Association Between Different Patterns of Opioid and Benzodiazepine Use and Risks of Emergency Department Visits and Hospitalizations: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Data Set
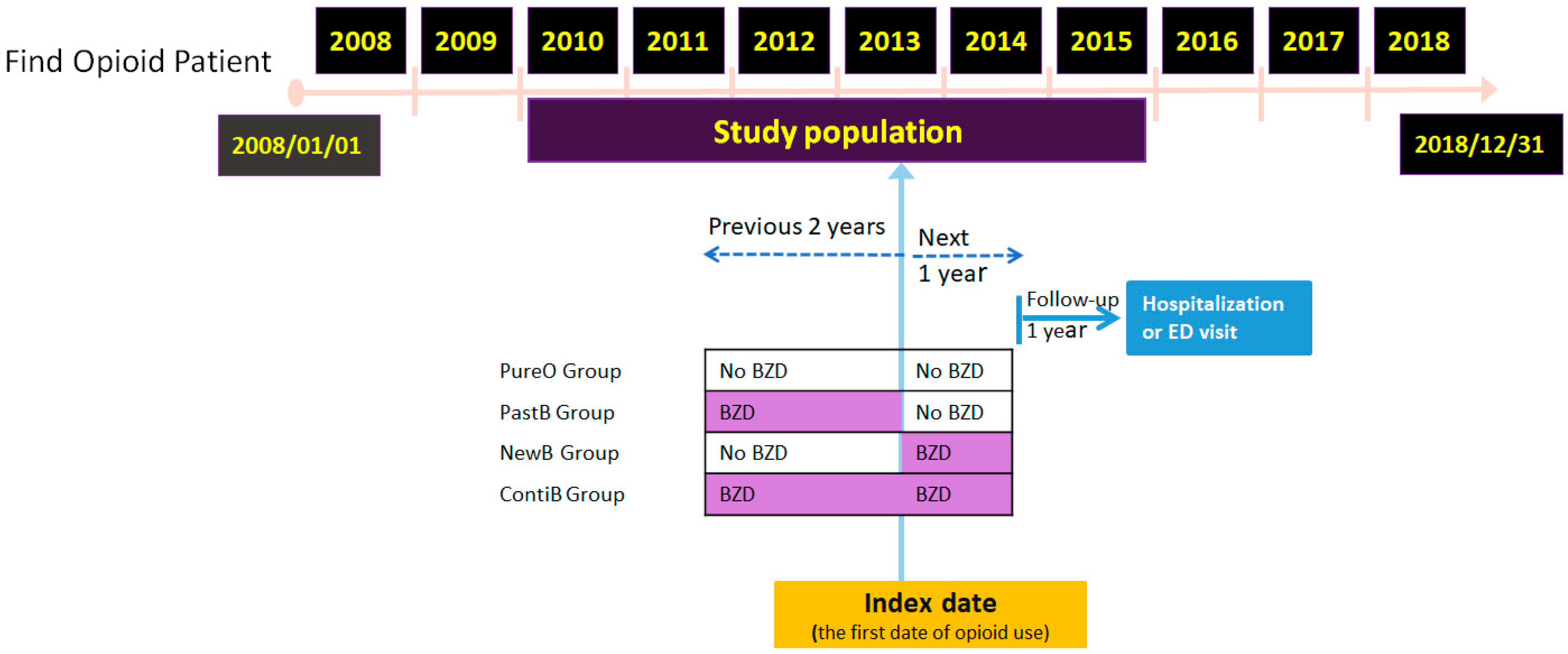
2.2. Study Design
2.3. Dependent Variables
2.4. Independent Variable
2.5. Control Variables
2.6. Data Analysis
3. Results
3.1. Demographics and Comorbidities of Opioid Users with and Without Benzodiazepine Use
3.2. Hospitalization Rate Among Patients in the Different Groups After 1-Year Follow-Up Study
3.3. Emergency Department Visit Rate Among Patients in the Different Groups After 1-Year Follow-Up Study
3.4. Odds Ratio of Hospitalizations in Each Group After 1-Year Follow-Up Study
3.5. Adjusted Odds Ratios for Emergency Department Visits After 1-Year Follow-Up
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BZD | Benzodiazepine |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| TIA | Transient ischemic attack |
References
- Freynhagen, R.; Geisslinger, G.; Schug, S.A. Opioids for chronic non-cancer pain. Br. Med. J. 2013, 346, f2937. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Pfau, G.; Vielhaber, S.; Haghikia, A.; Hachenberg, T.; Brinkers, M. Long-term opioid therapy and mental health comorbidity in patients with chronic pain. Pain Med. 2023, 24, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Santo, T.; Colledge-Frisby, S.; Mekonen, T.; Thomson, K.; Degenhardt, L.; Connor, J.P.; Hall, W.; Stjepanovic, D. Mood and Anxiety Symptoms in Persons Taking Prescription Opioids: A Systematic Review with Meta-Analyses of Longitudinal Studies. Pain Med. 2022, 23, 1442–1456. [Google Scholar] [CrossRef] [PubMed]
- Zin, C.S.; Ismail, F. Co-prescription of opioids with benzodiazepine and other co-medications among opioid users: Differential in opioid doses. J. Pain Res. 2017, 10, 249–257. [Google Scholar] [CrossRef]
- Lynch, N.; Lima, J.D.; Spinieli, R.L.; Kaur, S. Opioids, sleep, analgesia and respiratory depression: Their convergence on Mu (mu)-opioid receptors in the parabrachial area. Front. Neurosci. 2023, 17, 1134842. [Google Scholar] [CrossRef]
- Boon, M.; van Dorp, E.; Broens, S.; Overdyk, F. Combining opioids and benzodiazepines: Effects on mortality and severe adverse respiratory events. Ann. Palliat. Med. 2020, 9, 54257–54557. [Google Scholar] [CrossRef]
- Dowell, D. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR. Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. J. Am. Med. Assoc. 2016, 315, 1624–1645. [Google Scholar] [CrossRef]
- Molbournr. TGLec. Analgesia Guideline 2013. Available online: https://tgldcdp.tg.org.au/guideLine?guidelinePage=Analgesic&frompage=etgcomplete (accessed on 28 June 2018).
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Kharrazi, H.; Bodycombe, D.; Weiner, J.P.; Alexander, G.C. Healthcare costs and utilization associated with high-risk prescription opioid use: A retrospective cohort study. BMC Med. 2018, 16, 69. [Google Scholar] [CrossRef]
- Dasgupta, N.; Funk, M.J.; Proescholdbell, S.; Hirsch, A.; Ribisl, K.M.; Marshall, S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016, 17, 85–98. [Google Scholar] [CrossRef]
- Hung, A.; Bush, C.; Greiner, M.; Campbell, H.; Hammill, B.; Maciejewski, M.L.; McKethan, A. Risk Factors and Outcomes of Opioid Users with and Without Concurrent Benzodiazepine Use in the North Carolina Medicaid Population. J. Manag. Care Spec. Pharm. 2020, 26, 169–175. [Google Scholar] [CrossRef]
- Parent, S.; Nolan, S.; Fairbairn, N.; Ye, M.; Wu, A.; Montaner, J.; Barrios, R.; Ti, L.; Daly, P.; Gilbert, M. Correlates of opioid and benzodiazepine co-prescription among people living with HIV in British Columbia, Canada: A population-level cohort study. Int. J. Drug Policy 2019, 67, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, X.; Zhang, J.; Liang, J.; Wang, L. Association Between Opioid and Benzodiazepine Use and All-Cause Mortality in Individuals with Chronic Obstructive Pulmonary Disease: A Prospective Cohort Study. Int. J. Chronic Obstr. Pulm. Dis. 2024, 19, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M. Dangerously numb: Opioids, benzodiazepines, chronic pain, and posttraumatic stress disorder. Pain 2018, 159, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Kuo, L.F.; Cheng, I.C.; Chang, C.S.; Tsay, W.I. Trends in major opioid analgesic consumption in Taiwan, 2002–2014. J. Formos. Med. Assoc. 2017, 116, 529–535. [Google Scholar] [CrossRef]
- Wang, J.-J.; Teng, S.-F.; Chu, Y.-R.; Chu, C.-C.; Ho, C.-H.; Chu, L.-L. Evaluation of opioid consumption trends for pain in Taiwan and comparison with neighboring Asian countries. J. Food Drug Anal. 2022, 30, 104–110. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.-C. Data resource profile: The national health insurance research database (NHIRD). Epidemiol. Health 2018, 40, e2018062. [Google Scholar] [CrossRef]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef]
- Islam, M.M. Pattern and probability of dispensing of prescription opioids and benzodiazepines among the new users in Australia: A retrospective cohort study. BMJ Open 2019, 9, e030803. [Google Scholar] [CrossRef]
- Sung, H.G.; Li, J.; Nam, J.H.; Won, D.Y.; Choi, B.; Shin, J.Y. Concurrent use of benzodiazepines, antidepressants, and opioid analgesics with zolpidem and risk for suicide: A case-control and case-crossover study. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 1535–1544. [Google Scholar] [CrossRef]
- Sun, E.C.; Dixit, A.; Humphreys, K.; Darnall, B.D.; Baker, L.C.; Mackey, S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: Retrospective analysis. Br. Med. J. 2017, 356, j760. [Google Scholar] [CrossRef]
- Hernandez, I.; He, M.; Brooks, M.M.; Zhang, Y. Exposure-Response Association Between Concurrent Opioid and Benzodiazepine Use and Risk of Opioid-Related Overdose in Medicare Part D Beneficiaries. JAMA Netw. Open 2018, 1, e180919. [Google Scholar] [CrossRef]
- Romano, P.S.; Roos, L.L.; Jollis, J.G. Presentation adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J. Clin. Epidemiol. 1993, 46, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.D.; Beach, P.A.; Atalla, S.; Dietrich, M.S.; Bruehl, S.; Cowan, R.L.; Monroe, T.B. Gender differences in pain threshold, unpleasantness, and descending pain modulatory activation across the adult life span: A cross sectional study. J. Pain 2024, 25, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- M, S.; S, M.; Vadakkiniath, I.J.; A, G. Prevalence and correlates of stress, anxiety, and depression in patients with chronic diseases: A cross-sectional study. Middle East. Curr. Psychiatry 2023, 30, 1–14. [Google Scholar] [CrossRef]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Kastner, M.; Cardoso, R.; Lai, Y.; Treister, V.; Hamid, J.S.; Hayden, L.; Wong, G.; Ivers, N.M.; Liu, B.; Marr, S.; et al. Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: A systematic review and meta-analysis. Can. Med. Assoc. J. 2018, 190, E1004–E1012. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef]
- Gupta, A.; Eisenhauer, E.A.; Booth, C.M. The Time Toxicity of Cancer Treatment. J. Clin. Oncol. 2022, 40, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Colin, O.; Labreuche, J.; Deguil, J.; Mendyk, A.M.; Deken, V.; Cordonnier, C.; Deplanque, D.; Leys, D.; Bordet, R. Preadmission use of benzodiazepines and stroke outcomes: The Biostroke prospective cohort study. BMJ Open 2019, 9, e022720. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Park, S.; Choi, M.; Wijesinha, M.; Khokhar, B.; Simoni-Wastila, L. Respiratory events associated with concomitant opioid and sedative use among Medicare beneficiaries with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000483. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, D.; Hopman, W.M.; Holden, R.M. Association Between Sex and Opiate and Benzodiazepine Prescription Among Patients With CKD: Research Letter. Can. J. Kidney Health Dis. 2020, 7, 2054358120932673. [Google Scholar] [CrossRef]
- Yarborough, B.J.H.; Stumbo, S.P.; Stoneburner, A.; Smith, N.; Dobscha, S.K.; Deyo, R.A.; Morasco, B.J. Correlates of Benzodiazepine Use and Adverse Outcomes Among Patients with Chronic Pain Prescribed Long-term Opioid Therapy. Pain Med. 2019, 20, 1148–1155. [Google Scholar] [CrossRef]
- Maust, D.T.; Petzold, K.; Strominger, J.; Kim, H.M.; Bohnert, A.S.B. Benzodiazepine Discontinuation and Mortality Among Patients Receiving Long-Term Benzodiazepine Therapy. JAMA Netw. Open 2023, 6, e2348557. [Google Scholar] [CrossRef]
- Brunner, E.; Chen, C.-Y.A.; Klein, T.; Maust, D.; Mazer-Amirshahi, M.; Mecca, M.; Najera, D.; Ogbonna, C.; Rajneesh, K.F.; Roll, E. Joint Clinical Practice Guideline on Benzodiazepine Tapering: Considerations When Risks Outweigh Benefits. J. Gen. Intern. Med. 2025, 1–46. [Google Scholar] [CrossRef]
| Variable | All | PureO | PastB | NewB | ContiB | p a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | p | N | % | p | N | % | p | N | % | p | N | % | p | ||
| All | 418,549 | 12.56 | 264,789 | 10.04 | 53,016 | 17.50 | 68,845 | 15.57 | 31,899 | 18.79 | ||||||
| Gender | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.831 | ||||||||||
| Male | 197,256 | 13.58 | 124,250 | 10.95 | 22,366 | 18.93 | 35,965 | 16.66 | 14,675 | 20.19 | ||||||
| Female | 221,293 | 11.65 | 140,539 | 9.24 | 30,650 | 16.45 | 32,880 | 14.38 | 17,224 | 17.59 | ||||||
| Age Group | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| 20–44 | 131,481 | 9.63 | 105,455 | 8.48 | 7706 | 13.46 | 13,900 | 14.32 | 4420 | 15.52 | ||||||
| 45–64 | 155,651 | 12.06 | 92,828 | 9.45 | 20,068 | 15.45 | 29,317 | 15.38 | 13,438 | 17.79 | ||||||
| 65–74 | 64,064 | 15.32 | 33,374 | 12.73 | 11,316 | 19.43 | 12,677 | 15.95 | 6697 | 20.04 | ||||||
| 75–84 | 49,809 | 16.80 | 24,281 | 14.06 | 10,188 | 21.14 | 9810 | 16.73 | 5530 | 21.01 | ||||||
| ≥85 | 17,544 | 16.88 | 8851 | 13.63 | 3738 | 21.03 | 3141 | 17.77 | 1814 | 22.71 | ||||||
| Comorbidity | ||||||||||||||||
| Cancer | 110,470 | 15.40 | <0.001 | 63,157 | 12.33 | <0.001 | 14,141 | 19.45 | <0.001 | 22,971 | 18.81 | <0.001 | 10,201 | 21.13 | <0.001 | <0.001 |
| Depression | 18,736 | 18.26 | <0.001 | 2978 | 11.32 | 0.020 | 10,253 | 19.67 | <0.001 | 1923 | 17.21 | 0.044 | 3582 | 20.58 | 0.004 | 0.667 |
| Past history of stroke or TIA | 19,959 | 21.03 | <0.001 | 6593 | 16.91 | <0.001 | 6883 | 23.51 | <0.001 | 3418 | 19.89 | <0.001 | 3065 | 25.58 | <0.001 | <0.001 |
| COPD | 20,203 | 20.32 | <0.001 | 7833 | 15.84 | <0.001 | 5785 | 23.99 | <0.001 | 3713 | 20.87 | <0.001 | 2872 | 24.44 | <0.001 | 0.003 |
| CKD | 22,273 | 22.78 | <0.001 | 7969 | 18.53 | <0.001 | 6621 | 26.11 | <0.001 | 4517 | 22.03 | <0.001 | 3166 | 27.54 | <0.001 | <0.001 |
| CCI score | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| CCI = 0 | 250,012 | 8.58 | 188,002 | 7.78 | 19,152 | 10.78 | 31,301 | 10.76 | 11,557 | 12.17 | ||||||
| CCI = 1 | 53,389 | 13.19 | 27,569 | 10.90 | 10,541 | 15.83 | 9654 | 14.41 | 5625 | 17.37 | ||||||
| CCI = 2 | 50,555 | 18.11 | 24,693 | 15.17 | 8845 | 20.79 | 11,469 | 20.52 | 5548 | 21.92 | ||||||
| CCI ≥ 3 | 64,593 | 23.11 | 24,525 | 21.28 | 14,478 | 25.59 | 16,421 | 21.98 | 9169 | 26.10 | ||||||
| Variable | All | PureO | PastB | NewB | ContiB | p a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | p | N | % | p | N | % | p | N | % | p | N | % | p | ||
| All | 418,549 | 13.30 | 264,789 | 9.83 | 53,016 | 22.80 | 68,845 | 15.41 | 31,899 | 21.79 | ||||||
| Gender | <0.001 | <0.001 | 0.001 | <0.001 | 0.002 | <0.001 | ||||||||||
| Male | 197,256 | 14.11 | 124,250 | 10.86 | 22,366 | 23.56 | 35,965 | 15.97 | 14,675 | 22.56 | ||||||
| Female | 221,293 | 12.59 | 140,539 | 8.92 | 30,650 | 22.25 | 32,880 | 14.80 | 17,224 | 21.14 | ||||||
| Age Group | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| 20–44 | 131,481 | 9.66 | 105,455 | 7.82 | 7706 | 20.23 | 13,900 | 14.22 | 4420 | 20.86 | ||||||
| 45–64 | 155,651 | 12.32 | 92,828 | 9.04 | 20,068 | 20.06 | 29,317 | 14.02 | 13,438 | 19.67 | ||||||
| 65–74 | 64,064 | 15.71 | 33,374 | 12.01 | 11,316 | 23.10 | 12,677 | 15.54 | 6697 | 21.97 | ||||||
| 75–84 | 49,809 | 19.93 | 24,281 | 15.75 | 10,188 | 27.68 | 9810 | 19.18 | 5530 | 25.35 | ||||||
| ≥85 | 17,544 | 21.74 | 8851 | 17.58 | 3738 | 28.68 | 3141 | 21.43 | 1814 | 28.28 | ||||||
| Comorbidity | ||||||||||||||||
| Cancer | 110,470 | 13.24 | 0.467 | 63,157 | 9.85 | 0.838 | 14,141 | 20.72 | <0.001 | 22,971 | 15.12 | 0.131 | 10,201 | 19.62 | <0.001 | <0.001 |
| Depression | 18,736 | 24.86 | <0.001 | 2978 | 15.14 | <0.001 | 10,253 | 27.86 | <0.001 | 1923 | 21.63 | <0.001 | 3582 | 26.07 | <0.001 | 0.002 |
| Past history of stroke or TIA | 19,959 | 25.57 | <0.001 | 6593 | 20.08 | <0.001 | 6883 | 30.15 | <0.001 | 3418 | 23.08 | <0.001 | 3065 | 29.89 | <0.001 | <0.001 |
| COPD | 20,203 | 24.85 | <0.001 | 7833 | 19.28 | <0.001 | 5785 | 31.03 | <0.001 | 3713 | 23.92 | <0.001 | 2872 | 28.83 | <0.001 | <0.001 |
| CKD | 22,273 | 27.59 | <0.001 | 7969 | 21.37 | <0.001 | 6621 | 33.35 | <0.001 | 4517 | 25.73 | <0.001 | 3166 | 33.86 | <0.001 | <0.001 |
| CCI score | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| CCI = 0 | 250,012 | 9.11 | 188,002 | 7.64 | 19,152 | 15.95 | 31,301 | 11.12 | 11,557 | 16.30 | ||||||
| CCI = 1 | 53,389 | 17.10 | 27,569 | 13.21 | 10,541 | 23.53 | 9654 | 18.14 | 5625 | 22.40 | ||||||
| CCI = 2 | 50,555 | 18.15 | 24,693 | 13.94 | 8845 | 25.92 | 11,469 | 18.15 | 5548 | 24.48 | ||||||
| CCI ≥ 3 | 64,593 | 22.61 | 24,525 | 18.72 | 14,478 | 29.44 | 16,421 | 20.08 | 9169 | 26.71 | ||||||
| Variable | Univariate | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| cOR | 95% CI | p | aOR | 95% CI | p | aOR | 95% CI | p | |
| Group (ref: PureO) | |||||||||
| PastB | 1.90 | (1.85–1.95) | <0.001 | 1.54 | (1.50–1.59) | <0.001 | 1.43 | (1.39–1.46) | <0.001 |
| NewB | 1.65 | (1.61–1.69) | <0.001 | 1.48 | (1.44–1.51) | <0.001 | 1.31 | (1.28–1.35) | <0.001 |
| ContiB | 2.07 | (2.01–2.14) | <0.001 | 1.74 | (1.69–1.80) | <0.001 | 1.55 | (1.50–1.60) | <0.001 |
| Gender (ref: Female) | |||||||||
| Male | 1.19 | (1.17–1.21) | <0.001 | 1.17 | (1.14–1.19) | <0.001 | 1.09 | (1.07–1.11) | <0.001 |
| Age Group (ref: 20–44) | |||||||||
| 45–64 | 1.29 | (1.26–1.32) | <0.001 | 1.06 | (1.04–1.09) | <0.001 | 0.91 | (0.89–0.94) | <0.001 |
| 65–74 | 1.70 | (1.65–1.75) | <0.001 | 1.30 | (1.26–1.34) | <0.001 | 1.05 | (1.01–1.08) | 0.004 |
| 75–84 | 1.90 | (1.84–1.95) | <0.001 | 1.37 | (1.33–1.42) | <0.001 | 1.10 | (1.07–1.14) | <0.001 |
| ≥85 | 1.91 | (1.83–1.99) | <0.001 | 1.36 | (1.30–1.42) | <0.001 | 1.11 | (1.06–1.16) | <0.001 |
| Comorbidity | |||||||||
| Cancer | 1.40 | (1.37–1.42) | <0.001 | 1.35 | (1.32–1.37) | <0.001 | |||
| Depression | 1.59 | (1.53–1.66) | <0.001 | 1.16 | (1.11–1.20) | <0.001 | |||
| Past history of stroke or TIA | 1.93 | (1.86–2.00) | <0.001 | 1.37 | (1.32–1.42) | <0.001 | |||
| COPD | 1.84 | (1.78–1.91) | <0.001 | 1.32 | (1.27–1.37) | <0.001 | |||
| CKD | 2.17 | (2.10–2.24) | <0.001 | 1.64 | (1.59–1.70) | <0.001 | |||
| CCI score (ref: CCI = 0) | |||||||||
| CCI = 1 | 1.62 | (1.57–1.67) | <0.001 | 1.46 | (1.42–1.50) | <0.001 | |||
| CCI = 2 | 2.36 | (2.29–2.42) | <0.001 | 2.12 | (2.06–2.18) | <0.001 | |||
| CCI ≥ 3 | 3.20 | (3.13–3.28) | <0.001 | 2.72 | (2.65–2.79) | <0.001 | |||
| Variable | Univariate | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| cOR | 95%CI | p | aOR | 95% CI | p | aOR | 95% CI | p | |
| Group (ref: PureO) | |||||||||
| PastB | 2.71 | (2.65–2.78) | <0.001 | 2.04 | (1.99–2.10) | <0.001 | 2.07 | (2.02–2.12) | <0.001 |
| NewB | 1.67 | (1.63–1.71) | <0.001 | 1.51 | (1.48–1.55) | <0.001 | 1.37 | (1.34–1.41) | <0.001 |
| ContiB | 2.56 | (2.48–2.63) | <0.001 | 2.09 | (2.03–2.16) | <0.001 | 1.97 | (1.91–2.03) | <0.001 |
| Gender (ref: Female) | |||||||||
| Male | 1.14 | (1.12–1.16) | <0.001 | 1.11 | (1.09–1.13) | <0.001 | 1.08 | (1.06–1.10) | <0.001 |
| Age Group (ref: 20–44) | |||||||||
| 45–64 | 1.31 | (1.28–1.35) | <0.001 | 1.08 | (1.05–1.11) | <0.001 | 0.93 | (0.91–0.96) | <0.001 |
| 65–74 | 1.74 | (1.69–1.79) | <0.001 | 1.27 | (1.23–1.30) | <0.001 | 1.07 | (1.04–1.10) | <0.001 |
| 75–84 | 2.33 | (2.26–2.40) | <0.001 | 1.56 | (1.51–1.61) | <0.001 | 1.36 | (1.31–1.40) | <0.001 |
| ≥85 | 2.60 | (2.49–2.70) | <0.001 | 1.69 | (1.62–1.76) | <0.001 | 1.52 | (1.46–1.59) | <0.001 |
| Comorbidity | |||||||||
| Cancer | 0.99 | (0.97–1.01) | 0.467 | 0.94 | (0.92–0.96) | <0.001 | |||
| Depression | 2.26 | (2.19–2.34) | <0.001 | 1.40 | (1.34–1.45) | <0.001 | |||
| Past history of stroke or TIA | 2.36 | (2.29–2.44) | <0.001 | 1.43 | (1.38–1.49) | <0.001 | |||
| COPD | 2.27 | (2.20–2.35) | <0.001 | 1.49 | (1.44–1.54) | <0.001 | |||
| CKD | 2.67 | (2.59–2.75) | <0.001 | 1.84 | (1.78–1.90) | <0.001 | |||
| CCI score (ref: CCI = 0) | |||||||||
| CCI = 1 | 2.06 | (2.01–2.11) | <0.001 | 1.68 | (1.63–1.72) | <0.001 | |||
| CCI = 2 | 2.21 | (2.15–2.27) | <0.001 | 1.80 | (1.75–1.86) | <0.001 | |||
| CCI ≥ 3 | 2.92 | (2.85–2.98) | <0.001 | 2.16 | (2.10–2.21) | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, F.-Y.; Tsai, M.-C.; Ng, Y.-Y.; Wu, S.-C. Association Between Different Patterns of Opioid and Benzodiazepine Use and Risks of Emergency Department Visits and Hospitalizations: A Retrospective Cohort Study. Healthcare 2025, 13, 2073. https://doi.org/10.3390/healthcare13162073
Su F-Y, Tsai M-C, Ng Y-Y, Wu S-C. Association Between Different Patterns of Opioid and Benzodiazepine Use and Risks of Emergency Department Visits and Hospitalizations: A Retrospective Cohort Study. Healthcare. 2025; 13(16):2073. https://doi.org/10.3390/healthcare13162073
Chicago/Turabian StyleSu, Fang-Yu, Ming-Che Tsai, Yee-Yung Ng, and Shiao-Chi Wu. 2025. "Association Between Different Patterns of Opioid and Benzodiazepine Use and Risks of Emergency Department Visits and Hospitalizations: A Retrospective Cohort Study" Healthcare 13, no. 16: 2073. https://doi.org/10.3390/healthcare13162073
APA StyleSu, F.-Y., Tsai, M.-C., Ng, Y.-Y., & Wu, S.-C. (2025). Association Between Different Patterns of Opioid and Benzodiazepine Use and Risks of Emergency Department Visits and Hospitalizations: A Retrospective Cohort Study. Healthcare, 13(16), 2073. https://doi.org/10.3390/healthcare13162073







