Effectiveness and Adherence of Standalone Digital Tobacco Cessation Modalities: A Systematic Review of Systematic Reviews
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
- Population (P): Current daily adult (≥18 years old) smokers of combustible tobacco, as referred to by the WHO [19];
- Intervention (I): Standalone individually administered digital tobacco cessation modalities (mobile text messaging, smartphone apps, Internet-based websites and programs, AI-based interventions) from all providers and setting types;
- Comparison (C): Standalone combined administered digital tobacco cessation modalities (combination of mobile text messaging and/or smartphone apps and/or Internet-based websites and programs and/or AI-based interventions) from all providers and setting types;
- Outcomes (O):
- -
- Primary outcome(s): Point prevalence abstinence (PPA) and/or continuous abstinence rates (CARs) at least ≥6 months from the start of the digital tobacco cessation intervention, biochemically verified (e.g., cotinine or carbon monoxide test) and/or self-reported, as referred to the Russell Standard and the Society for Research on Nicotine and Tobacco [20,21].
- If reported, the secondary outcome(s) extracted were:
- -
- Secondary outcome(s): Adherence, satisfaction, and acceptability to digital tobacco cessation modalities; medical (cardiovascular/pneumological/metabolic/psychological) and oral (periodontal/peri-implant/mucosal lesions) parameters before and after digital tobacco cessation modalities.
2.2. Search Strategy
2.3. Study Selection and Eligibility Criteria
2.4. Data Extraction and Collection
- Study features: First author, year, journal, included study’s number and design, meta-analysis or no meta-analysis, assessed quality, funding information (if any);
- Population features: Sample size (n.), mean and/or range age, gender ratio (male/female), comorbidities, smoked cigarettes per day, nicotine addiction severity, quit smoking motivation;
- Intervention and comparison features: Type and duration of digital tobacco cessation modalities;
- Outcome(s):
- -
- Primary outcome(s): PPA and/or CARs, smoked cigarettes per day, failure reasons (if any);
- -
- Secondary outcome(s): Adherence, satisfaction, and acceptability to digital tobacco cessation modalities; medical (cardiovascular/pneumological/metabolic/psychological) and oral (periodontal/peri-implant/mucosal lesions) parameters before and after digital tobacco cessation modalities.
2.5. Data Synthesis
- Evaluate long-term effectiveness (≥6 months) of the different standalone digital tobacco cessation modalities (mobile text messaging, smartphone apps, Internet-based websites and programs, AI-based interventions);
- Compare long-term effectiveness (≥6 months) of individual vs. combined standalone digital tobacco cessation modalities;
- Evaluate adherence/satisfaction/acceptability of the different standalone digital tobacco cessation modalities (mobile text messaging, smartphone apps, Internet-based websites and programs, AI-based interventions);
- Compare adherence/satisfaction/acceptability of the different standalone digital tobacco cessation modalities;
- Evaluate long-term effectiveness (≥6 months) of digital tobacco cessation modalities on medical (cardiovascular/pneumological/metabolic/psychological) and oral (periodontal/peri-implant/mucosal lesions) parameters before and after digital tobacco cessation modalities;
- Compare long-term effectiveness (≥6 months) of digital tobacco cessation modalities on medical (cardiovascular/pneumological/metabolic/psychological) and oral (periodontal/peri-implant/mucosal lesions) parameters before and after individual vs. combined different digital tobacco cessation modalities.
2.6. Quality Assessment and Overlap Management
3. Results
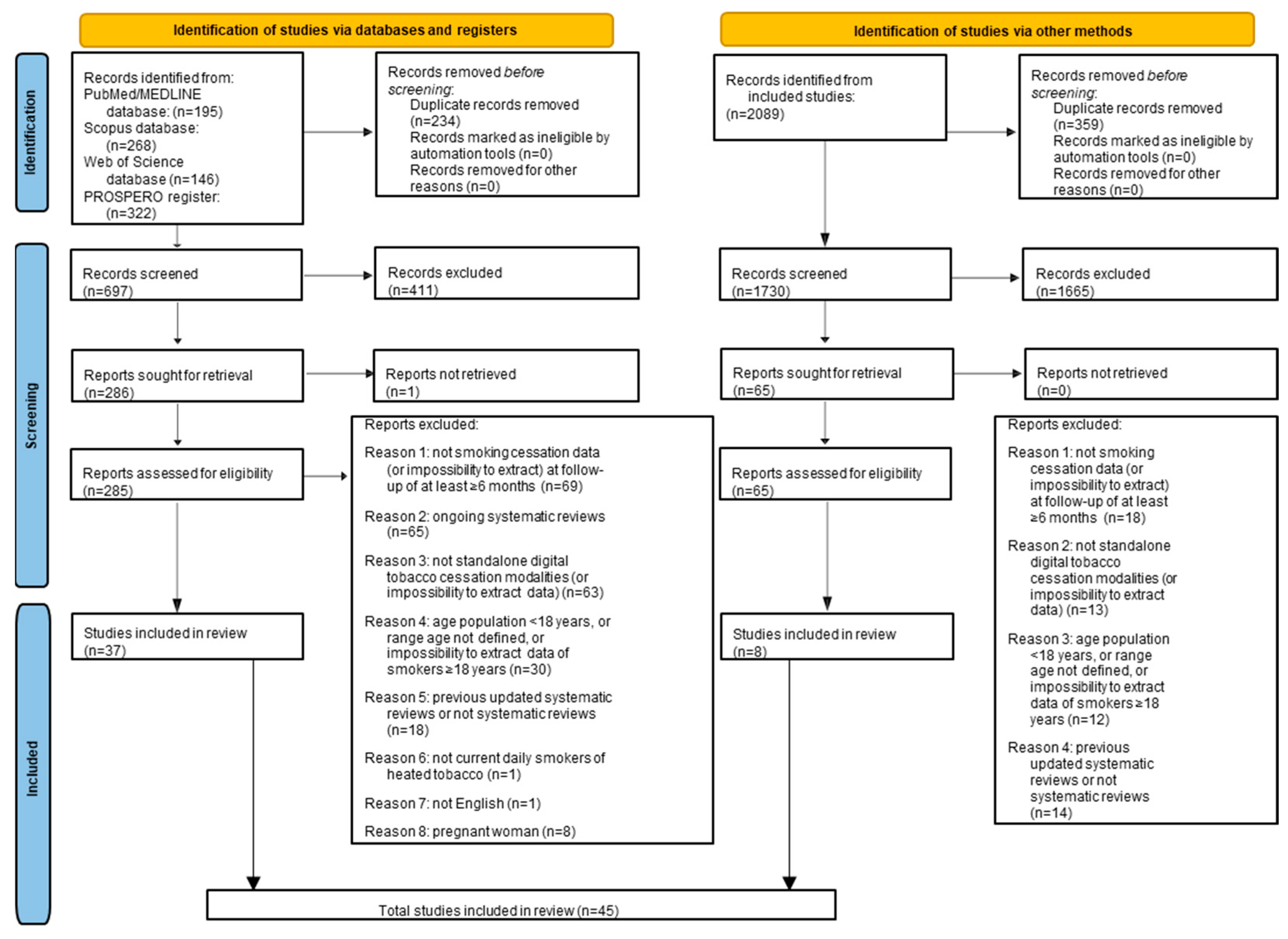
3.1. Study Selection
3.2. Study Characteristics and Qualitative Synthesis
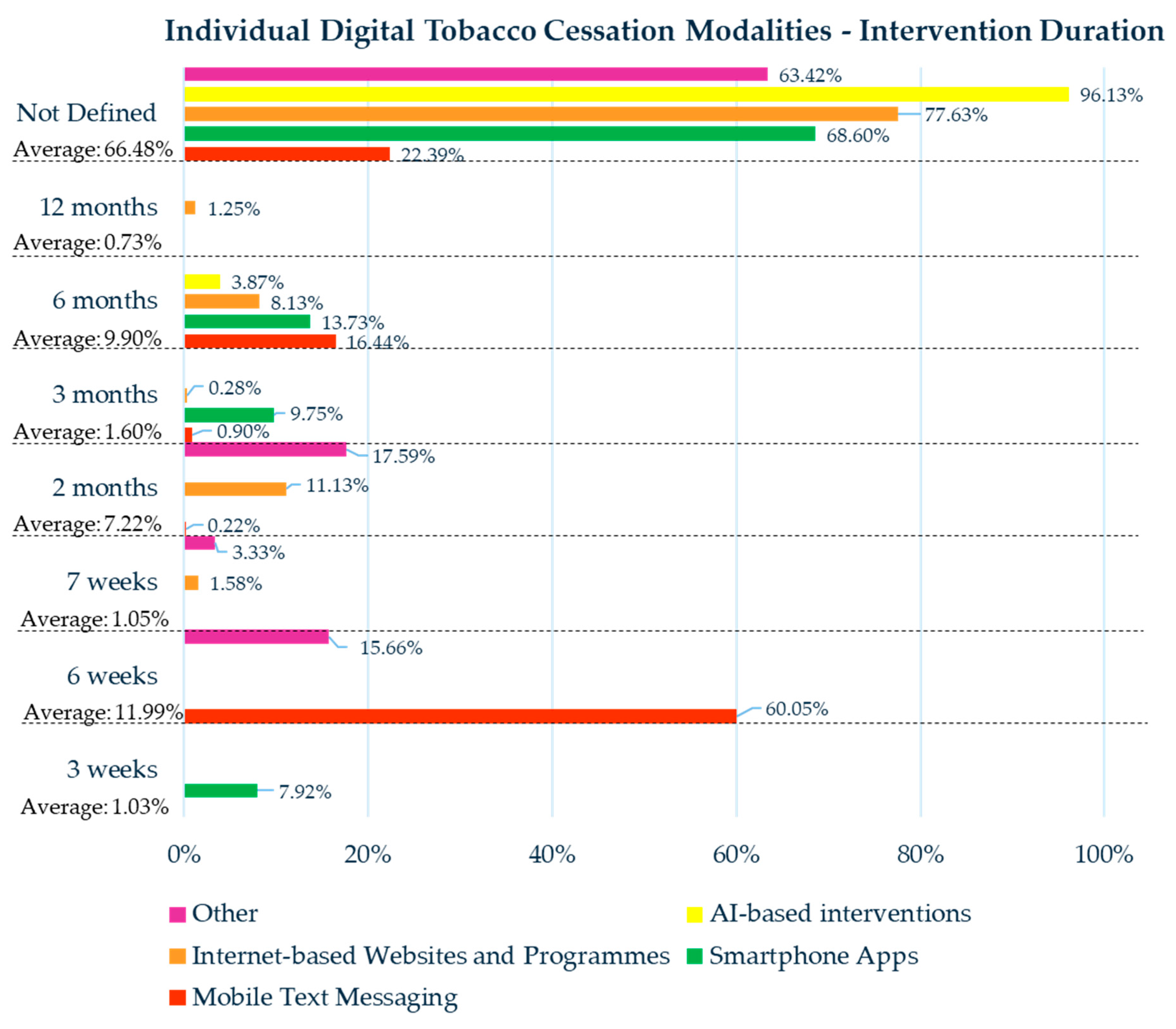
3.3. Individual Digital Tobacco Cessation Modalities
3.3.1. Mobile Text Messaging
3.3.2. Smartphone Apps
3.3.3. Internet-Based Websites and Programs
3.3.4. AI-Based Interventions
3.3.5. Other Digital Tobacco Modalities
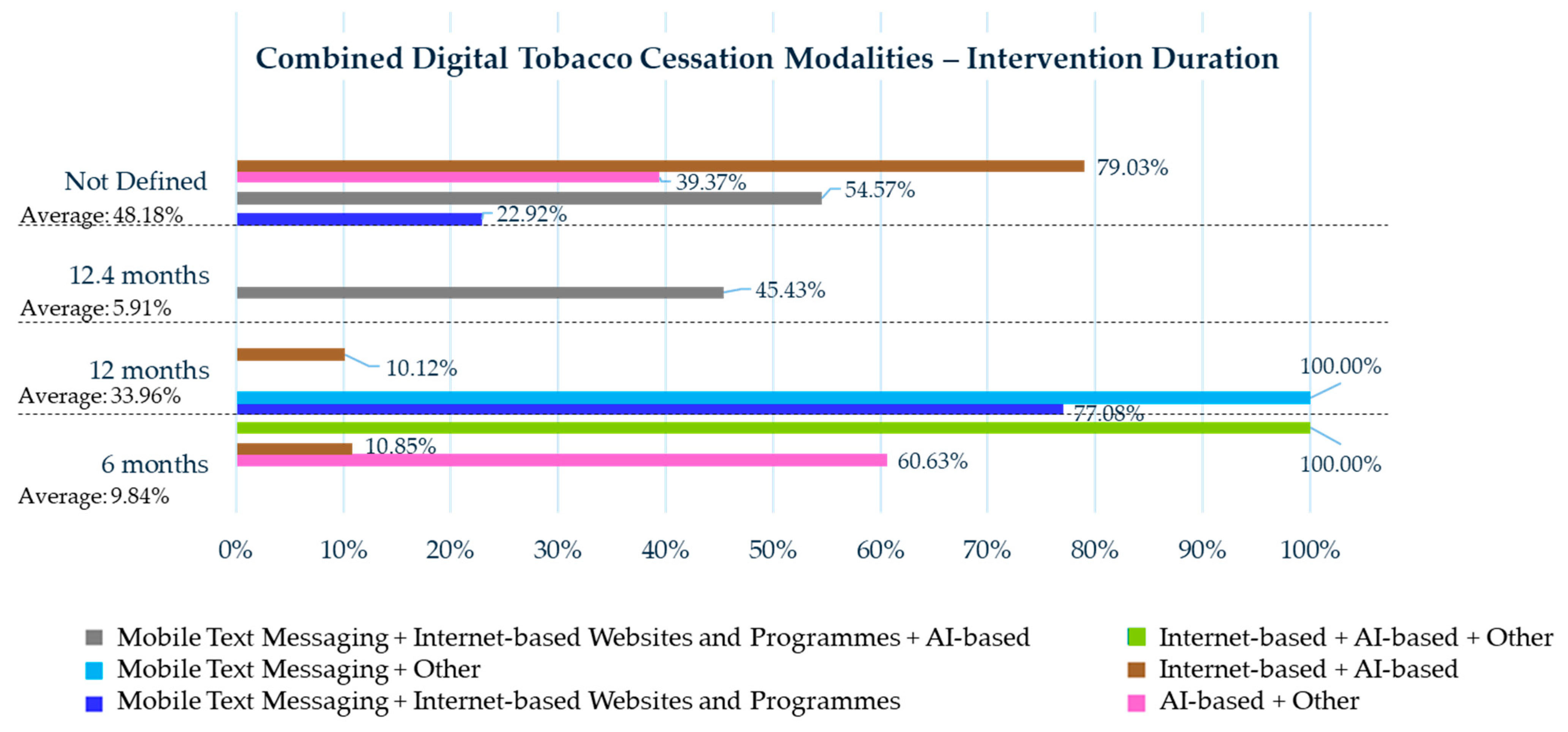
3.4. Combined Digital Tobacco Cessation Modalities
3.4.1. Mobile Text Messaging Plus Internet-Based Websites and Programs
3.4.2. Mobile Text Messaging Plus Other
3.4.3. Mobile Text Messaging Plus Internet-Based Websites and Programs Plus AI-Based Interventions
3.4.4. Smartphone App Plus Other
3.4.5. AI-Based Plus Other
3.4.6. Internet-Based Plus AI-Based
3.4.7. Internet-Based Plus Other
3.4.8. Internet-Based Plus AI-Based Plus Other
3.5. Overall: Individual and Combined Standalone Digital Tobacco Cessation Modalities
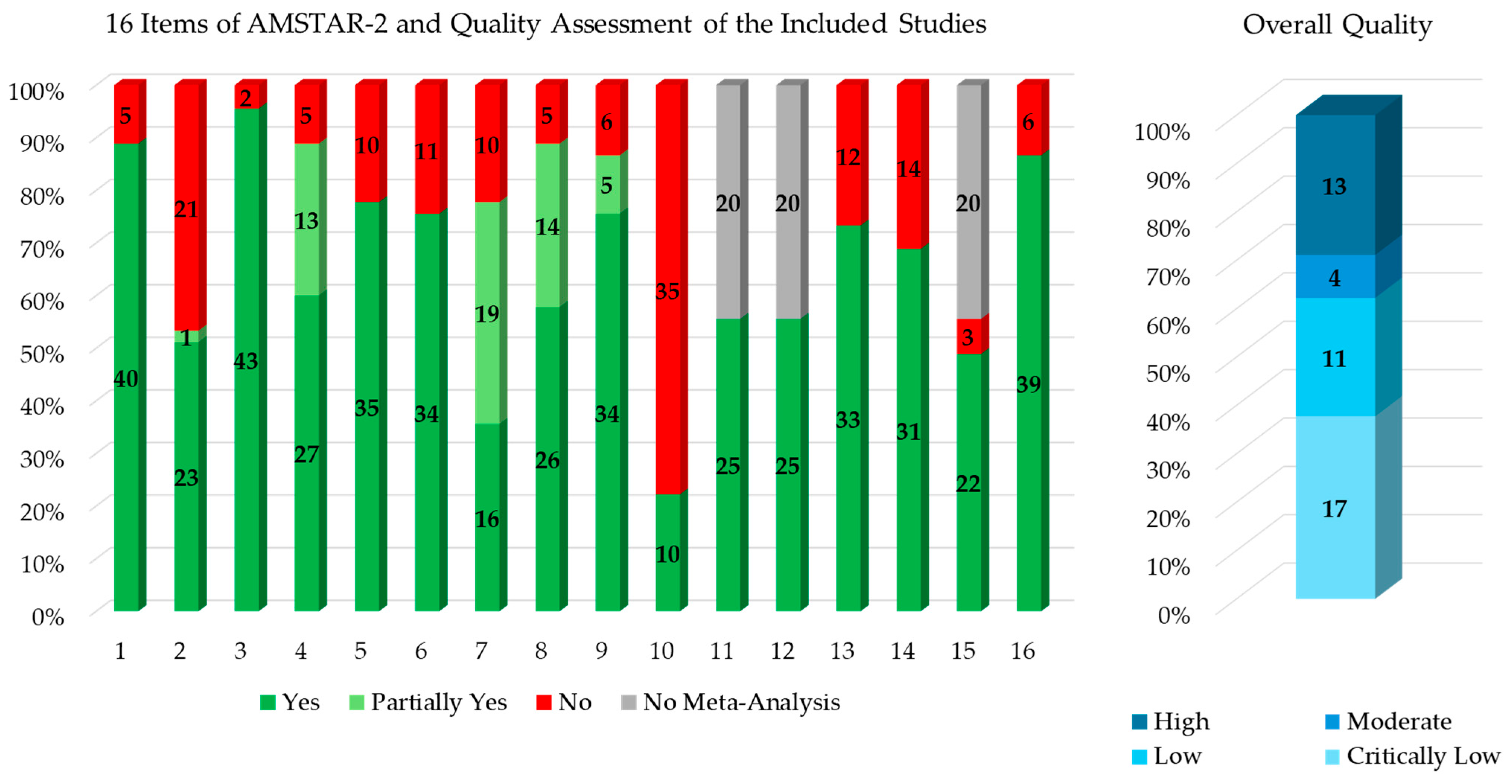
3.6. Quality Assessment and Overlap Management
4. Discussion
4.1. Effectiveness
4.1.1. Effectiveness of Individual Standalone Digital Tobacco Cessation Modalities
4.1.2. Effectiveness of Individual vs. Combined Standalone Digital Tobacco Cessation Modalities
4.2. Adherence
4.2.1. Adherence of Individual Standalone Digital Tobacco Cessation Modalities
4.2.2. Adherence of Individual vs. Combined Standalone Digital Tobacco Cessation Modalities
4.3. Relationship Between Effectiveness and Adherence of Digital Tobacco Cessation Modalities
4.4. Limitations, Unaddressed Knowledge Gaps, and Future Directions
- The lack of funding sources for the primary studies, which may undermine the assessment of potential conflicts of interest;
- The absence of a registered protocol, which raises concerns about potential reporting biases;
- The failure to discuss the heterogeneity observed in the results, which may potentially mislead the synthesis of the evidence.
- The clear explanations of the study design selection, which enhance transparency;
- The adherence to the PICO models and the establishment of a research question, which ensures a rigorous methodological approach to evidence synthesis and facilitates reproducibility;
- The clarification about any potential sources of conflict of interest, which contributes to the trustworthiness of the findings.
4.5. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 20 February 2025).
- West, R. The Clinical Significance of ‘Small’ Effects of Smoking Cessation Treatments. Addiction 2007, 102, 506–509. [Google Scholar] [CrossRef]
- Pisano, M.; Bramanti, A.; De Benedetto, G.; Martin Carreras-Presas, C.; Di Spirito, F. The Use of Audiovisual Distraction Tools in the Dental Setting for Pediatric Subjects with Special Healthcare Needs: A Review and Proposal of a Multi-Session Model for Behavioral Management. Children 2024, 11, 1077. [Google Scholar] [CrossRef] [PubMed]
- Akanbi, M.O.; Carroll, A.J.; Achenbach, C.; O’Dwyer, L.C.; Jordan, N.; Hitsman, B.; Bilaver, L.A.; McHugh, M.C.; Murphy, R. The Efficacy of Smoking Cessation Interventions in Low- and Middle-income Countries: A Systematic Review and Meta-analysis. Addiction 2019, 114, 620–635. [Google Scholar] [CrossRef]
- Tzelepis, F.; Paul, C.L.; Williams, C.M.; Gilligan, C.; Regan, T.; Daly, J.; Hodder, R.K.; Byrnes, E.; Byaruhanga, J.; McFadyen, T.; et al. Real-Time Video Counselling for Smoking Cessation. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef]
- Giansanti, D. The Future of Healthcare Is Digital: Unlocking the Potential of Mobile Health and E-Health Solutions. Healthcare 2025, 13, 802. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Clinical Treatment Guideline for Tobacco Cessation in Adults; WHO: Geneva, Switzerland, 2024; pp. 1–53. [Google Scholar]
- Di Spirito, F.; Di Palo, M.P.; Garofano, M.; Del Sorbo, R.; Allegretti, G.; Rizki, I.; Bartolomeo, M.; Giordano, M.; Amato, M.; Bramanti, A. Effectiveness and Adherence of Pharmacological vs. Non-Pharmacological Technology-Supported Smoking Cessation Interventions: An Umbrella Review. Healthcare 2025, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Lee, E. Effectiveness of Mobile Health Application Use to Improve Health Behavior Changes: A Systematic Review of Randomized Controlled Trials. Healthc. Inform. Res. 2018, 24, 207. [Google Scholar] [CrossRef] [PubMed]
- Raupach, T.; Brown, J.; Herbec, A.; Brose, L.; West, R. A Systematic Review of Studies Assessing the Association Between Adherence to Smoking Cessation Medication and Treatment Success. Addiction 2014, 109, 35–43. [Google Scholar] [CrossRef]
- Shiffman, S. Use of More Nicotine Lozenges Leads to Better Success in Quitting Smoking. Addiction 2007, 102, 809–814. [Google Scholar] [CrossRef]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef]
- Saracen, A. Cigarette Smoking and Respiratory System Diseases in Adolescents. Adv. Exp. Med. Biol. 2017, 944, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Burgess, S. Appraising the Causal Role of Smoking in Multiple Diseases: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies. EBioMedicine 2022, 82, 104154. [Google Scholar] [CrossRef]
- Zięba, S.; Błachnio-Zabielska, A.; Maciejczyk, M.; Pogodzińska, K.; Szuta, M.; Lo Giudice, G.; Lo Giudice, R.; Zalewska, A. Impact of Smoking on Salivary Lipid Profile and Oxidative Stress in Young Adults: A Comparative Analysis between Traditional Cigarettes, E-Cigarettes, and Heat-Not-Burn Products. Med. Sci. Monit. 2023, 30, e942507. [Google Scholar] [CrossRef]
- Goldenberg, M.; Danovitch, I.; IsHak, W.W. Quality of Life and Smoking. Am. J. Addict. 2014, 23, 540–562. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The Well-Built Clinical Question: A Key to Evidence-Based Decisions. ACP J. Club 1995, 123, A12-3. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO global report on trends in prevalence of tobacco use 2000–2030. Available online: https://iris.who.int/bitstream/handle/10665/375711/9789240088283-eng.pdf?sequence=1 (accessed on 18 November 2024).
- West, R.; Hajek, P.; Stead, L.; Stapleton, J. Outcome Criteria in Smoking Cessation Trials: Proposal for a Common Standard. Addiction 2005, 100, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Keely, J.; Niaura, R.; Ossip-Klein, D.; Richmond, R.; Swan, G. Measures of Abstinence in Clinical Trials: Issues and Recommendations. Nicotine Tob. Res. 2003, 5, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Elkins, M.R.; Moseley, A.M. Intention-to-Treat Analysis. J. Physiother. 2015, 61, 165–167. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Pieper, D.; Antoine, S.-L.; Mathes, T.; Neugebauer, E.A.M.; Eikermann, M. Systematic Review Finds Overlapping Reviews Were Not Mentioned in Every Other Overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef]
- Barroso-Hurtado, M.; Suárez-Castro, D.; Martínez-Vispo, C.; Becoña, E.; López-Durán, A. Smoking Cessation Apps: A Systematic Review of Format, Outcomes, and Features. Int. J. Environ. Res. Public Health 2021, 18, 11664. [Google Scholar] [CrossRef]
- Bendotti, H.; Lawler, S.; Chan, G.C.K.; Gartner, C.; Ireland, D.; Marshall, H.M. Conversational Artificial Intelligence Interventions to Support Smoking Cessation: A Systematic Review and Meta-Analysis. Digit. Health 2023, 9, 20552076231211634. [Google Scholar] [CrossRef]
- Boland, V.C.; Stockings, E.A.; Mattick, R.P.; McRobbie, H.; Brown, J.; Courtney, R.J. The Methodological Quality and Effectiveness of Technology-Based Smoking Cessation Interventions for Disadvantaged Groups: A Systematic Review and Meta-Analysis. Nicotine Tob. Res. 2018, 20, 276–285. [Google Scholar] [CrossRef]
- Byambasuren, O.; Greenwood, H.; Bakhit, M.; Atkins, T.; Clark, J.; Scott, A.M.; Glasziou, P. Comparison of Telephone and Video Telehealth Consultations: Systematic Review. J. Med. Internet Res. 2023, 25, e49942. [Google Scholar] [CrossRef] [PubMed]
- Byaruhanga, J.; Atorkey, P.; McLaughlin, M.; Brown, A.; Byrnes, E.; Paul, C.; Wiggers, J.; Tzelepis, F. Effectiveness of Individual Real-Time Video Counseling on Smoking, Nutrition, Alcohol, Physical Activity, and Obesity Health Risks: Systematic Review. J. Med. Internet Res. 2020, 22, e18621. [Google Scholar] [CrossRef]
- Cartujano-Barrera, F.; Rodríguez-Bolaños, R.; Arana-Chicas, E.; Allaham, F.; Sandoval, L.; Rubado, M.; Gallegos-Carrillo, K.; Colugnati, F.A.B.; Galil, A.G.; Mejia, R.M.; et al. Smoking Cessation Mobile Interventions in Latin America: A Systematic Review. Hisp. Health Care Int. 2022, 20, 122–132. [Google Scholar] [CrossRef]
- Chhabra, D.; Tushya; Penberthy, J.K.; Dang, S. Effectiveness of Remotely Delivered Mindfulness and Acceptance and Commitment Therapy-Based Smoking Cessation Programs: A Systematic Review. Curr. Psychol. 2024, 43, 8158–8172. [Google Scholar] [CrossRef]
- Cobos-Campos, R.; Sáez de Lafuente, A.; Apiñaniz, A.; Parraza, N.; Pérez Llanos, I.; Orive, G. Effectiveness of Mobile Applications to Quit Smoking: Systematic Review and Meta-Analysis. Tob. Prev. Cessat. 2020, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, L.M.; de Macêdo, Â.C.D.A.D.; Lanzieri, I.O.; de Oliveira Andrade, R.; Richter, K.P.; Leite, I.C.G. Promoting Cessation in Hospitalized Smoking Patients: A Systematic Review. Rev. Assoc. Med. Bras. 2020, 66, 849–860. [Google Scholar] [CrossRef]
- Eghdami, S.; Ahmadkhaniha, H.R.; Baradaran, H.R.; Hirbod-Mobarakeh, A. Ecological Momentary Interventions for Smoking Cessation: A Systematic Review and Meta-Analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2023, 58, 1431–1445. [Google Scholar] [CrossRef]
- Fang, Y.E.; Zhang, Z.; Wang, R.; Yang, B.; Chen, C.; Nisa, C.; Tong, X.; Yan, L.L. Effectiveness of EHealth Smoking Cessation Interventions: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2023, 25, e45111. [Google Scholar] [CrossRef] [PubMed]
- Gainsbury, S.; Blaszczynski, A. A Systematic Review of Internet-Based Therapy for the Treatment of Addictions. Clin. Psychol. Rev. 2011, 31, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, J.M.; Duran, C.; Slatore, C.G.; Wiener, R.S.; Kathuria, H. Combining Smoking Cessation Interventions with LDCT Lung Cancer Screening: A Systematic Review. Prev. Med. 2019, 121, 24–32. [Google Scholar] [CrossRef]
- Krishnan, N.; Gu, J.; Abroms, L.C. Mobile Phone-Based Messaging for Tobacco Cessation in Low and Middle-Income Countries: A Systematic Review. Addict. Behav. 2021, 113, 106676. [Google Scholar] [CrossRef]
- Li, S.; Qu, Z.; Li, Y.; Ma, X. Efficacy of E-Health Interventions for Smoking Cessation Management in Smokers: A Systematic Review and Meta-Analysis. eClinicalMedicine 2024, 68, 102412. [Google Scholar] [CrossRef]
- Liu, S.; Feng, W.; Chhatbar, P.Y.; Liu, Y.; Ji, X.; Ovbiagele, B. Mobile Health as a Viable Strategy to Enhance Stroke Risk Factor Control: A Systematic Review and Meta-Analysis. J. Neurol. Sci. 2017, 378, 140–145. [Google Scholar] [CrossRef]
- Luo, T.; Li, M.; Williams, D.; Phillippi, S.; Yu, Q.; Kantrow, S.; Kao, Y.; Celestin, M.; Lin, W.; Tseng, T. Using Social Media for Smoking Cessation Interventions: A Systematic Review. Perspect. Public Health 2021, 141, 50–63. [Google Scholar] [CrossRef]
- McCrabb, S.; Baker, A.L.; Attia, J.; Skelton, E.; Twyman, L.; Palazzi, K.; McCarter, K.; Ku, D.; Bonevski, B. Internet-Based Programs Incorporating Behavior Change Techniques Are Associated With Increased Smoking Cessation in the General Population: A Systematic Review and Meta-Analysis. Ann. Behav. Med. 2019, 53, 180–195. [Google Scholar] [CrossRef]
- Mersha, A.G.; Bryant, J.; Booth, K.; Watson, L.; Kennedy, M. The Effectiveness of Internet-Based Group Behavioural Interventions on Lifestyle Modifications: A Systematic Review. Prev. Med. 2024, 186, 108099. [Google Scholar] [CrossRef] [PubMed]
- Naslund, J.A.; Kim, S.J.; Aschbrenner, K.A.; McCulloch, L.J.; Brunette, M.F.; Dallery, J.; Bartels, S.J.; Marsch, L.A. Systematic Review of Social Media Interventions for Smoking Cessation. Addict. Behav. 2017, 73, 81–93. [Google Scholar] [CrossRef]
- Nguyen, A.; Eschiti, V.; Bui, T.C.; Nagykaldi, Z.; Dwyer, K. Mobile Health Interventions to Improve Health Behaviors and Healthcare Services among Vietnamese Individuals: A Systematic Review. Healthcare 2023, 11, 1225. [Google Scholar] [CrossRef]
- O’Logbon, J.; Wickersham, A.; Williamson, C.; Leightley, D. The Effectiveness of Digital Health Technologies for Reducing Substance Use among Young People: A Systematic Review & Meta-Analysis. J. Ment. Health 2024, 33, 645–673. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Zhou, Y.; Chen, C.; Chacko, T.; Mahoney, M.; Chang, Y.-P. Systematic Review: Interventions to Quit Tobacco Products for Young Adults. BMC Public Health 2023, 23, 1233. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, B.; Simmons, V.N.; Palmer, A.M.; Correa, J.B.; Brandon, T.H. Smoking Cessation Interventions within the Context of Low-Dose Computed Tomography Lung Cancer Screening: A Systematic Review. Lung Cancer 2016, 98, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ricker, A.B.; Manning, D.; Smith, K.E.; Warren, Y.E.; Matthews, B.D.; Reinke, C.E. Preoperative Intervention for Smoking Cessation: A Systematic Review. Am. J. Surg. 2024, 227, 175–182. [Google Scholar] [CrossRef]
- Saroj, S.K.; Bhardwaj, T. Non-Pharmacological Interventions for Tobacco Cessation: A Systematic Review of Existing Practices and Their Effectiveness. Monaldi Arch. Chest Dis. 2022, 92. [Google Scholar] [CrossRef]
- Sawyer, C.; McKeon, G.; Hassan, L.; Onyweaka, H.; Martinez Agulleiro, L.; Guinart, D.; Torous, J.; Firth, J. Digital Health Behaviour Change Interventions in Severe Mental Illness: A Systematic Review. Psychol. Med. 2023, 53, 6965–7005. [Google Scholar] [CrossRef]
- Shahab, L.; McEwen, A. Online Support for Smoking Cessation: A Systematic Review of the Literature. Addiction 2009, 104, 1792–1804. [Google Scholar] [CrossRef]
- Spanakis, P.; Peckham, E.; Young, B.; Heron, P.; Bailey, D.; Gilbody, S. A Systematic Review of Behavioural Smoking Cessation Interventions for People with Severe Mental Ill Health—What Works? Addiction 2022, 117, 1526–1542. [Google Scholar] [CrossRef]
- Staiger, P.K.; O’Donnell, R.; Liknaitzky, P.; Bush, R.; Milward, J. Mobile Apps to Reduce Tobacco, Alcohol, and Illicit Drug Use: Systematic Review of the First Decade. J. Med. Internet Res. 2020, 22, e17156. [Google Scholar] [CrossRef]
- Tatnell, P.; Atorkey, P.; Tzelepis, F. The Effectiveness of Virtual Reality Interventions on Smoking, Nutrition, Alcohol, Physical Activity and/or Obesity Risk Factors: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10821. [Google Scholar] [CrossRef]
- Taylor, G.M.J.; Dalili, M.N.; Semwal, M.; Civljak, M.; Sheikh, A.; Car, J. Internet-Based Interventions for Smoking Cessation. Cochrane Database Syst. Rev. 2017, 2017, CD007078. [Google Scholar] [CrossRef]
- Villanti, A.C.; West, J.C.; Klemperer, E.M.; Graham, A.L.; Mays, D.; Mermelstein, R.J.; Higgins, S.T. Smoking-Cessation Interventions for U.S. Young Adults: Updated Systematic Review. Am. J. Prev. Med. 2020, 59, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y. Mobile Phone-Based Interventions for Smoking Cessation. Cochrane Database Syst. Rev. 2016, 59, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.; McRobbie, H.; Bullen, C.; Rodgers, A.; Gu, Y.; Dobson, R. Mobile Phone Text Messaging and App-Based Interventions for Smoking Cessation. Cochrane Database Syst. Rev. 2019, 10, CD006611. [Google Scholar] [CrossRef]
- Williams, P.J.; Philip, K.E.; Alghamdi, S.M.; Perkins, A.M.; Buttery, S.C.; Polkey, M.I.; Laverty, A.A.; Hopkinson, N.S. Strategies to Deliver Smoking Cessation Interventions during Targeted Lung Health Screening—A Systematic Review and Meta-Analysis. Chron. Respir. Dis. 2023, 20, 14799731231183446. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, X.; Cheng, A.; Liu, Z.; Su, Z.; Li, J.; Qin, R.; Zhao, L.; Xie, Y.; Huang, Z.; et al. Mobile Phone–Based Interventions for Smoking Cessation Among Young People: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2023, 11, e48253. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.; Ding, H.; Hay, K.E.; Yang, I.A.; Bowman, R.V.; Fong, K.M.; Marshall, H.M. The Effectiveness of Smartphone Applications to Aid Smoking Cessation: A Meta-Analysis. Clin. Ehealth 2020, 3, 69–81. [Google Scholar] [CrossRef]
- Brown, J. A Review of the Evidence on Technology-Based Interventions for the Treatment of Tobacco Dependence in College Health. Worldviews Evid. Based Nurs. 2013, 10, 150–162. [Google Scholar] [CrossRef]
- Graham, A.; Carpenter, K.; Cha, S.; Cole, S.; Jacobs, M.; Raskob, M.; Cole-Lewis, H. Systematic Review and Meta-Analysis of Internet Interventions for Smoking Cessation among Adults. Subst. Abus. Rehabil. 2016, 7, 55–69. [Google Scholar] [CrossRef]
- Hutton, H.E.; Wilson, L.M.; Apelberg, B.J.; Avila Tang, E.; Odelola, O.; Bass, E.B.; Chander, G. A Systematic Review of Randomized Controlled Trials: Web-Based Interventions for Smoking Cessation Among Adolescents, College Students, and Adults. Nicotine Tob. Res. 2011, 13, 227–238. [Google Scholar] [CrossRef]
- Kant, R.; Yadav, P.; Bairwa, M. Effectiveness of the Internet-Based Versus Face-to-Face Interaction on Reduction of Tobacco Use Among Adults: A Meta-Analysis. Cureus 2021, 13, e19380. [Google Scholar] [CrossRef]
- Lindson-Hawley, N.; Hartmann-Boyce, J.; Fanshawe, T.R.; Begh, R.; Farley, A.; Lancaster, T. Interventions to Reduce Harm from Continued Tobacco Use. Cochrane Database Syst. Rev. 2016, 2016, CD005231. [Google Scholar] [CrossRef] [PubMed]
- Matkin, W.; Ordóñez-Mena, J.M.; Hartmann-Boyce, J. Telephone Counselling for Smoking Cessation. Cochrane Database Syst. Rev. 2019, 2019, CD002850. [Google Scholar] [CrossRef]
- Stead, L.F.; Carroll, A.J.; Lancaster, T. Group Behaviour Therapy Programmes for Smoking Cessation. Cochrane Database Syst. Rev. 2017, 2017, CD001007. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Bernert, J.T.; Foulds, J.; Hecht, S.S.; Jacob, P.; Jarvis, M.J.; Joseph, A.; Oncken, C.; Piper, M.E. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob. Res. 2020, 22, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.L.; Cheadle, A.; Thompson, D.C.; Diehr, P.; Koepsell, T.; Kinne, S. The Validity of Self-Reported Smoking: A Review and Meta-Analysis. Am. J. Public Health 1994, 84, 1086–1093. [Google Scholar] [CrossRef]
- Pisano, M.; Bramanti, A.; Di Spirito, F.; Di Palo, M.P.; De Benedetto, G.; Amato, A.; Amato, M. Reviewing Mobile Dental Apps for Children with Cognitive and Physical Impairments and Ideating an App Tailored to Special Healthcare Needs. J. Clin. Med. 2025, 14, 2105. [Google Scholar] [CrossRef]
- Mersha, A.G.; Bovill, M.; Eftekhari, P.; Erku, D.A.; Gould, G.S. The Effectiveness of Technology-based Interventions for Smoking Cessation: An Umbrella Review and Quality Assessment of Systematic Reviews. Drug Alcohol. Rev. 2021, 40, 1294–1307. [Google Scholar] [CrossRef]
- Willcox, J.C.; Dobson, R.; Whittaker, R. Old-Fashioned Technology in the Era of “Bling”: Is There a Future for Text Messaging in Health Care? J. Med. Internet Res. 2019, 21, e16630. [Google Scholar] [CrossRef]
- Pisano, M.; Bramanti, A.; Menditti, D.; Sangiovanni, G.; Santoro, R.; Amato, A. Modern Approaches to Providing Telematics Oral Health Services in Pediatric Dentistry: A Narrative Review. Appl. Sci. 2023, 13, 8331. [Google Scholar] [CrossRef]
- Martín Cantera, C.; Puigdomènech, E.; Ballvé, J.L.; Arias, O.L.; Clemente, L.; Casas, R.; Roig, L.; Pérez-Tortosa, S.; Díaz-Gete, L.; Granollers, S. Effectiveness of Multicomponent Interventions in Primary Healthcare Settings to Promote Continuous Smoking Cessation in Adults: A Systematic Review. BMJ Open 2015, 5, e008807. [Google Scholar] [CrossRef]
- Rasmussen, M.; Lauridsen, S.V.; Pedersen, B.; Backer, V.; Tønnesen, H. Intensive versus Short Face-to-Face Smoking Cessation Interventions: A Meta-Analysis. Eur. Respir. Rev. 2022, 31, 220063. [Google Scholar] [CrossRef]
- Brandon, T.H.; Simmons, V.N.; Sutton, S.K.; Unrod, M.; Harrell, P.T.; Meade, C.D.; Craig, B.M.; Lee, J.-H.; Meltzer, L.R. Extended Self-Help for Smoking Cessation. Am. J. Prev. Med. 2016, 51, 54–62. [Google Scholar] [CrossRef]
- Pacek, L.R.; McClernon, F.J.; Bosworth, H.B. Adherence to Pharmacological Smoking Cessation Interventions: A Literature Review and Synthesis of Correlates and Barriers. Nicotine Tob. Res. 2018, 20, 1163–1172. [Google Scholar] [CrossRef]
- Free, C.; Knight, R.; Robertson, S.; Whittaker, R.; Edwards, P.; Zhou, W.; Rodgers, A.; Cairns, J.; Kenward, M.G.; Roberts, I. Smoking Cessation Support Delivered via Mobile Phone Text Messaging (Txt2stop): A Single-Blind, Randomised Trial. Lancet 2011, 378, 49–55. [Google Scholar] [CrossRef]
- Abroms, L.C.; Yousefi, A.; Wysota, C.N.; Wu, T.-C.; Broniatowski, D.A. Assessing the Adherence of ChatGPT Chatbots to Public Health Guidelines for Smoking Cessation: Content Analysis. J. Med. Internet Res. 2025, 27, e66896. [Google Scholar] [CrossRef]
- Topol, E.J. High-Performance Medicine: The Convergence of Human and Artificial Intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Bouton, M.E. Why Behavior Change Is Difficult to Sustain. Prev. Med. 2014, 68, 29–36. [Google Scholar] [CrossRef]
- Yardley, L.; Morrison, L.; Bradbury, K.; Muller, I. The Person-Based Approach to Intervention Development: Application to Digital Health-Related Behavior Change Interventions. J. Med. Internet Res. 2015, 17, e30. [Google Scholar] [CrossRef]
- Mohr, D.C.; Cuijpers, P.; Lehman, K. Supportive Accountability: A Model for Providing Human Support to Enhance Adherence to EHealth Interventions. J. Med. Internet Res. 2011, 13, e30. [Google Scholar] [CrossRef]
- Robin DiMatteo, M.; Giordani, P.J.; Lepper, H.S.; Croghan, T.W. Patient Adherence and Medical Treatment Outcomes. Med. Care 2002, 40, 794–811. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, F.; Di Spirito, F.; De Caro, F.; Lanza, A.; Passarella, D.; Sbordone, L. Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance. Healthcare 2022, 10, 1636. [Google Scholar] [CrossRef]
- Kulhánek, A.; Lukavska, K.; Gabrhelík, R.; Novák, D.; Burda, V.; Prokop, J.; Holter, M.T.S.; Brendryen, H. Comparing Reminders Sent via SMS Text Messaging and Email for Improving Adherence to an Electronic Health Program: Randomized Controlled Trial. JMIR Mhealth Uhealth 2022, 10, e31040. [Google Scholar] [CrossRef]
- Kwon, D.M.; Santiago-Torres, M.; Mull, K.E.; Sullivan, B.M.; Bricker, J.B. Older Adults Who Smoke: Do They Engage with and Benefit from Web-Based Smoking Cessation Interventions? Prev. Med. 2022, 161, 107118. [Google Scholar] [CrossRef]
- Haimi, M.; Goren, U.; Grossman, Z. Barriers and Challenges to Telemedicine Usage among the Elderly Population in Israel. Eur. J. Public Health 2024, 34, ckae144.1163. [Google Scholar] [CrossRef]
- Caggiano, M.; Gasparro, R.; D’Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Cannatà, D.; Galdi, M.; Russo, A.; Scelza, C.; Michelotti, A.; Martina, S. Reliability and Educational Suitability of TikTok Videos as a Source of Information on Sleep and Awake Bruxism: A Cross-Sectional Analysis. J. Oral. Rehabil. 2025, 52, 434–442. [Google Scholar] [CrossRef]
| Database | Date of Search | Search String | Filters |
|---|---|---|---|
| MEDLINE/ PubMed | 10 Oct 2024 | ((“smoking cessation”[All Fields] OR “stopping smoking”[All Fields] OR “quitting smoking”[All Fields] OR “ex-smokers”[All Fields] OR “giving up smoking”[All Fields]) AND (“cell-phone”[All Fields] OR “text messaging”[All Fields] OR (“smartphone”[MeSH Terms] OR “smartphone”[All Fields] OR “smartphones”[All Fields] OR “smartphone s”[All Fields]) OR “social media”[All Fields] OR “computers”[All Fields] OR “online systems”[All Fields] OR “computer handheld”[All Fields] OR ((“mobile”[All Fields] OR “mobiles”[All Fields]) AND “appli cations”[All Fields]) OR “technology”[All Fields] OR “virtual reality”[All Fields] OR (“telemedicine”[MeSH Terms] OR “telemedicine”[All Fields] OR “telemedicine s”[All Fields]) OR “augmented reality”[All Fields] OR (“multimedia”[MeSH Terms] OR “multimedia”[All Fields] OR “multimedium”[All Fields]) OR “internet-based intervention”[All Fields] OR “electronic mail”[All Fields]) AND “systematic review”[All Fields]) AND ((systematicreview[Filter]) AND (english[Filter])) | Systematic Review; English |
| Scopus | 10 Oct 2024 | TITLE-ABS-KEY ((“smoking cessation” OR “stopping smoking” OR “quitting smoking” OR “ex-smokers” OR “giving up smoking”) AND (“cell-phone” OR “text messaging” OR smartphone OR “social media” OR “computers” OR “online systems” OR “computer handheld” OR “mobile appli-cations” OR “technology” OR “virtual reality” OR telemedicine OR “augmented reality” OR multimedia OR “internet-based intervention” OR “electronic mail”) AND (“systematic review”)) AND (LIMIT-TO (DOCTYPE, “re”)) AND (LIMIT-TO (LANGUAGE, “English”)) | Review; English |
| Web of Science | 10 Oct 2024 | (“smoking cessation” OR “stopping smoking” OR “quitting smoking” OR “ex-smokers” OR “giving up smoking”) AND (“cell-phone” OR “text messaging” OR smartphone OR “social media” OR “computers” OR “online systems” OR “computer handheld” OR “mobile appli-cations” OR “technology” OR “virtual reality” OR telemedicine OR “augmented reality” OR multimedia OR “internet-based intervention” OR “electronic mail”) AND (“systematic review”) (All Fields) and Review Article (Document Types) and English (Languages) | Review; English |
| PROSPERO | 30 Dec 2024 | (“smoking cessation” OR “stopping smoking” OR “quitting smoking” OR “ex-smokers” OR “giving up smoking”) AND (“cell-phone” OR “text messaging” OR smartphone OR “social media” OR “computers” OR “online systems” OR “computer handheld” OR “mobile appli-cations” OR “technology” OR “virtual reality” OR telemedicine OR “augmented reality” OR multimedia OR “internet-based intervention” OR “electronic mail”) AND (“systematic review”) (All Fields) and English (Languages) | English |
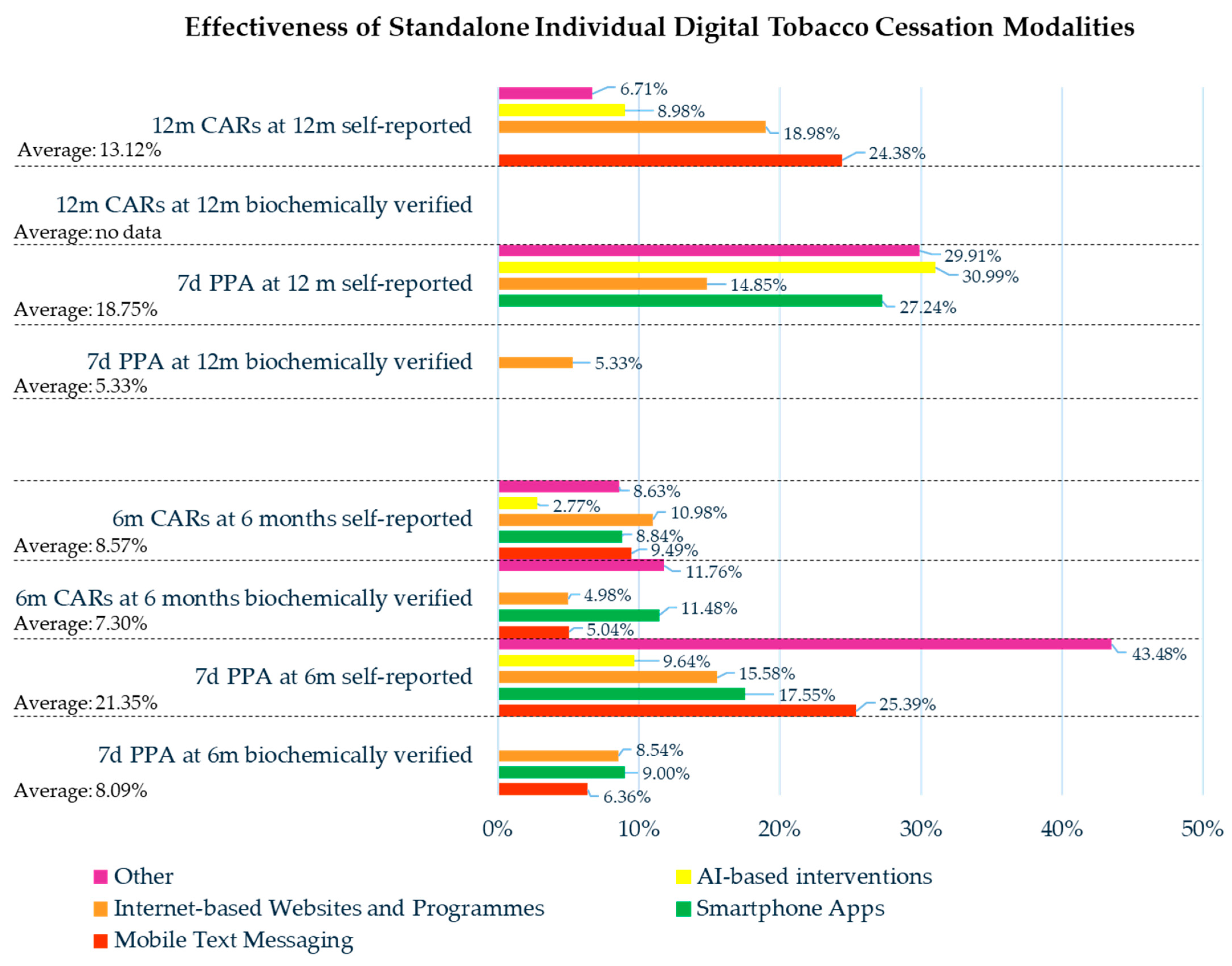
| Mobile Text Messaging | Smartphone Apps | Internet-Based Websites and Programs | AI-Based Interventions | Other Digital Tobacco Modalities | |
|---|---|---|---|---|---|
| Effectiveness: CARs Former Smokers/Smokers Assessed (Former Smoker %) | |||||
| For 3 m at 6 m Self-reported | — | 465/1798 (25.86%) | — | — | — |
| For 3 m at 6 m N/D methods | — | — | — | — | 24/430 (5.58%) |
| For 6 m at 6 m Biochemically verified | 138/2738 (5.04%) | 225/1960 (11.48%) | 48/964 (4.98%) | — | 6/51 (11.76%) |
| For 6 m at 6 m Self-reported | 237/2738 (9.49%) | 890/10,070 (8.84%) | 388/3534 (10.98%) | 55/1982 (2.77%) | 78/904 (8.63%) |
| For 6 m at 7 m Biochemically verified | — | — | 1828/18,452 (9.91%) | — | — |
| For 6 m at 7 m Self-reported | 104/1688 (6.16%) | — | 552/6256 (8.82%) | — | — |
| For 6.5 m at 6.5 m Self-reported | — | 104/850 (12.24%) | — | — | — |
| For 6 m at 12 m Self-reported | — | — | — | 14/163 (8.59%) | — |
| For 12 m at 12 m Self-reported | 78/320 (24.38%) | — | 703/3703 (18.98%) | 139/1548 (8.98%) | 198/2952 (6.71%) |
| For 18 m at 18 m Self-reported | — | — | 159/3990 (3.98%) | — | — |
| For 24 m at 24 m Self-reported | — | — | 234/1926 (12.15%) | — | — |
| Effectiveness: PPA Former Smokers/Smokers Assessed (Former Smoker %) | |||||
| 7 d PPA at 6 m Biochemically verified | 96/1509 (6.36%) | 144/1600 (9.00%) | 218/2554 (8.54%) | — | — |
| 7 d PPA at 6 m Self-reported | 5135/20,226 (25.39%) | 1308/7452 (17.55%) | 697/4473 (15.58%) | 236/2447 (9.64%) | 20/46 (43.48%) |
| 30 d PPA at 6 m Biochemically verified | — | — | 75/420 (17.86%) | — | — |
| 30 d PPA at 6 m Self-reported | 58/317 (18.30%) | 504/4920 (10.24%) | 318/2427 (13.10%) | 265/814 (32.55%) | 436/1727 (25.25%) |
| N/D time PPA at 6 m Biochemically verified | — | — | 108/964 (11.20%) | — | — |
| N/D time PPA at 6 m Self-reported | — | — | 2792/18,686 (14.94%) | — | — |
| N/D methods and time at 6 m | — | — | — | 27/205 (13.17%) | 54/80 (67.50%) |
| 7 d PPA at 6.5 m Self-reported | — | 134/850 (15.76%) | — | — | — |
| 7 d PPA at 7 m Self-reported | 340/1688 (20.14%) | — | 1527/5581 (27.36%) | — | — |
| 30 d PPA at 7 m Biochemically verified | — | — | 308/1820 (16.92%) | — | — |
| 30 d PPA at 7 m Self-reported | — | — | 420/1820 (23.08%) | — | — |
| N/D methods and time at 7 m | — | — | 17/65 (26.15%) | — | — |
| 30 d PPA at 9 m Self-reported | — | — | 71/307 (23.13%) | — | — |
| 7 d PPA at 11.5 m Self-reported | — | — | 102/272 (37.50%) | — | — |
| 30 d PPA at 11.5 m Self-reported | — | — | 183/1686 (10.85%) | — | — |
| 7 d PPA at 12 m Biochemically verified | — | — | 84/1577 (5.33%) | — | — |
| 7 d PPA at 12 m Self-reported | — | 1316/4830 (27.24%) | 2103/14,160 (14.85%) | 181/584 (30.99%) | 189/632 (29.91%) |
| 30 d PPA at 12 m Biochemically verified | — | — | 357/1953 (18.28%) | — | 22/171 (12.87%) |
| 30 d PPA at 12 m Self-reported | — | — | 874/4674 (18.70%) | — | 168/757 (22.19%) |
| N/D time PPA at 12 m Biochemically verified | — | — | 24/952 (2.52%) | — | — |
| N/D time PPA at 12 m Self-reported | — | — | 56/952 (5.88%) | — | — |
| N/D methods and time at 12 m | — | — | 14/312 (4.49%) | — | — |
| 30 d PPA at 13 m Self-reported | — | — | 1436/12,904 (11.13%) | — | — |
| N/D methods and time at 13 m | — | — | 594/5404 (10.99%) | — | — |
| 30 d PPA at 18 m Self-reported | — | — | 726/3990 (18.20%) | — | — |
| 7 d PPA at 24 m Biochemically verified | — | — | 255/1926 (13.24%) | — | — |
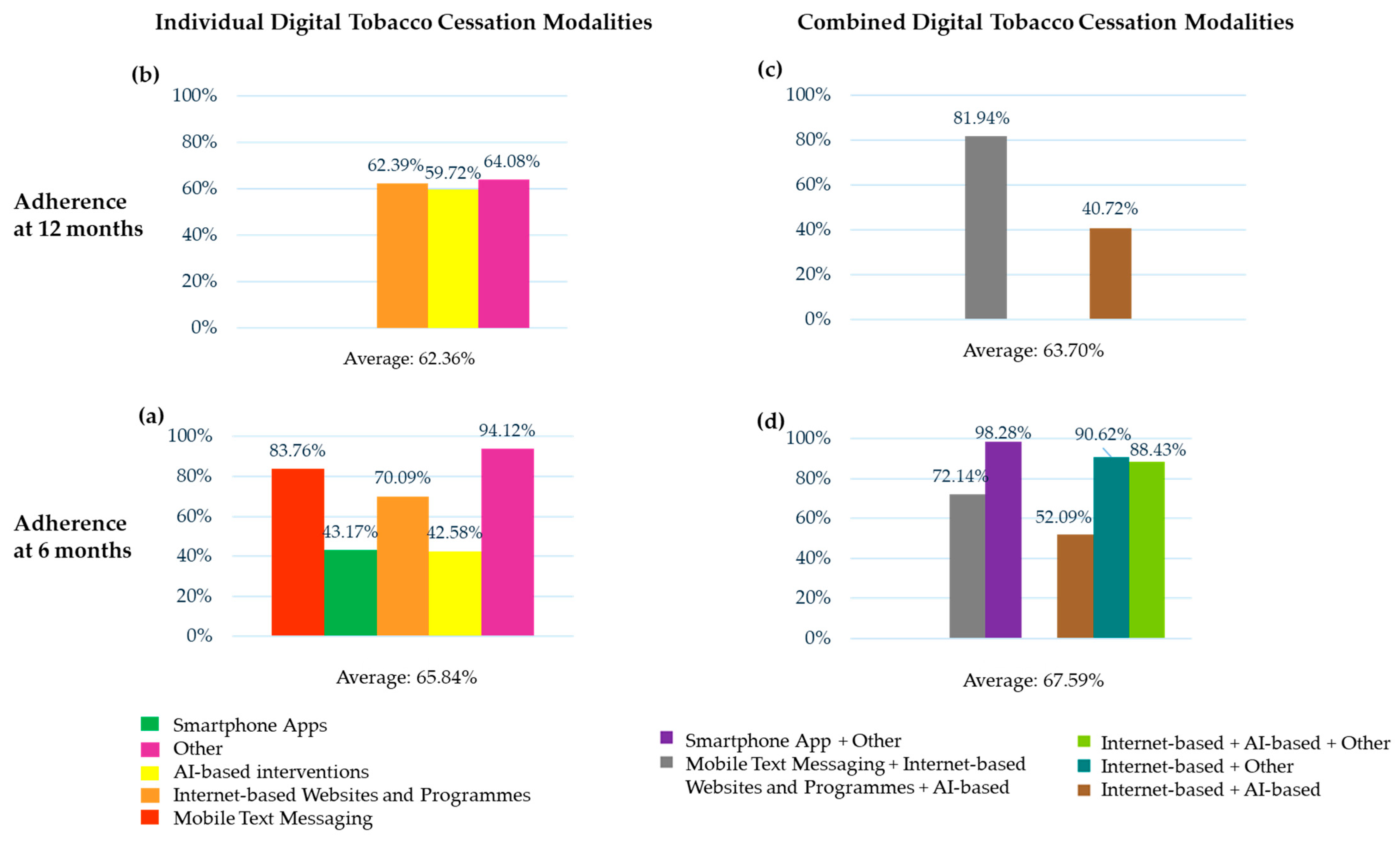
| Adherence | |||||
| At 6 m | 9312/11,118 (83.76%) | 5809/13,456 (43.17%) | 18,995/27,101 (70.09%) | 195/458 (42.58%) | 48/51 (94.12%) |
| At 7 m | 1424/1688 (84.36%) | — | 7144/8076 (88.46%) | — | — |
| At 11.5 m | — | — | 816/1686 (48.40%) | — | — |
| At 12 m | — | — | 5719/9167 (62.39%) | 943/1579 (59.72%) | 1438/2244 (64.08%) |
| At 18 m | — | — | 2745/3990 (68.80%) | — | — |
| Satisfaction | |||||
| At 6 m | 256/320 (80.00%) satisfied or totally satisfied | — | 8.59 mean of the Perceived Usefulness and Ease of Use Scale in 486 subjects with schizophrenia | — | — |
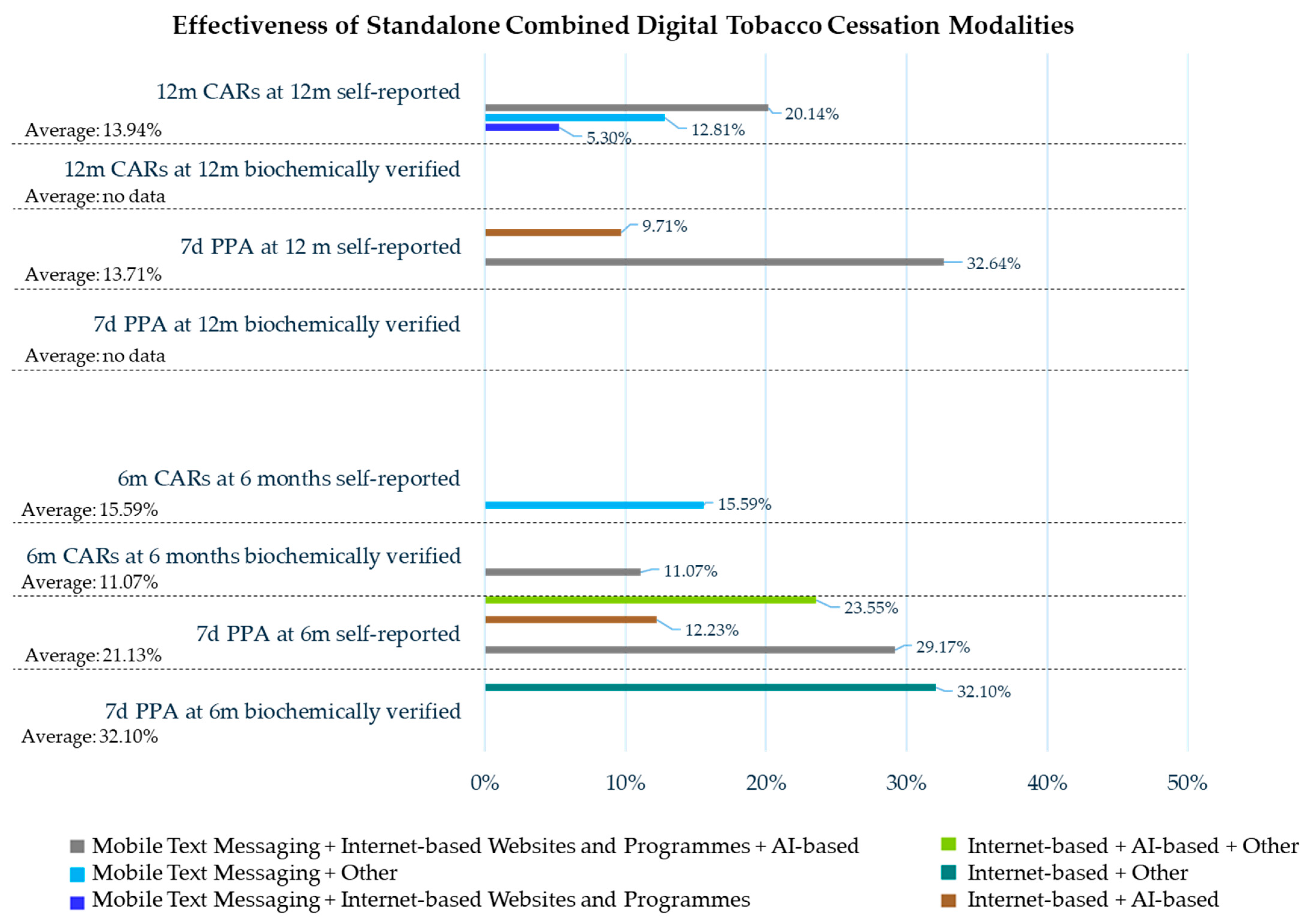
| Mobile Text Messaging + Internet-Based | Mobile Text Messaging + Other | Mobile Text Messaging + Internet-Based + AI-Based | Smartphone App + Other | AI-Based + Other | Internet-Based + AI-Based | Internet-Based + Other | Internet-Based + AI-Based Other | |
|---|---|---|---|---|---|---|---|---|
| Effectiveness: CARs Former Smokers/Smokers Assessed (Former Smoker %) | ||||||||
| For 3 m at 6 m Self-reported | — | — | — | — | — | 56/877 (6.39%) | — | — |
| For 4 m at 6 m Biochemically verified | — | — | — | 43/58 (74.14%) | — | — | — | — |
| For 4.6 m at 6 m Self-reported | — | — | — | — | — | 46/1104 (4.16%) | — | — |
| For 6 m at 6 m Biochemically verified | — | — | 116/1048 (11.07%) | — | — | — | — | — |
| For 6 m at 6 m Self-reported | — | 566/3631 (15.59%) | — | — | — | — | — | — |
| For 9 m at 9 Self-reported | — | 523/3631 (14.40%) | — | — | — | — | — | — |
| For 12 m at 12 m Self-reported | 24/453 (5.30%) | 465/3631 (12.81%) | 261/1296 (20.14%) | — | — | — | — | — |
| Effectiveness: PPA Former Smokers/Smokers Assessed (Former Smoker %) | ||||||||
| 7 d PPA at 6 m Biochemically verified | — | — | — | — | — | — | 130/405 (32.10%) | — |
| 7 d PPA at 6 m Self-reported | — | — | 378/1296 (29.17%) | — | — | 167/1366 (12.23%) | — | 171/726 (23.55%) |
| 30 d PPA at 6 m Biochemically verified | — | — | — | — | — | — | 90/405 (22.22%) | — |
| 30 d PPA at 6 m Self-reported | 451/2570 (17.55%) | — | — | — | — | — | — | — |
| N/D time PPA at 6 m Biochemically verified | — | — | 164/1048 (15.65%) | — | — | — | — | — |
| 7 d PPA at 7 m Self-reported | — | — | 150/509 (29.47%) | — | — | 1064/1799 (59.14%) | — | — |
| 30 d PPA at 7 m Biochemically verified | — | — | — | — | — | 595/1799 (33.07%) | — | — |
| 30 d PPA at 7 m Self-reported | — | — | — | — | — | 728/1799 (40.47%) | — | — |
| 30 d PPA at 9 m Self-reported | 72/311 (23.15%) | — | — | — | — | — | — | — |
| 1 d PPA at 12 m Self-reported | — | — | — | — | 18/213 (8.45%) | — | — | — |
| 7 d PPA at 12 m Self-reported | — | — | 423/1296 (32.64%) | — | — | 595/6130 (9.71%) | — | — |
| 30 d PPA at 12 m Biochemically verified | — | 13/221 (5.88%) | — | — | — | — | — | — |
| 30 d PPA at 12 m Self-reported | 569/2570 (22.14%) | 90/221 (40.72%) | — | — | — | — | — | — |
| N/D methods and time at 12 m | — | — | — | — | 22/328 (6.71%) | — | — | — |
| Adherence | ||||||||
| At 2 w | — | — | — | 57/58 (98.28%) | — | — | — | — |
| At 1 m | — | — | — | 57/58 (98.28%) | — | — | — | — |
| At 2 m | — | — | — | 57/58 (98.28%) | — | — | — | — |
| At 3 m | — | — | — | 57/58 (98.28%) | — | — | — | — |
| At 6 m | — | — | 756/1048 (72.14%) | 57/58 (98.28%) | — | 1032/1981 (52.09%) | 367/405 (90.62%) | 639/726 (88.43%) |
| At 7 m | — | — | — | — | — | 1673/1799 (93.00%) | — | — |
| At 12 m | — | — | 1062/1296 (81.94%) | — | — | 419/1029 (40.72%) | — | — |
| Individual Overall | Combined Overall | Overall (Individual Plus Combined) | |
|---|---|---|---|
| Effectiveness: CARs | |||
| For 3 m at 6 m Self-reported | 465/1798 (25.86%) | 56/877 (6.39%) | 521/2675 (19.48%) |
| N/D methods for 3 m at 6 m | 24/430 (5.58%) | — | 24/430 (5.58%) |
| For 4 m at 6 m Biochemically verified | — | 43/58 (74.14%) | 43/58 (74.14%) |
| For 4.6 m at 6 m Self-reported | — | 46/1104 (4.16%) | 46/1104 (4.16%) |
| For 6 m at 6 m Biochemically verified | 417/5713 (7.30%) | 116/1048 (11.07%) | 533/6761 (7.88%) |
| For 6 m at 6 m Self-reported | 1648/19,228 (8.57%) | 566/3631 (15.59%) | 2214/22,859 (9.69%) |
| For 6 m at 7 m Biochemically verified | 1828/18,452 (9.91%) | — | 1828/18,452 (9.91%) |
| For 6 m at 7 m Self-reported | 656/7944 (8.26%) | — | 656/7944 (8.26%) |
| For 6.5 m at 6.5 m Self-reported | 104/850 (12.24%) | — | 104/850 (12.24%) |
| For 9 m at 9 m Self-reported | — | 523/3631 (14.40%) | 523/3631 (14.40%) |
| For 6 m at 12 m Self-reported | 14/163 (8.59%) | — | 14/163 (8.59%) |
| For 12 m at 12 m Self-reported | 1118/8523 (13.12%) | 750/5380 (13.94%) | 1868/13,903 (13.44%) |
| For 18 m at 18 m Self-reported | 159/3990 (3.98%) | — | 159/3990 (3.98%) |
| For 24 m at 24 m Self-reported | 234/1926 (12.15%) | — | 234/1926 (12.15%) |
| Effectiveness: PPA | |||
| 7 d PPA at 6 m Biochemically verified | 458/5663 (8.09%) | 130/405 (32.10%) | 588/6068 (9.69%) |
| 7 d PPA at 6 m Self-reported | 7396/34,644 (21.35%) | 716/3388 (21.13%) | 8112/38,032 (21.33%) |
| 30 d PPA at 6 m Biochemically verified | 75/420 (17.86%) | 90/405 (22.22%) | 165/825 (20.00%) |
| 30 d PPA at 6 m Self-reported | 1581/10,205 (15.49%) | 451/2570 (17.55%) | 2032/12,775 (15.91%) |
| N/D time PPA at 6 m Biochemically verified | 108/964 (11.20%) | 164/1048 (15.65%) | 272/2012 (13.52%) |
| N/D time PPA at 6 m Self-reported | 2792/18,686 (14.94%) | — | 2792/18,686 (14.94%) |
| N/D methods and time at 6 m | 81/285 (28.42%) | — | 81/285 (28.42%) |
| 7 d PPA at 6.5 m Self-reported | 134/850 (15.76%) | — | 134/850 (15.76%) |
| 7 d PPA at 7 m Self-reported | 1867/7269 (25.68%) | 1214/2308 (52.73%) | 3081/9577 (32.17%) |
| 30 d PPA at 7 m Biochemically verified | 308/1820 (16.92%) | 595/1799 (33.07%) | 903/3619 (24.95%) |
| 30 d PPA at 7 m Self-reported | 420/1820 (23.08%) | 728/1799 (40.47%) | 1148/3619 (31.72%) |
| N/D methods and time at 7 m | 17/65 (26.15%) | — | 17/65 (26.15%) |
| 30 d PPA at 9 m Self-reported | 71/307 (23.13%) | 72/311 (23.15%) | 143/618 (23.14%) |
| 7 d PPA at 11.5 m Self-reported | 102/272 (37.50%) | — | 102/272 (37.50%) |
| 30 d PPA at 11.5 m Self-reported | 183/1686 (10.85%) | — | 183/1686 (10.85%) |
| 1 d PPA at 12 m Self-reported | — | 18/213 (8.45%) | 18/213 (8.45%) |
| 7 d PPA at 12 m Biochemically verified | 84/1577 (5.33%) | — | 84/1577 (5.33%) |
| 7 d PPA at 12 m Self-reported | 3789/20,206 (18.75%) | 1018/7426 (13.71%) | 4807/27,632 (17.40%) |
| 30 d PPA at 12 m Biochemically verified | 379/2124 (17.84%) | 13/221 (5.88%) | 392/2345 (16.72%) |
| 30 d PPA at 12 m Self-reported | 1042/5431 (19.19%) | 659/2791 (23.61%) | 1701/8222 (20.69%) |
| N/D time PPA at 12 m Biochemically verified | 24/952 (2.52%) | — | 24/952 (2.52%) |
| N/D time PPA at 12 m Self-reported | 56/952 (5.88%) | — | 56/952 (5.88%) |
| N/D methods and time at 12 m | 14/312 (4.49%) | 22/328 (6.71%) | 36/640 (5.63%) |
| 30 d PPA at 13 m Self-reported | 1436/12,904 (11.13%) | — | 1436/12,904 (11.13%) |
| N/D methods and time at 13 m | 594/5404 (10.99%) | — | 594/5404 (10.99%) |
| 30 d PPA at 18 m Self-reported | 726/3990 (18.20%) | — | 726/3990 (18.20%) |
| 7 d PPA at 24 m Biochemically verified | 255/1926 (13.24%) | — | 255/1926 (13.24%) |
| Adherence | |||
| At 2 w | — | 57/58 (98.28%) | 57/58 (98.28%) |
| At 1 m | — | 57/58 (98.28%) | 57/58 (98.28%) |
| At 2 m | — | 57/58 (98.28%) | 57/58 (98.28%) |
| At 3 m | — | 57/58 (98.28%) | 57/58 (98.28%) |
| At 6 m | 34,359/52,184 (65.84%) | 2851/4218 (67.59%) | 37,210/56,402 (65.97%) |
| At 7 m | 8568/9764 (87.75%) | 1673/1799 (93.00%) | 10,241/11,563 (88.57%) |
| At 11.5 m | 816/1686 (48.40%) | — | 816/1686 (48.40%) |
| At 12 m | 8100/12,990 (62.36%) | 1481/2325 (63.70%) | 9581/15,315 (62.56%) |
| At 18 m | 2745/3990 (68.80%) | — | 2745/3990 (68.80%) |
| Satisfaction | |||
| At 6 m | 256/320 (80.00%) satisfied or totally satisfied; 8.59 mean of the Perceived Usefulness and Ease of Use Scale in 486 subjects with schizophrenia | — | 256/320 (80.00%) satisfied or totally satisfied; 8.59 mean of the Perceived Usefulness and Ease of Use Scale in 486 subjects with schizophrenia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Palo, M.P.; Di Spirito, F.; Garofano, M.; Del Sorbo, R.; Caggiano, M.; Giordano, F.; Bartolomeo, M.; Pessolano, C.; Giordano, M.; Amato, M.; et al. Effectiveness and Adherence of Standalone Digital Tobacco Cessation Modalities: A Systematic Review of Systematic Reviews. Healthcare 2025, 13, 2125. https://doi.org/10.3390/healthcare13172125
Di Palo MP, Di Spirito F, Garofano M, Del Sorbo R, Caggiano M, Giordano F, Bartolomeo M, Pessolano C, Giordano M, Amato M, et al. Effectiveness and Adherence of Standalone Digital Tobacco Cessation Modalities: A Systematic Review of Systematic Reviews. Healthcare. 2025; 13(17):2125. https://doi.org/10.3390/healthcare13172125
Chicago/Turabian StyleDi Palo, Maria Pia, Federica Di Spirito, Marina Garofano, Rosaria Del Sorbo, Mario Caggiano, Francesco Giordano, Marianna Bartolomeo, Colomba Pessolano, Massimo Giordano, Massimo Amato, and et al. 2025. "Effectiveness and Adherence of Standalone Digital Tobacco Cessation Modalities: A Systematic Review of Systematic Reviews" Healthcare 13, no. 17: 2125. https://doi.org/10.3390/healthcare13172125
APA StyleDi Palo, M. P., Di Spirito, F., Garofano, M., Del Sorbo, R., Caggiano, M., Giordano, F., Bartolomeo, M., Pessolano, C., Giordano, M., Amato, M., & Bramanti, A. (2025). Effectiveness and Adherence of Standalone Digital Tobacco Cessation Modalities: A Systematic Review of Systematic Reviews. Healthcare, 13(17), 2125. https://doi.org/10.3390/healthcare13172125







