. Med. 2023, 14, 27. [Google Scholar] [CrossRef]
- Boettger, S.F.; Angersbach, B.; Klimek, C.N.; Wanderley, A.L.M.; Shaibekov, A.; Sieske, L.; Wang, B.; Zuchowski, M.; Wirth, R.; Pourhassan, M. Prevalence and predictors of vitamin D-deficiency in frail older hospitalized patients. BMC Geriatr. 2018, 18, 219. [Google Scholar] [CrossRef]
- Kiebzak, G.M.; Moore, N.L.; Margolis, S.; Hollis, B.; Kevorkian, C.G. Vitamin D status of patients admitted to a hospital rehabilitation unit: Relationship to function and progress. Am. J. Phys. Med. Rehabil. 2007, 86, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Bucurica, S.; Prodan, I.; Pavalean, M.; Taubner, C.; Bucurica, A.; Socol, C.; Calin, R.; Ionita-Radu, F.; Jinga, M. Association of Vitamin D Deficiency and Insufficiency with Pathology in Hospitalized Patients. Diagnostics 2023, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.S.; Blumel, J.E.; Arteaga, E.; Aedo, S.; Tapia, V.; Araos, A.; Sciaraffia, C.; Castelo-Branco, C. Gender differences in the prevalence of vitamin D deficiency in a southern Latin American country: A pilot study. Climacteric 2020, 23, 410–416. [Google Scholar] [CrossRef]
- Houston, D.K.; Tooze, J.A.; Neiberg, R.H.; Hausman, D.B.; Johnson, M.A.; Cauley, J.A.; Bauer, D.C.; Cawthon, P.M.; Shea, M.K.; Schwartz, G.G.; et al. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: The Health, Aging, and Body Composition Study. Am. J. Epidemiol. 2012, 176, 1025–1034. [Google Scholar] [CrossRef]
- Ceglia, L.; Niramitmahapanya, S.; da Silva Morais, M.; Rivas, D.A.; Harris, S.S.; Bischoff-Ferrari, H.; Fielding, R.A.; Dawson-Hughes, B. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J. Clin. Endocrinol. Metab. 2013, 98, E1927–E1935. [Google Scholar] [CrossRef]
- Janssen, H.C.; Samson, M.M.; Verhaar, H.J. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: A randomized controlled trial on vitamin D and calcium supplementation. Aging Clin. Exp. Res. 2010, 22, 78–84. [Google Scholar] [CrossRef]
- Magalhães, P.M.; Cruz, S.P.d.; Carneiro, O.A.; Teixeira, M.T.; Ramalho, A. Vitamin D Inadequacy and Its Relation to Body Fat and Muscle Mass in Adult Women of Childbearing Age. Nutrients 2024, 16, 1267. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.C. Malnutrition and sarcopenia. Aging Clin. Exp. Res. 2019, 31, 793–798. [Google Scholar] [CrossRef]
- Ajabshir, S.; Asif, A.; Nayer, A. The effects of vitamin D on the renin-angiotensin system. J. Nephropathol. 2014, 3, 41–43. [Google Scholar] [CrossRef]
- Neagu, M.; Neagu, A. A Decade of Progress in Ultrasound Assessments of Subcutaneous and Total Body Fat: A Scoping Review. Life 2025, 15, 236. [Google Scholar] [CrossRef]
- Durak, A.; Binay Safer, V.; Catikkas, N.M. The relationship between pressure injuries and ultrasonographically measured rectus femoris muscle thickness. J. Tissue Viability 2024, 33, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Miazek, K.; Selmi, A.; Balcerczyk, A.; Sliwinska, A. The Action of Vitamin D in Adipose Tissue: Is There the Link between Vitamin D Deficiency and Adipose Tissue-Related Metabolic Disorders? Int. J. Mol. Sci. 2022, 23, 956. [Google Scholar] [CrossRef] [PubMed]
- Smith, G. Step away from stepwise. J. Big Data 2018, 5, 32. [Google Scholar] [CrossRef]

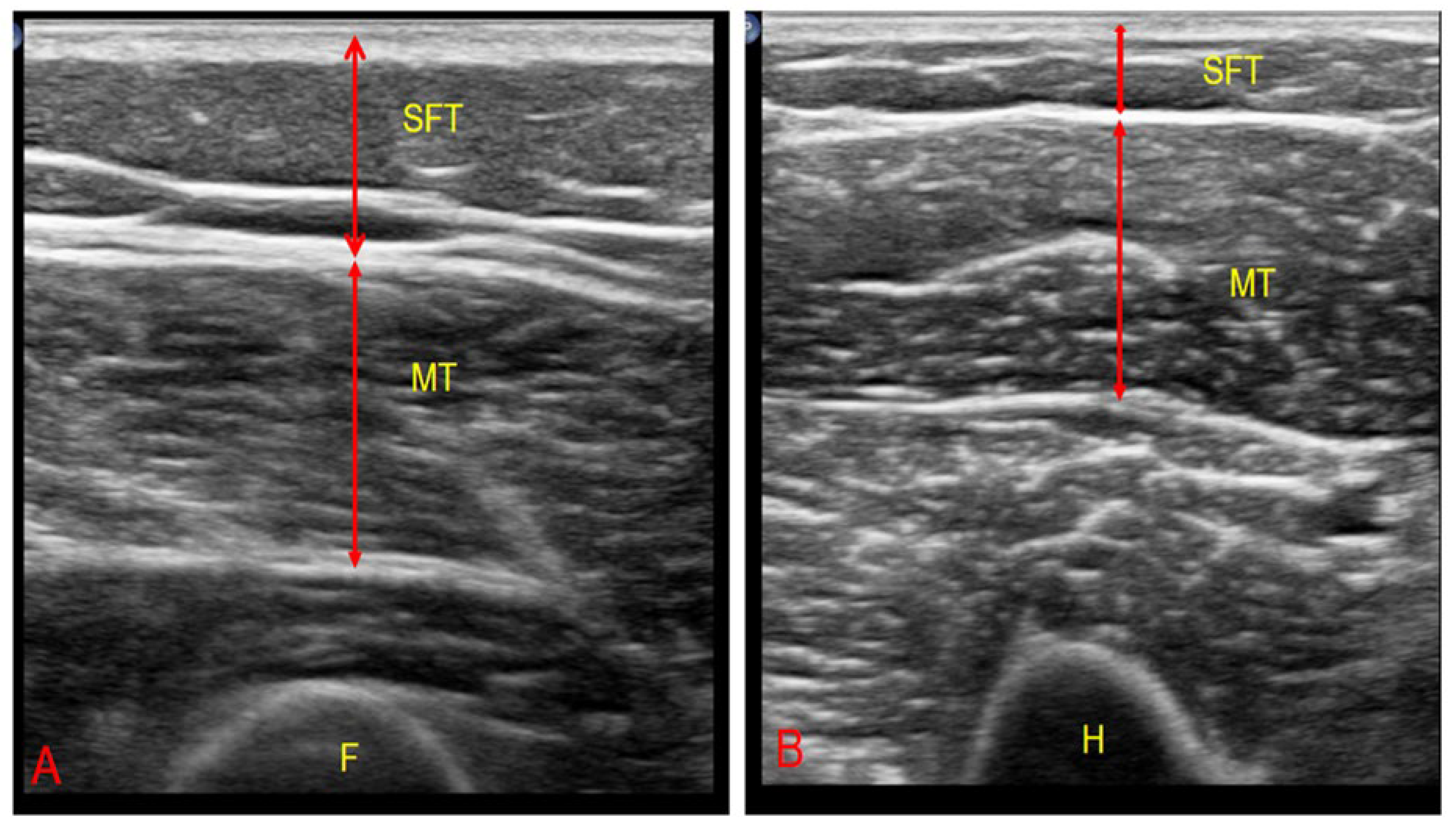
| Patients (n = 187) | Mean ± SD a | Number (%) | |
|---|---|---|---|
| Median (Min-Max) b | |||
| Age a | 75.1 ± 14.4 | ||
| Sex (n, %) | female | 104 (55.6%) | |
| male | 83 (44.4%) | ||
| ADLs (n,%) | independent | 13 (7%) | |
| dependent | 174 (93%) | ||
| DM (n, %) | no | 130 (69.5%) | |
| yes | 57 (30.5%) | ||
| Cancer (n, %) | no | 137 (73.3%) | |
| yes | 50 (26.7%) | ||
| CVO (n, %) | no | 129 (69%) | |
| yes | 58 (31%) | ||
| Dementia (n, %) | no | 121 (64.7%) | |
| yes | 66 (35.3%) | ||
| HT (n, %) | no | 88 (47.1%) | |
| yes | 99 (52.9%) | ||
| BMI (kg/m2) b | 22.1 (15.6–44.4) | ||
| CC (cm) b | 28 (13–45) | ||
| MUAC (cm) b | 24 (13–46) | ||
| 25-0H vitamin D (nmol/L) b | 13 (3–58) | ||
| Ultrasonography measurments | |||
| RF-SFT (cm) b | 0.66 (0.1–2.6) | ||
| RF-MT (cm) b | 0.56 (0.2–1.5) | ||
| RF-MT/SFT b | 0.89 (0.2–3.0) | ||
| RF-CSA (cm2) b | 1.57 (0.3–4.7) | ||
| BB-SFT (cm) b | 0.3 (0.1–1.3) | ||
| BB-MT (cm) b | 0.9 (0.4–2) | ||
| BB-MT/SFT b | 3.0 (0.86–8.0) | ||
| BB-CSA (cm2)b | 2.6 (0.8–6.7) |
| Vitamin D | ||||
|---|---|---|---|---|
| Control Group (n = 60) | Deficient Group (n = 127) | p | ||
| Age a | 76 ± 13.7 | 74.7 ± 14.9 | 0.558 | |
| Sex (n, %) | female | 40 (66.7%) | 64 (50.4%) | 0.037 χ2 |
| male | 20 (33.3%) | 63 (49.6%) | ||
| ADLs (n, %) | independent | 10 (16.7%) | 3 (2.4%) | <0.001 m |
| dependent | 50 (83.3%) | 124 (97.6%) | ||
| DM (n, %) | no | 38 (63.3%) | 92 (72.4%) | 0.207 |
| yes | 22 (36.7%) | 35 (27.6%) | ||
| Cancer (n, %) | no | 47 (78.3%) | 90 (70.9%) | 0.218 |
| yes | 13 (21.7%) | 37 (29.1%) | ||
| CVO (n, %) | no | 44 (73.3%) | 85 (66.9%) | 0.377 |
| yes | 16 (26.7%) | 42 (33.1%) | ||
| Dementia (n, %) | no | 39 (65%) | 82 (64.6%) | 0.954 |
| yes | 21 (35%) | 45 (35.4%) | ||
| HT (n, %) | no | 22 (36.7%) | 66 (52%) | 0.05 χ2 |
| yes | 38 (63.3%) | 61 (48%) | ||
| BMI (kg/m2) b | 23.5 (17–38.1) | 22 (15.6–44.4) | 0.02 m | |
| CC (cm) b | 29 (13–45) | 27 (18–39) | 0.006 m | |
| MUAC (cm) b | 26 (13–46) | 24 (15–40) | 0.057 | |
| 25-OH vit D (nmol/L) b | 61.2 (50–145) | 22.5 (7.5–47.5) | <0.001 m | |
| Ultrasonography measurements | ||||
| RF-SFT (cm) b | 0.84 (0.1–2.6) | 0.59 (0.1–2) | <0.001 m | |
| RF-MT (cm) b | 0.65 (0.2–1.5) | 0.53 (0.2–1.5) | <0.001 m | |
| RF-MT/SFT b | 0.810 (0.25–1.77) | 0.920 (0.22–3.0) | 0.049 m | |
| RF-CSA (cm2) b | 1.89 (0.3–4.5) | 1.45 (0.4–4.7) | <0.001 m | |
| BB-SFT (cm) b | 0.33 (0.1–1.3) | 0.3 (0.1–1) | 0.210 | |
| BB-MT (cm) b | 0.65 (0.2–1.5) | 0.5 (0.2–1.5) | 0.009 m | |
| BB-MT/BB-SFT | 3.07 (0.86–7.94) | 3.06 (0.86–8.03) | 0.968 | |
| BB-CSA (cm2) b | 3.05 (1.4–5.8) | 2.6 (0.8–6.7) | 0.002 m | |
| Univariable | Multivariable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | OR | %95 CI | p | β | OR | %95 CI | p | |||||
| Sex | 0.677 | 1.96 | 1.04 | - | 3.73 | 0.038 * | - | - | - | - | ||
| Age | −0.006 | 0.99 | 0.972 | - | 1.02 | 0.57 | - | - | - | - | ||
| ADL dependency | 2.122 | 8.26 | 2.183 | - | 31.3 | 0.002 * | 2.182 | 8.86 | 2.28 | - | 34.43 | 0.002 * |
| HT | −0.625 | 0.53 | 0.285 | - | 1.01 | 0.052 | - | - | - | - | ||
| RF-MT/SFT | 0.920 | 2.51 | 1.179 | - | 5.344 | 0.017 * | 0.994 | 2.70 | 1.21 | - | 6.03 | 0.015 * |
| BB-MT | −1.307 | 0.27 | 0.094 | - | 0.779 | 0.015 * | - | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, A.; Safer, U. Association of Vitamin D Deficiency with Local Muscle–Fat Ratio in Geriatric Palliative Care Patients: An Ultrasonographic Study. Healthcare 2025, 13, 2188. https://doi.org/10.3390/healthcare13172188
Durak A, Safer U. Association of Vitamin D Deficiency with Local Muscle–Fat Ratio in Geriatric Palliative Care Patients: An Ultrasonographic Study. Healthcare. 2025; 13(17):2188. https://doi.org/10.3390/healthcare13172188
Chicago/Turabian StyleDurak, Ayfer, and Umut Safer. 2025. "Association of Vitamin D Deficiency with Local Muscle–Fat Ratio in Geriatric Palliative Care Patients: An Ultrasonographic Study" Healthcare 13, no. 17: 2188. https://doi.org/10.3390/healthcare13172188
APA StyleDurak, A., & Safer, U. (2025). Association of Vitamin D Deficiency with Local Muscle–Fat Ratio in Geriatric Palliative Care Patients: An Ultrasonographic Study. Healthcare, 13(17), 2188. https://doi.org/10.3390/healthcare13172188






