Frequency, Grades of Toxicity, and Predictors of Hepatotoxicity and Acute Kidney Injury with Remdesivir in COVID-19 Patients: A Multicenter Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Included and Excluded Patients
2.3. Outcomes
2.4. Data Collection
2.5. Statistical Analysis
2.6. Power Calculation for Sample Size
2.7. Definitions
- Hepatotoxicity: elevation of AST or ALT more than 3 times the upper limit of normal if the baseline was normal, or 1.5–3.0× if the baseline was abnormal; elevation of bilirubin more than 1.5× the upper limit of normal if the baseline was normal, or more than 1.0–1.5× if the baseline was abnormal [18].
- Acute kidney injury: Increase in serum creatinine (Scr) by ≥0.3 mg/dL (≥26.5 µmol/L) within 48 h; or increase in SCr to ≥1.5 times baseline occurring within 7 days; or Urine volume of <0.5 mL/kg/h for 6 h [19].
- Grade 1 toxicity: Asymptomatic or mild symptoms without the need for intervention [18].
- Grade 2 toxicity: Moderate symptoms that need local or noninvasive intervention [18].
- Grade 3 toxicity: Severe or significant but not life-threatening, which warrants hospitalization or prolongation of the hospitalization course [18].
- Grade 4 toxicity: Results in life-threatening implications that warrant urgent intervention [18].
- Grade 5 toxicity: death related to the adverse drug reaction (ADR) [18].
3. Results
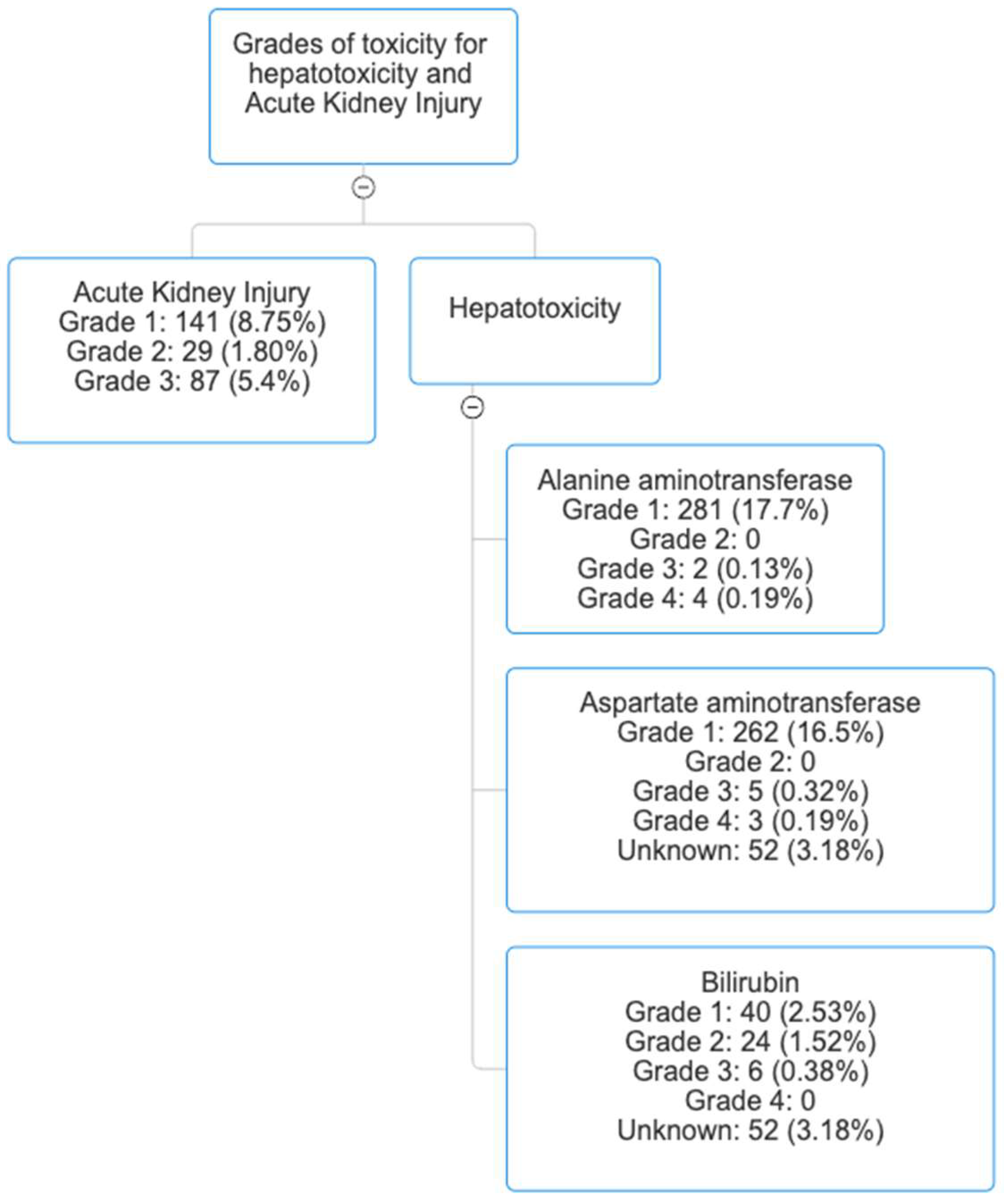
3.1. The Frequency and Grades of Remdesivir-Associated Hepatotoxicity
3.2. Predictors of Remdesivir-Associated Hepatotoxicity
3.3. The Frequency and Grades of Remdesivir-Associated Acute Kidney Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Director-General’s Opening Remarks at the Media Briefing on COVID-19. 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 30 June 2025).
- Centers for Disease Control and Prevention. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 30 June 2025).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) In Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Gilead. Remdesivir. Available online: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf (accessed on 23 August 2022).
- Gulick, R.M.; Pau, A.K.; Daar, E.; Evans, L.; Gandhi, R.T.; Tebas, P.; Ridzon, R.; Masur, H.; Lane, H.C.; Panel, N.C.-T.G.; et al. National Institutes of Health COVID-19 Treatment Guidelines Panel: Perspectives and Lessons Learned. Ann. Intern. Med. 2024, 177, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Alsowaida, Y.S.; Shehadeh, F.; Kalligeros, M.; Mylonakis, E. Incidence and potential risk factors for remdesivir-associated bradycardia in hospitalized patients with COVID-19: A retrospective cohort study. Front. Pharmacol. 2023, 14, 1106044. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, B.; Ma, J.; Zhang, S. Safety profile of the antiviral drug remdesivir: An update. Biomed. Pharmacother. 2020, 130, 110532. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Lankala, C.R.; Kalyankar, P.; Ishak, A.; Mekhail, M.; Sestacovschi, C.; Kima, E. An Updated Systematic Review on Remdesivir’s Safety and Efficacy in Patients Afflicted With COVID-19. Cureus 2023, 15, e43060. [Google Scholar] [CrossRef]
- Ackley, T.W.; McManus, D.; Topal, J.E.; Cicali, B.; Shah, S. A Valid Warning or Clinical Lore: An Evaluation of Safety Outcomes of Remdesivir in Patients with Impaired Renal Function from a Multicenter Matched Cohort. Antimicrob. Agents Chemother. 2021, 65, e02290-20. [Google Scholar] [CrossRef]
- Kim, M.S.; Jung, S.Y.; Lee, S.W.; Li, H.; Koyanagi, A.; Kronbichler, A.; Dragioti, E.; Tizaoui, K.; Wasuwanich, P.; Hong, S.H.; et al. Hepatobiliary Adverse Drug Reactions Associated With Remdesivir: The WHO International Pharmacovigilance Study. Clin. Gastroenterol. Hepatol. 2021, 19, 1970–1972.e3. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Gaggl, M.; Kinlaw, A.C.; Gou, Z.; Xu, Y.; Wei, J.; Wang, T. Remdesivir for COVID-19 and acute kidney injury: Disproportionality analysis of data from the U.S. Food and Drug Administration Adverse Event Reporting System. Int. J. Clin. Pharm. 2023, 45, 509–514. [Google Scholar] [CrossRef]
- Shah, S.; Ackley, T.W.; Topal, J.E. Renal and Hepatic Toxicity Analysis of Remdesivir Formulations: Does What Is on the Inside Really Count? Antimicrob. Agents Chemother. 2021, 65, e0104521. [Google Scholar] [CrossRef]
- Leegwater, E.; Strik, A.; Wilms, E.B.; Bosma, L.B.E.; Burger, D.M.; Ottens, T.H.; van Nieuwkoop, C. Drug-induced Liver Injury in a Patient With Coronavirus Disease 2019: Potential Interaction of Remdesivir With P-Glycoprotein Inhibitors. Clin. Infect. Dis. 2021, 72, 1256–1258. [Google Scholar] [CrossRef]
- Zampino, R.; Mele, F.; Florio, L.L.; Bertolino, L.; Andini, R.; Galdo, M.; De Rosa, R.; Corcione, A.; Durante-Mangoni, E. Liver injury in remdesivir-treated COVID-19 patients. Hepatol. Int. 2020, 14, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Reeves, A.A.; Hopefl, R.; Bejusca, R. ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential. Pharmaceuticals 2021, 14, 655. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Kebriaei, R.; Dresser, L.D. Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19. Pharmacotherapy 2020, 40, 659–671. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 21 October 2021).
- KDIGO. AKI. Available online: https://kdigo.org/guidelines/acute-kidney-injury/ (accessed on 30 June 2025).
- Farinde, A. Lab Values, Normal Adult. Available online: https://emedicine.medscape.com/article/2172316-overview (accessed on 30 June 2025).
- Wong, C.K.H.; Au, I.C.H.; Cheng, W.Y.; Man, K.K.C.; Lau, K.T.K.; Mak, L.Y.; Lui, S.L.; Chung, M.S.H.; Xiong, X.; Lau, E.H.Y.; et al. Remdesivir use and risks of acute kidney injury and acute liver injury among patients hospitalised with COVID-19: A self-controlled case series study. Aliment. Pharmacol. Ther. 2022, 56, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Jinda, T.; Mizuno, S.; Tatami, S.; Kasai, M.; Ishida, T. Risk factors for liver enzyme elevation with remdesivir use in the treatment of paediatric COVID-19. J. Paediatr. Child. Health 2024, 60, 299–302. [Google Scholar] [CrossRef]
- Seyedalipour, F.; Alipour, S.; Mehdinezhad, H.; Akrami, R.; Shirafkan, H. Incidence of Elevated Liver Enzyme Levels in Patients Receiving Remdesivir and Its Effective Factors. Middle East J. Dig. Dis. 2024, 16, 109–113. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, K.J.; Yang, H.R.; Chang, J.Y.; Moon, J.S.; Khang, Y.H.; Ko, J.S. Prevalence and risk factors of elevated alanine aminotransferase among Korean adolescents: 2001–2014. BMC Public Health 2018, 18, 617. [Google Scholar] [CrossRef]
- Cohen, E.B.; Patwardhan, M.; Raheja, R.; Alpers, D.H.; Andrade, R.J.; Avigan, M.I.; Lewis, J.H.; Rockey, D.C.; Chui, F.; Iacob, A.M.; et al. Correction to: Drug-Induced Liver Injury in the Elderly: Consensus Statements and Recommendations from the IQ-DILI Initiative. Drug Saf. 2024, 47, 513. [Google Scholar] [CrossRef]
- Huang, C.Y.; You, Y.S.; Lai, J.M.; Lin, C.L.; Hsu, H.Y.; Hsieh, Y.W. The Association Between Antidepressant Use and Drug-Induced Liver Injury: A Nationwide, Population-Based Case-Control Study in Taiwan. Drugs Real World Outcomes 2024, 11, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Todorovic Vukotic, N.; Dordevic, J.; Pejic, S.; Dordevic, N.; Pajovic, S.B. Antidepressants- and antipsychotics-induced hepatotoxicity. Arch. Toxicol. 2021, 95, 767–789. [Google Scholar] [CrossRef]
- Amacher, D.E. Female gender as a susceptibility factor for drug-induced liver injury. Hum. Exp. Toxicol. 2014, 33, 928–939. [Google Scholar] [CrossRef]
| Total (N = 1635; 100.0%) | Hepatotoxicity (N = 337; 20.6%) | Normal Liver Function (N = 1298; 79.4%) | p-Value | |
|---|---|---|---|---|
| Patient age, years, mean (SD) | 67.098 (16.184) | 63.270 (15.513) | 68.092 (16.213) | <0.001 |
| Patient age groups | ||||
| 94 (5.7%) 579 (35.4%) 962 (58.8%) | 26 (7.7%) 145 (43.0%) 166 (49.3%) | 68 (5.2%) 434 (33.4%) 796 (61.3%) | <0.001 |
| Patient Sex | ||||
| 790 (48.3%) 845 (51.7%) | 118 (35.0%) 219 (65.0%) | 672 (51.8%) 626 (48.2%) | <0.001 |
| Patient race | ||||
| 295 (18.0%) 155 (9.5%) 1069 (65.4%) 116 (7.1%) | 64 (19.0%) 33 (9.8%) 209 (62.0%) 31 (9.2%) | 231 (17.8%) 122 (9.4%) 860 (66.3%) 85 (6.5%) | 0.303 |
| Non-invasive Positive Pressure Ventilation | 581 (35.5%) | 134 (39.8%) | 447 (34.4%) | 0.069 |
| ICU | 299 (18.3%) | 78 (23.1%) | 221 (17.0%) | 0.010 |
| Ventilator | 114 (7.0%) | 46 (13.6%) | 68 (5.2%) | <0.001 |
| Comorbidities | ||||
| Coronary heart disease | 414 (25.3%) | 58 (17.2%) | 356 (27.4%) | <0.001 |
| Congestive heart failure | 189 (11.6%) | 21 (6.2%) | 168 (12.9%) | <0.001 |
| Cardiac arrhythmias | 300 (18.3%) | 41 (12.2%) | 259 (20.0%) | <0.001 |
| Valvular disease | 104 (6.4%) | 19 (5.6%) | 85 (6.5%) | 0.542 |
| Pulmonary circulation disorders | 83 (5.1%) | 11 (3.3%) | 72 (5.5%) | 0.089 |
| Peripheral vascular disorders | 126 (7.7%) | 21 (6.2%) | 105 (8.1%) | 0.255 |
| Chronic pulmonary disease | 379 (23.2%) | 56 (16.6%) | 323 (24.9%) | 0.001 |
| Hypertension | 982 (60.1%) | 184 (54.6%) | 798 (61.5%) | 0.022 |
| Diabetes | 509 (31.1%) | 85 (25.2%) | 424 (32.7%) | 0.009 |
| Hypothyroidism | 147 (9.0%) | 25 (7.4%) | 122 (9.4%) | 0.257 |
| Obesity | 450 (27.5%) | 79 (23.4%) | 371 (28.6%) | 0.060 |
| Coagulopathy | 78 (4.8%) | 14 (4.2%) | 64 (4.9%) | 0.551 |
| Deficiency anemia | 61 (3.7%) | 8 (2.4%) | 53 (4.1%) | 0.140 |
| Renal failure | 171 (10.5%) | 23 (6.8%) | 148 (11.4%) | 0.014 |
| Liver disease | 64 (3.9%) | 16 (4.7%) | 48 (3.7%) | 0.376 |
| Peptic ulcer disease | 22 (1.3%) | 1 (0.3%) | 21 (1.6%) | 0.061 |
| Rheumatoid arthritis | 71 (4.3%) | 11 (3.3%) | 60 (4.6%) | 0.276 |
| HIV | 8 (0.5%) | 3 (0.9%) | 5 (0.4%) | 0.237 |
| Lymphoma | 26 (1.6%) | 4 (1.2%) | 22 (1.7%) | 0.507 |
| Metastatic cancer | 55 (3.4%) | 8 (2.4%) | 47 (3.6%) | 0.258 |
| Solid tumor | 171 (10.5%) | 26 (7.7%) | 145 (11.2%) | 0.065 |
| Paralysis * | 6 (0.4%) | 3 (0.9%) | 3 (0.2%) | 0.075 |
| Neurological disorders | 135 (8.3%) | 20 (5.9%) | 115 (8.9%) | 0.082 |
| Depression | 297 (18.2%) | 46 (13.6%) | 251 (19.3%) | 0.016 |
| Alcohol abuse | 35 (2.1%) | 8 (2.4%) | 27 (2.1%) | 0.740 |
| Drug abuse | 27 (1.7%) | 4 (1.2%) | 23 (1.8%) | 0.453 |
| Laboratory parameters | ||||
| ALT in unit/Liter, mean (SD) $ | 6.039 (4.255) | 7.189 (5.657) | 5.743 (3.759) | <0.001 |
| AST in unit/Liter, mean (SD) # | 6.064 (4.264) | 7.189 (5.652) | 5.775 (3.775) | <0.001 |
| Bilirubin in micromole/Liter, mean (SD) ^ | 5.991 (4.177) | 7.120 (5.578) | 5.700 (3.679) | <0.001 |
| Creatinine in micromole/Liter, mean (SD) & | 9.623 (11.096) | 12.231 (13.957) | 8.951 (10.130) | <0.001 |
| LOS, mean (SD) | 8.390 (8.476) | 10.395 (10.515) | 7.869 (7.782) | <0.001 |
| Fluid and electrolyte abnormalities | 209 (12.8%) | 27 (8.0%) | 182 (14.0%) | 0.003 |
| COVID-19 therapeutics | ||||
| Remdesivir duration, days, mean (SD) | 4.314 (1.405) | 4.318 (1.493) | 4.314 (1.382) | 0.963 |
| Dexamethasone use | 1610 (98.5%) | 333 (98.8%) | 1277 (98.4%) | 0.566 |
| Tocilizumab | 27 (1.7%) | 11 (3.3%) | 16 (1.2%) | 0.009 |
| Other concurrent medications | ||||
| Mycobacterium tuberculosis drugs | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | 0.612 |
| Antiarrhythmic drugs | 47 (3.4%) | 18 (6.4%) | 29 (2.7%) | 0.002 |
| Antifungal agents | 38 (2.8%) | 10 (3.6%) | 28 (2.6%) | 0.364 |
| Statins dyslipidemia drugs | 818 (59.8%) | 154 (55.0%) | 664 (61.0%) | 0.069 |
| Macrolide antibiotics | 224 (16.4%) | 60 (21.4%) | 164 (15.1%) | 0.010 |
| Quinolone antibiotics | 77 (5.6%) | 20 (7.1%) | 57 (5.2%) | 0.216 |
| Selective serotonin reuptake inhibitors | 300 (21.9%) | 42 (15.0%) | 258 (23.7%) | 0.002 |
| Sympathetic agents | 39 (2.8%) | 18 (6.4%) | 21 (1.9%) | <0.001 |
| Tricyclic antidepressants | 42 (3.1%) | 6 (2.1%) | 36 (3.3%) | 0.314 |
| Vancomycin | 280 (20.5%) | 74 (26.4%) | 206 (18.9%) | 0.005 |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Patient age | 1.02 | 1–1.04 | 0.02 |
| Male gender | 0.63 | 0.47–0.87 | 0.005 |
| Race | |||
| 1.07 0.86 0.77 | 0.7–1.62 0.5–1.45 0.42–1.38 | 0.7 0.57 0.38 |
| ICU admission | 1.9 | 0.9–4.04 | 0.09 |
| Length of hospital stay | 0.97 | 0.94–1.01 | 0.17 |
| Ventilator use | 0.24 | 0.06–0.86 | 0.02 |
| SSRIs use | 1.73 | 1.13–2.65 | 0.01 |
| Liver disease * | 0.68 | 0.33–1.38 | 0.28 |
| ALT | 0.84 | 0.43–1.64 | 0.61 |
| AST | 1.61 | 0.77–3.35 | 0.2 |
| Bilirubin | 0.7 | 0.43–1.12 | 0.13 |
| Serum creatinine | 1.01 | 0.98–1.05 | 0.2 |
| Duration of remdesivir and hepatotoxicity | 0.99 | 0.91–1.08 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsowaida, Y.S.; Alowais, S.A.; Alsowaida, D.; Alshammari, A.; Alshoumr, B.; Alshurtan, K.; Almunef, M.; Almangour, T.A.; Alshaya, O.A.; Bin Saleh, K. Frequency, Grades of Toxicity, and Predictors of Hepatotoxicity and Acute Kidney Injury with Remdesivir in COVID-19 Patients: A Multicenter Retrospective Cohort Study. Healthcare 2025, 13, 2143. https://doi.org/10.3390/healthcare13172143
Alsowaida YS, Alowais SA, Alsowaida D, Alshammari A, Alshoumr B, Alshurtan K, Almunef M, Almangour TA, Alshaya OA, Bin Saleh K. Frequency, Grades of Toxicity, and Predictors of Hepatotoxicity and Acute Kidney Injury with Remdesivir in COVID-19 Patients: A Multicenter Retrospective Cohort Study. Healthcare. 2025; 13(17):2143. https://doi.org/10.3390/healthcare13172143
Chicago/Turabian StyleAlsowaida, Yazed Saleh, Shuroug A. Alowais, Dalal Alsowaida, Alia Alshammari, Bader Alshoumr, Kareemah Alshurtan, Mohammed Almunef, Thamer A. Almangour, Omar A. Alshaya, and Khalid Bin Saleh. 2025. "Frequency, Grades of Toxicity, and Predictors of Hepatotoxicity and Acute Kidney Injury with Remdesivir in COVID-19 Patients: A Multicenter Retrospective Cohort Study" Healthcare 13, no. 17: 2143. https://doi.org/10.3390/healthcare13172143
APA StyleAlsowaida, Y. S., Alowais, S. A., Alsowaida, D., Alshammari, A., Alshoumr, B., Alshurtan, K., Almunef, M., Almangour, T. A., Alshaya, O. A., & Bin Saleh, K. (2025). Frequency, Grades of Toxicity, and Predictors of Hepatotoxicity and Acute Kidney Injury with Remdesivir in COVID-19 Patients: A Multicenter Retrospective Cohort Study. Healthcare, 13(17), 2143. https://doi.org/10.3390/healthcare13172143






