Healthcare Spending Before and After Mild Cognitive Impairment Diagnosis: Evidence from the NHIS–NHID in Korea
Abstract
1. Introduction
2. Materials and Methods
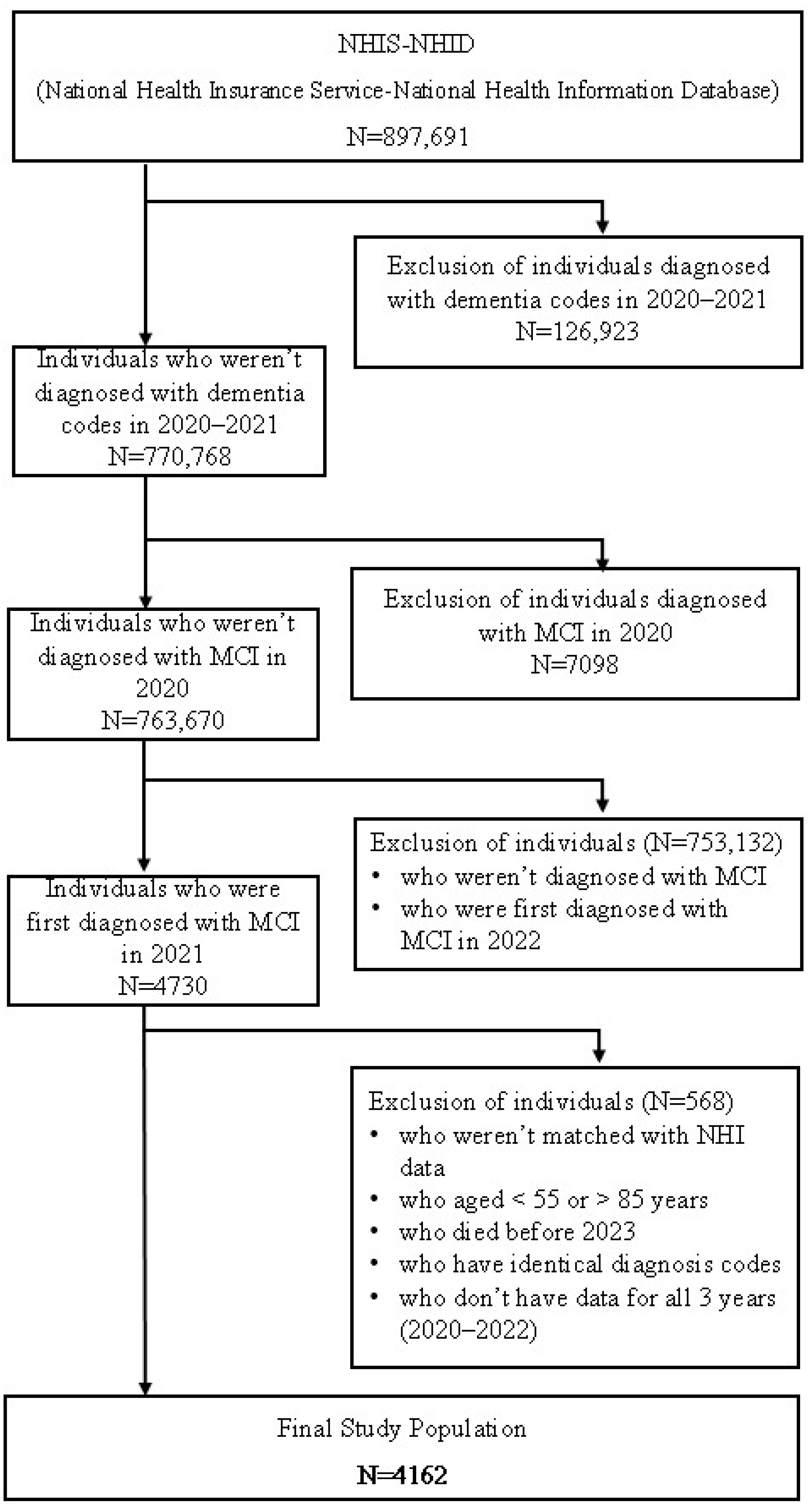
2.1. Data and Study Population
2.2. Variables
2.3. Statistical Analysis
2.4. Ethical Consideration
3. Results
3.1. Characteristics of the Study Population
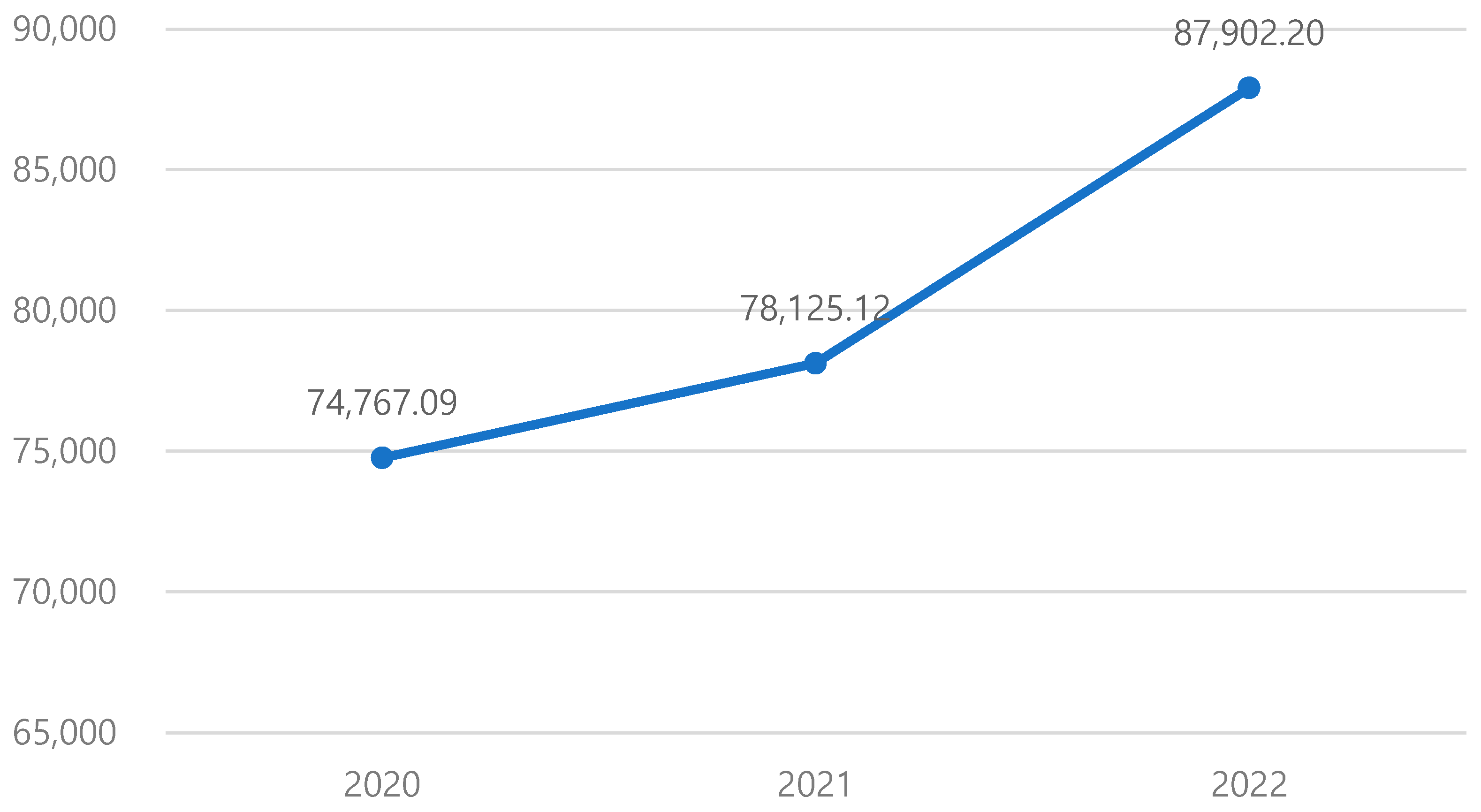
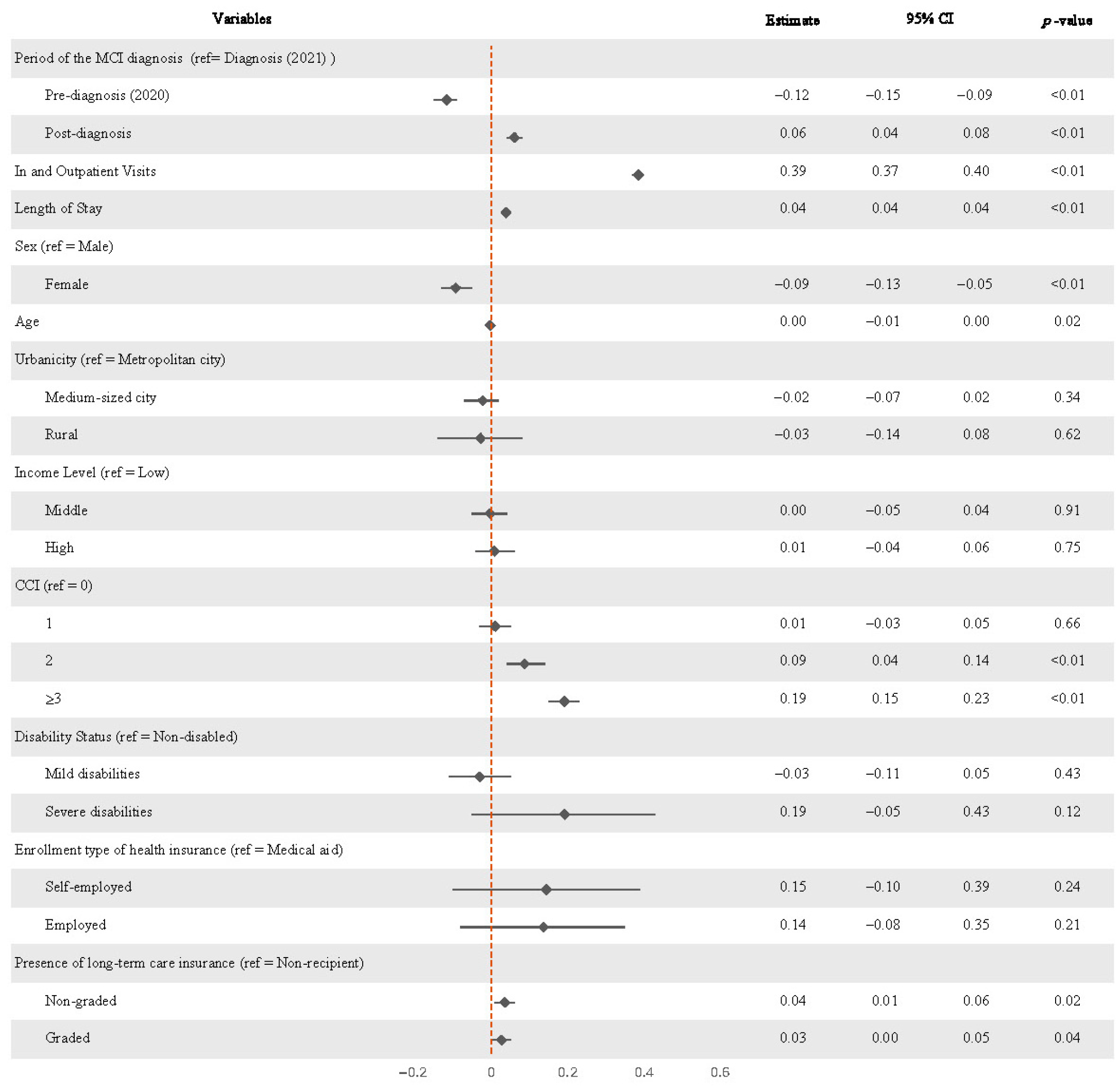
3.2. Changes in Medical Expenditures Before and After MCI Diagnosis
3.3. Changes in Medical Expenditures Before and After MCI Diagnosis, Stratified by Sex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCI | Mild Cognitive Impairment |
| NHIS | National Health Insurance Service |
| NHID | National Health Information Database |
| ICD-10 | International Classification of Diseases, 10th Revision |
| CCI | Charlson Comorbidity Index |
| LTCI | Long-Term Care Insurance |
| GEE | Generalized Estimating Equation |
References
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild cognitive impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Han, J.W.; So, Y.S.; Seo, J.Y.; Kim, K.Y.; Kim, K.W. Prevalence and trends of dementia in Korea: A systematic review and meta-analysis. J. Korean Med. Sci. 2014, 29, 903. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef] [PubMed]
- Leibson, C.L.; Long, K.H.; Ransom, J.E.; Roberts, R.O.; Hass, S.L.; Duhig, A.M.; Smith, C.Y.; Emerson, J.A.; Pankratz, V.S.; Petersen, R.C. Direct medical costs and source of cost differences across the spectrum of cognitive decline: A population-based study. Alzheimer’s Dement. 2015, 11, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.J.; Zhong, Y.; Fillit, H.M.; Chen, E.; Neumann, P.J. Medicare expenditures of individuals with Alzheimer’s disease and related dementias or mild cognitive impairment before and after diagnosis. J. Am. Geriatr. Soc. 2016, 64, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.H.; Lewis, L.; Blackwell, A.D.; Taylor, M. Early intervention in Alzheimer’s disease: A health economic study of the effects of diagnostic timing. BMC Neurol. 2014, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Korea, S. 2023 Elderly Population Statistics; Statistics Korea: Dajeon, Republic of Korea, 2023. [Google Scholar]
- Quentin, W.; Riedel-Heller, S.; Luppa, M.; Rudolph, A.; König, H.H. Cost-of-illness studies of dementia: A systematic review focusing on stage dependency of costs. Acta Psychiatr. Scand. 2010, 121, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.I.; Ha, S.; et al. Data resource profile: The national health information database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Nam, J.Y. Effects of changes in multiple chronic conditions on medical costs among older adults in South Korea. Healthcare 2022, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.L.; Solomon, P.J.; Peisach, A.R.; Martin, J. New models for old questions: Generalized linear models for cost prediction. J. Eval. Clin. Pract. 2007, 13, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. The future of data analysis. In Breakthroughs in Statistics: Methodology and Distribution; Springer: Berlin/Heidelberg, Germany, 1962; pp. 408–552. [Google Scholar]
- Gläser, E.; Kilimann, I.; Platen, M.; Hoffmann, W.; Brosseron, F.; Buerger, K.; Coenjaerts, M.; Düzel, E.; Ewers, M.; Fliessbach, K.; et al. The economic burden of subjective cognitive decline, mild cognitive impairment and Alzheimer’s dementia: Excess costs and associated clinical and risk factors. Alzheimer’s Res. Ther. 2025, 17, 142. [Google Scholar] [CrossRef]
- Frech, F.H.; Li, G.; Juday, T.; Ding, Y.; Mattke, S.; Khachaturian, A.; Rosenberg, A.S.; Ndiba-Markey, C.; Rava, A.; Batrla, R.; et al. Economic impact of progression from mild cognitive impairment to Alzheimer disease in the United States. J. Prev. Alzheimer’s Dis. 2024, 11, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Hong, J.; Jung, J. Projection of Future Medical Expenses Based on Medical Needs and Physician Availability. J. Korean Med. Sci. 2024, 40, e121. [Google Scholar] [CrossRef]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. eClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Choi, S. Trajectories of preventive health care utilization among older Koreans: The role of social relationships. Health Educ. Behav. 2023, 50, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kahng, S.K. Factors associated with the preventive healthcare service use among older adults in Korea: Focusing on age variation. Asian Soc. Work. Policy Rev. 2021, 15, 24–34. [Google Scholar] [CrossRef]
- Chandler, J.M.; Rentz, D.M.; Zagar, A.; Kim, Y.; Schwartz, R.L.; Fillit, H. Disease progression and costs at the 3-year follow-up of the GERAS-US study. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12430. [Google Scholar] [CrossRef] [PubMed]
- Mudrazija, S.; Aranda, M.P.; Gaskin, D.J.; Monroe, S.; Richard, P. Economic Burden of Alzheimer Disease and Related Dementias by Race and Ethnicity, 2020 to 2060. JAMA Netw. Open 2025, 8, e2513931. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Lee, M.; Choi, M.; Park, S. The impact of the expanded health insurance coverage policy on healthcare spending: Evidence from Korea. Int. J. Equity Health 2024, 23, 126. [Google Scholar] [CrossRef] [PubMed]
| Variables | N/Mean + S.D. | % | |
|---|---|---|---|
| In and Outpatient Visits | 1.06 ± 1.02 | ||
| Length of Stay | 2.03 ± 6.77 | ||
| Sex | Male | 1380 | 33.16 |
| Female | 2782 | 66.84 | |
| Age | 70.59 ± 7.53 | ||
| Urbanicity | Metropolitan city | 1747 | 41.98 |
| Medium-sized city | 1913 | 45.96 | |
| Rural | 502 | 12.06 | |
| Income Level | Low | 2011 | 48.32 |
| Middle | 950 | 22.83 | |
| High | 1201 | 28.86 | |
| CCI | 0 | 1185 | 28.47 |
| 1 | 677 | 16.27 | |
| 2 | 630 | 15.14 | |
| ≥3 | 1670 | 40.12 | |
| Disability Status | Non-disabled | 3644 | 87.55 |
| Mild disabilities | 415 | 9.97 | |
| Severe disabilities | 103 | 2.47 | |
| Enrollment type of health insurance | Medical aid | 1322 | 31.76 |
| Self-employed | 2611 | 62.73 | |
| Employed | 229 | 5.5 | |
| Presence of long-term care insurance | Non-recipient | 3814 | 91.64 |
| Non-graded | 257 | 6.17 | |
| Graded | 91 | 2.19 | |
| Variables | Mean (KRW) | S.D | Max (KRW) | Min (KRW) | |
|---|---|---|---|---|---|
| Period of the MCI diagnosis | Diagnosis (2021) | 78,125.12 ($57.61) | 126,093.39 | 5460 ($4.03) | 2,705,468.33 ($1995.18) |
| Pre-diagnosis (2020) | 74,767.09 ($55.13) | 174,991.36 | 4670 ($3.44) | 6,183,749.09 ($4560.29) | |
| Post-diagnosis (2022) | 87,902.2 ($64.82) | 171,618.41 | 8160 ($6.02) | 3,691,224.29 ($2722.14) | |
| Variables | Estimate | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| LL | UL | ||||
| Period of the MCI diagnosis | Diagnosis (2021) | Ref. | |||
| Pre-diagnosis (2020) | −0.117 | −0.15 | −0.09 | <0.01 | |
| Post-diagnosis | 0.061 | 0.04 | 0.08 | <0.01 | |
| In and Outpatient Visits | 0.385 | 0.37 | 0.40 | <0.01 | |
| Length of Stay | 0.039 | 0.03 | 0.04 | <0.01 | |
| Sex | Male | Ref. | |||
| Female | −0.093 | −0.13 | −0.05 | <0.01 | |
| Age | −0.003 | −0.01 | −0.00 | 0.02 | |
| Urbanicity | Metropolitan city | Ref. | |||
| Medium-sized city | −0.023 | −0.07 | 0.02 | 0.34 | |
| Rural | −0.028 | −0.14 | 0.08 | 0.62 | |
| Income Level | Low | Ref. | |||
| Middle | −0.003 | −0.05 | 0.04 | 0.91 | |
| High | 0.008 | −0.04 | 0.06 | 0.75 | |
| CCI | 0 | Ref. | |||
| 1 | 0.010 | −0.03 | 0.05 | 0.66 | |
| 2 | 0.088 | 0.04 | 0.14 | <0.01 | |
| ≥3 | 0.192 | 0.15 | 0.23 | <0.01 | |
| Disability Status | Non-disabled | Ref. | |||
| Mild disabilities | −0.030 | −0.11 | 0.05 | 0.43 | |
| Severe disabilities | 0.192 | −0.05 | 0.43 | 0.12 | |
| Enrollment type of health insurance | Medical aid | Ref. | |||
| Self-employed | 0.145 | −0.10 | 0.39 | 0.24 | |
| Employed | 0.137 | −0.08 | 0.35 | 0.21 | |
| Presence of long-term care insurance | Non-recipient | Ref. | |||
| Non-graded | 0.035 | 0.01 | 0.06 | 0.02 | |
| Graded | 0.027 | 0.00 | 0.05 | 0.04 | |
| Variables | Estimate | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| LL | UL | ||||
| Period of the MCI diagnosis | Diagnosis (2021) | Ref. | |||
| Pre-diagnosis (2020) | −0.142 | −0.19 | −0.09 | <0.01 | |
| Post-diagnosis | 0.053 | 0.01 | 0.10 | 0.02 | |
| In and Outpatient Visits | 0.385 | 0.407 | 0.39 | <0.01 | |
| Length of Stay | 0.039 | 0.042 | 0.03 | <0.01 | |
| Age | −0.003 | −0.002 | −0.01 | 0.51 | |
| Urbanicity | Metropolitan city | Ref. | |||
| Medium-sized city | −0.075 | −0.14 | −0.01 | 0.03 | |
| Rural | −0.069 | −0.17 | 0.04 | 0.20 | |
| Income Level | Low | Ref. | |||
| Middle | 0.016 | −0.08 | 0.11 | 0.74 | |
| High | −0.028 | −0.11 | 0.06 | 0.52 | |
| CCI | 0 | Ref. | |||
| 1 | −0.013 | −0.09 | 0.07 | 0.76 | |
| 2 | 0.076 | −0.02 | 0.17 | 0.12 | |
| ≥3 | 0.237 | 0.15 | 0.32 | <0.01 | |
| Disability Status | Non-disabled | Ref. | |||
| Mild disabilities | −0.012 | −0.15 | 0.13 | 0.87 | |
| Severe disabilities | 0.251 | −0.10 | 0.60 | 0.16 | |
| Enrollment type of health insurance | Medical aid | Ref. | |||
| Self-employed | 0.105 | −0.09 | 0.3 | 0.29 | |
| Employed | 0.133 | −0.05 | 0.31 | 0.15 | |
| Presence of long-term care insurance | Non-recipient | Ref. | |||
| Non-graded | 0.059 | −0.01 | 0.12 | 0.08 | |
| Graded | 0.062 | 0.00 | 0.12 | 0.05 | |
| Variables | Estimate | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| LL | UL | ||||
| Period of the MCI diagnosis | Diagnosis (2021) | Ref. | |||
| Pre-diagnosis (2020) | −0.104 | −0.14 | −0.07 | <0.01 | |
| Post-diagnosis | 0.063 | 0.04 | 0.09 | <0.01 | |
| In and Outpatient Visits | 0.369 | 0.35 | 0.39 | <0.01 | |
| Length of Stay | 0.037 | 0.03 | 0.04 | <0.01 | |
| Age | −0.003 | −0.01 | 0.00 | 0.02 | |
| Urbanicity | Metropolitan city | ||||
| Medium-sized city | 0.005 | −0.08 | 0.09 | 0.91 | |
| Rural | 0.039 | −0.17 | 0.25 | 0.72 | |
| Income Level | Low | Ref. | |||
| Middle | −0.003 | −0.05 | 0.05 | 0.92 | |
| High | 0.036 | −0.03 | 0.1 | 0.26 | |
| CCI | 0 | Ref. | |||
| 1 | 0.026 | −0.03 | 0.08 | 0.33 | |
| 2 | 0.105 | 0.05 | 0.16 | <0.01 | |
| ≥3 | 0.183 | 0.13 | 0.23 | <0.01 | |
| Disability Status | Non-disabled | Ref. | |||
| Mild disabilities | −0.044 | −0.12 | 0.03 | 0.24 | |
| Severe disabilities | −0.038 | −0.15 | 0.07 | 0.50 | |
| Enrollment type of health insurance | Medical aid | Ref. | |||
| Self-employed | 0.204 | −0.19 | 0.6 | 0.31 | |
| Employed | 0.183 | −0.18 | 0.54 | 0.32 | |
| Presence of long-term care insurance | Non-recipient | Ref. | |||
| Non-graded | 0.025 | −0.01 | 0.06 | 0.20 | |
| Graded | 0.018 | −0.01 | 0.05 | 0.23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Jeon, H.; Noh, Y.; Noh, J.-W. Healthcare Spending Before and After Mild Cognitive Impairment Diagnosis: Evidence from the NHIS–NHID in Korea. Healthcare 2025, 13, 2076. https://doi.org/10.3390/healthcare13162076
Ma S, Jeon H, Noh Y, Noh J-W. Healthcare Spending Before and After Mild Cognitive Impairment Diagnosis: Evidence from the NHIS–NHID in Korea. Healthcare. 2025; 13(16):2076. https://doi.org/10.3390/healthcare13162076
Chicago/Turabian StyleMa, Sujin, Huiwon Jeon, Yoohun Noh, and Jin-Won Noh. 2025. "Healthcare Spending Before and After Mild Cognitive Impairment Diagnosis: Evidence from the NHIS–NHID in Korea" Healthcare 13, no. 16: 2076. https://doi.org/10.3390/healthcare13162076
APA StyleMa, S., Jeon, H., Noh, Y., & Noh, J.-W. (2025). Healthcare Spending Before and After Mild Cognitive Impairment Diagnosis: Evidence from the NHIS–NHID in Korea. Healthcare, 13(16), 2076. https://doi.org/10.3390/healthcare13162076






