Telerehabilitation After Surgery in Adolescent Idiopathic Scoliosis: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Size and Randomization
2.3. Telerehabilitation Group
2.4. Control Group
2.5. Measurements
2.6. Statistical Analysis
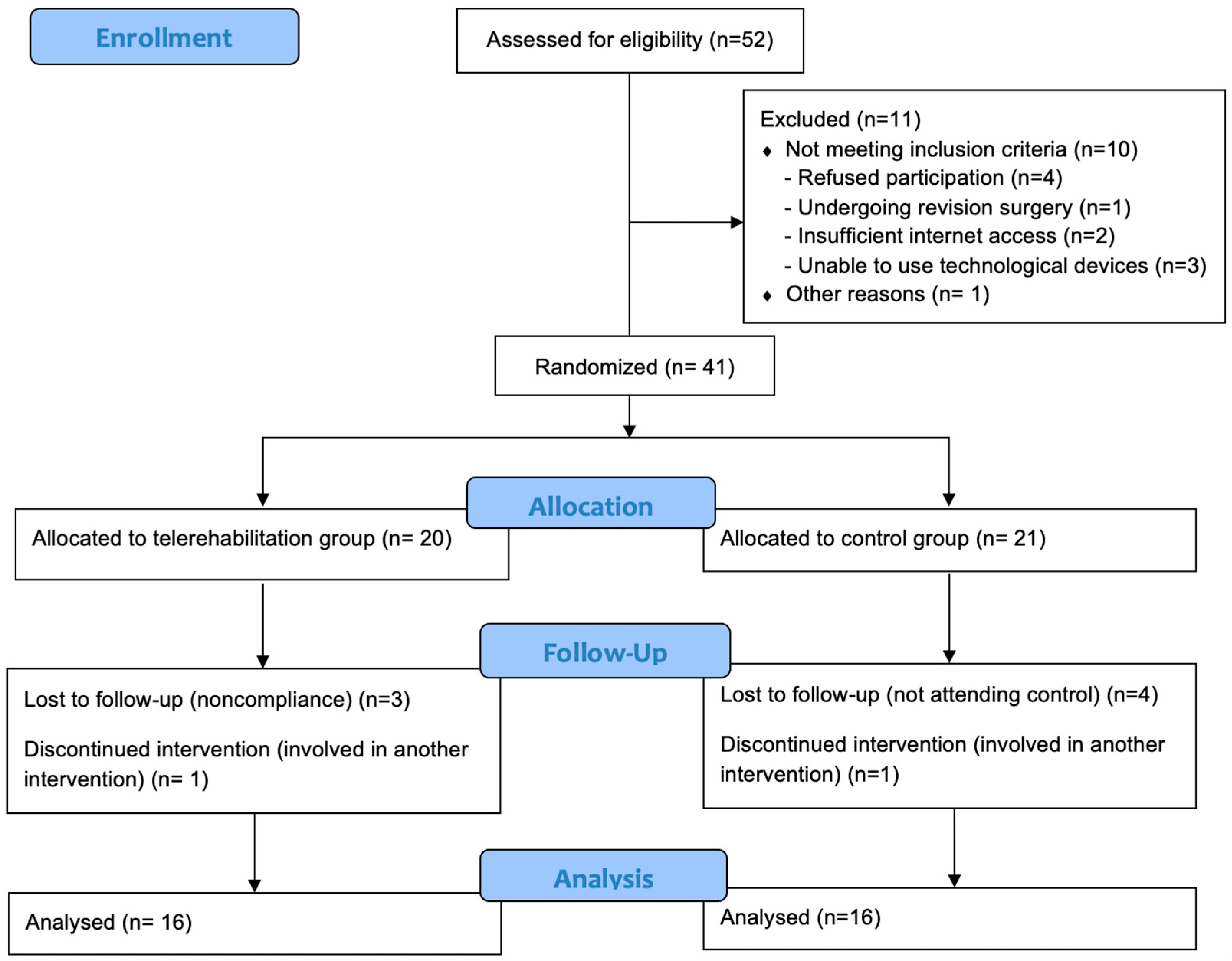
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | Adolescent Idiopathic Scoliosis |
| BMI | Body Mass Index |
| CG | Control Group |
| L | Lumbar |
| PSFI | Posterior Spinal Fusion and Instrumentation |
| PTR | Physiotherapy and Rehabilitation |
| SAQ | Spinal Appearance Questionnaire |
| SRS-30 | Scoliosis Research Society-30 |
| T | Thoracic |
| TG | Telerehabilitation Group |
| TL | Thoracolumbar |
| TrA | Transversus Abdominis |
Appendix A. Exercise Protocol
- Step counting in place;
- Lifting arms forward, sideways, and diagonally while counting steps in place;
- Step counting with reciprocal lower- and upper-extremity movements;
- Soldier march;
- Jogging in place at a light pace.

| Warm-up program |
| Core muscle activation consisting of 8 s isometric contractions together with diaphragmatic breathing while maintaining the neutral position of the spine and pelvis was applied in the static positions specified below (Figure A2). Core muscle activation:
|
| Cool-down–stretching exercises. |

| Warm-up program |
Transition to dynamic phase—while core muscle activation was achieved, the following exercises were applied (Figure A3):
|
| Cool down–stretching exercises. |

| Warm-up program |
Advanced dynamic phase—while core muscle activation was achieved, the following exercises were applied (Figure A4):
|
| Cool-down–stretching exercises. |

| Warm-up program |
Advanced dynamic phase—while core muscle activation was achieved, the following exercises were applied (Figure A5):
|
| Cool-down–stretching exercises. |

| Warm-up program |
While core muscle activation was achieved, the following exercises were applied (dumbbells weighing 1 to 2 kg included) (Figure A6):
|
| Cool-down–stretching exercises. |

| Warm-up program |
While core muscle activation was achieved, the following exercises were applied (medium-resistance elastic band included) (Figure A7):
|
| Cool-down–stretching exercises. |

| Warm-up program |
While core muscle activation was achieved, the following exercises were applied (advanced phase in postural control, strength, and endurance training) (Figure A8):
|
| Cool-down–stretching exercises. |

| Warm-up program |
While core muscle activation was achieved, the following exercises were applied (functional training in bipedal stance and final phase with short general repetitions) (Figure A9):
|
| Cool-down–stretching exercises. |

- Lumbar extensor stretching;
- Iliopsoas stretching;
- Piriformis stretching;
- Hamstring stretching;
- Rectus femoris stretching;
- Hip adductor stretching;
- Upper extremity capsular stretching;
- Pectoral stretching and cervical stretching as needed.

References
- Lambrechts, M.J.; Boeyer, M.E.; Tweedy, N.M.; Gupta, S.K.; Kimchi, E.T.; Hoernschemeyer, D.G. Team Integrated Enhanced Recovery (TIGER) Protocol after adolescent idiopathic scoliosis correction lowers direct cost and length of stay while increasing daily contribution margins. Mo Med. 2022, 119, 152–157. [Google Scholar]
- Upasani, V.V.; Hedequist, D.J.; Hresko, M.T.; Karlin, L.I.; Emans, J.B.; Glotzbecker, M.P. Spinal deformity progression after posterior segmental instrumentation and fusion for idiopathic scoliosis. J. Child. Orthop. 2015, 9, 29–37. [Google Scholar] [CrossRef]
- Danielsson, A.J.; Romberg, K.; Nachemson, A.L. Spinal range of motion, muscle endurance, and back pain and function at least 20 years after fusion or brace treatment for adolescent idiopathic scoliosis: A case-control study. Spine 2006, 31, 275–283. [Google Scholar] [CrossRef]
- Engsberg, J.R.; Lenke, L.G.; Uhrich, M.L.; Ross, S.A.; Bridwell, K.H. Prospective comparison of gait and trunk range of motion in adolescents with idiopathic thoracic scoliosis undergoing anterior or posterior spinal fusion. Spine 2003, 28, 1993–2000. [Google Scholar] [CrossRef]
- Lorente, A.; Barrios, C.; Burgos, J.; Hevia, E.; Fernández-Pineda, L.; Lorente, R.; Rosa, B.; Pérez-Encinas, C. Cardiorespiratory function does not improve 2 years after posterior surgical correction of adolescent idiopathic scoliosis. Spine 2017, 42, 1391–1397. [Google Scholar] [CrossRef]
- Danielsson, A.J.; Wiklund, I.; Pehrsson, K.; Nachemson, A.L. Health-related quality of life in patients with adolescent idiopathic scoliosis: A matched follow-up at least 20 years after treatment with brace or surgery. Eur. Spine J. 2001, 10, 278–288. [Google Scholar] [CrossRef]
- Weiss, H.R. Rehabilitation of scoliosis patients with pain after surgery. Stud. Health Technol. Inform. 2002, 88, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Laurentowska, M.; Glowacki, M.; Deskur-Śmielecka, E.; Barinow-Wojewódzki, A. Effects of rehabilitation based on endurance training in adolescent girls with surgically treated scoliosis. Biol. Sport 2009, 26, 45–53. [Google Scholar] [CrossRef]
- Marti, C.L.; Glassman, S.D.; Knott, P.T.; Carreon, L.Y.; Hresko, M.T. Scoliosis Research Society members attitudes towards physical therapy and physiotherapeutic scoliosis specific exercises for adolescent idiopathic scoliosis. Scoliosis 2015, 10, 16. [Google Scholar] [CrossRef]
- Cottrell, M.A.; Galea, O.A.; O’Leary, S.P.; Hill, A.J.; Russell, T.G. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: A systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 625–638. [Google Scholar] [CrossRef]
- Simmich, J.; Ross, M.H.; Russell, T. Real-time video telerehabilitation shows comparable satisfaction and similar or better attendance and adherence compared with in-person physiotherapy: A systematic review. J. Physiother. 2024, 70, 181–192. [Google Scholar] [CrossRef]
- Pastora-Bernal, J.M.; Martín-Valero, R.; Barón-López, F.J.; Estebanez-Pérez, M.J. Evidence of benefit of telerehabitation after orthopedic surgery: A systematic review. J. Med. Internet Res. 2017, 19, e142. [Google Scholar] [CrossRef] [PubMed]
- van Egmond, M.A.; van der Schaaf, M.; Vredeveld, T.; Vollenbroek-Hutten, M.M.R.; van Berge Henegouwen, M.I.; Klinkenbijl, J.H.G.; EngelbertRHH. Effectiveness of physiotherapy with telerehabilitation in surgical patients: A systematic review and meta-analysis. Physiotherapy 2018, 104, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Diwan, S.; Kohan, L.; Rosenblum, D.; Gharibo, C.; Soin, A.; Sulindro, A.; Quinn Nguyen, Q.; Provenzano, D.A. The technological impact of COVID-19 on the future of education and health care delivery. Pain Physician 2020, 23, S367–S380. [Google Scholar] [CrossRef] [PubMed]
- Tanner, K.; Bican, R.; Boster, J.; Christensen, C.; Coffman, C.; Fallieras, K.; Long, R.; Mansfield, C.; O’ROurke, S.; Pauline, L.; et al. Feasibility and acceptability of clinical pediatric telerehabilitation services. Int. J. Telerehabilitation 2020, 12, 43–52. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- McGregor, A.H.; Probyn, K.; Cro, S.; Doré, C.J.; Burton, A.K.; Balagué, F.; Pincus, T.; Fairbank, J. Rehabilitation following surgery for lumbar spinal stenosis: A Cochrane review. Spine 2014, 39, 1044–1054. [Google Scholar] [CrossRef]
- Scandelli, F.; De Leo, D.; Marino, G.; De Martino, E.; Cannizzaro, D.; Adamo, P.; Temporiti, F. Effects of Supervised Rehabilitation on Psychosocial and Participation-Related Outcomes after Lumbar Spine Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 7246. [Google Scholar] [CrossRef]
- Kernc, D.; Strojnik, V.; Vengust, R. Early initiation of a strength training-based rehabilitation after lumbar spine fusion improves core muscle strength: A randomized controlled trial. J. Orthop. Surg. Res. 2018, 13, 151. [Google Scholar] [CrossRef]
- McGill, S.M.; Karpowicz, A. Exercises for spine stabilization: Motion/motor patterns, stability progressions, and clinical technique. Arch. Phys. Med. Rehabil. 2009, 90, 118–126. [Google Scholar] [CrossRef]
- Canbulat, N. Rehabilitation after surgery of the spinal deformity. Türk Nöroşirürji Dergisi 2013, 23, 106–113. [Google Scholar]
- Paul, S.M. Scoliosis and Other Spinal Deformities. In DeLisa Physical Medicine and Rehabilitation: Principles and Practice, 5th ed.; Frontera, W.R., Ed.; Güneş Tıp Kitabevleri: Istanbul, Turkey, 2007; pp. 883–906. [Google Scholar]
- McGill, S.M.; Childs, A.; Liebenson, C. Endurance times for low back stabilization exercises: Clinical targets for testing and training from a normal database. Arch. Phys. Med. Rehabil. 1999, 80, 941–944. [Google Scholar] [CrossRef]
- Biering-Sørensen, F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine 1984, 9, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Shirado, O.; Suzuki, H.; Takahashi, M.; Kaneda, K.; Strax, T.E. Lumbar trunk muscle endurance testing: An inexpensive alternative to a machine for evaluation. Arch. Phys. Med. Rehabil. 1996, 77, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Evcik, D. Kas Boyu Ölçüm Testleri ve Esneklik. In Kas İskelet Sisteminde Pratik Ölçme ve Değerlendirme; Evcik, D., Ed.; Pelikan Publishing: Ankara, Turkey, 2008; pp. 111–123. [Google Scholar]
- Otman, A.S.; Köse, N. Esneklik ve Değerlendirmesi. In Tedavi Hareketlerinde Temel Değerlendirme Prensipleri, 11th ed.; Otman, A.S., Köse, N., Eds.; Hipokrat Publishing: Ankara, Turkey, 2019; pp. 47–52. [Google Scholar]
- Troosters, T.; Gosselink, R.; Decramer, M. Six-minute walk test: A valuable test, when properly standardized. Phys. Ther. 2002, 82, 826–828. [Google Scholar] [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef]
- Yapar, A.; Yapar, D.; Ergisi, Y.; Kaptan, A.Y.; Tokgoz, M.A.; Senkoylu, A. Reliability and validity of the adapted Turkish version of the Spinal Appearance Questionnaire. Spine Deform. 2021, 9, 57–66. [Google Scholar] [CrossRef]
- Aksekili, M.A.E.; Demir, P.; Iyigun, A.; Akcaalan, S.; Korkmazer, S.; Tecimel, O.; Senkoylu, A. Turkish validity and reliability study of Scoliosis Research Society-30 questionnaire in adolescent idiopathic scoliosis patients. Spine 2021, 46, E1058–E1064. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Rosenthal, R. Parametric measures of effect size. In The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Cooper, H., Hedges, L.V., Valentine, J.C., Eds.; Russell Sage Foundation: New York, NY, USA, 2009; pp. 231–244. [Google Scholar]
- Öztürk, F.; Deniz, H.G.; Ayvaz, M.; Demirkıran, H.G.; Kınıklı, G.İ. The relationship between trunk assessments and quality of life in adolescent idiopathic scoliosis following surgery. Turk. J. Physiother. Rehabil. 2020, 31, 36–44. [Google Scholar] [CrossRef]
- Bayraktar, D.; Özyürek, S.; Genç, A. The relationship between isometric trunk muscle endurance and physical activity related energy expenditure in healthy young adults. J. Back Musculoskelet. Rehabil. 2015, 28, 859–864. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Fan, C.; Yang, Y.; He, C.; Wong, M. Could the clinical effectiveness be improved under the integration of orthotic intervention and scoliosis-specific exercise in managing adolescent idiopathic scoliosis? A randomized controlled trial study. Am. J. Phys. Med. Rehabil. 2019, 98, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Parent, E.C.; Moez, E.K.; Hedden, D.M.; Hill, D.; Moreau, M.J.; Lou, E.; Watkins, E.M.; Southon, S.C. The effect of Schroth exercises added to the standard of care on the quality of life and muscle endurance in adolescents with idiopathic scoliosis—An assessor and statistician blinded randomized controlled trial: “SOSORT 2015 Award Winner”. Scoliosis 2015, 10, 16. [Google Scholar] [CrossRef]
- Hides, J.; Wilson, S.; Stanton, W.; McMahon, S.; Keto, H.; McMahon, K.; Bryant, M.; Richardson, C. An MRI investigation into the function of the transversus abdominis muscle during “drawing-in” of the abdominal wall. Spine 2006, 31, E175–E178. [Google Scholar] [CrossRef]
- Kim, Y.; Shim, J.K.; Son, J.; Pyeon, H.Y.; Yoon, B. A neuromuscular strategy to prevent spinal torsion: Backward perturbation alters asymmetry of transversus abdominis muscle thickness into symmetry. Gait Posture 2013, 38, 231–235. [Google Scholar] [CrossRef]
- Mehkri, Y.; Hernandez, J.; McQuerry, J.L.; Carmona, J.; Ihnow, S. Global spine range of motion in patients with adolescent idiopathic scoliosis before and after corrective surgery. Cureus 2021, 13, e19362. [Google Scholar] [CrossRef]
- Engsberg, J.R.; Lenke, L.; Reitenbach, A.K.; Hollander, K.W.; Bridwell, K.H.; Blanke, K. Prospective evaluation of trunk range of motion in adolescents with idiopathic scoliosis undergoing spinal fusion surgery. Spine 2002, 27, 1346–1354. [Google Scholar] [CrossRef]
- Nokariya, S.; Kotani, T.; Sakuma, T.; Iijima, Y.; Okumura, T.; Katogi, T.; Okuwaki, S.; Miyagi, M.; Inoue, G.; Akazawa, T.; et al. Trunk flexibility using a sit-and-reach test after surgery for adolescent idiopathic scoliosis. Spine Deform. 2023, 11, 297–303. [Google Scholar] [CrossRef]
- Sanchez-Raya, J.; Bago, J.; Pellise, F.; Cuxart, A.; Villanueva, C. Does the lower instrumented vertebra have an effect on lumbar mobility, subjective perception of trunk flexibility, and quality of life in patients with idiopathic scoliosis treated by spinal fusion? J. Spinal Disord. Tech. 2021, 25, 437–442. [Google Scholar] [CrossRef]
- Marks, M.; Newton, P.; Petcharaporn, M.; Bastrom, T.P.; Shah, S.; Betz, R.; Lonner, R.; Miyanji, F. Postoperative segmental motion of the unfused spine distal to the fusion in 100 patients with adolescent idiopathic scoliosis. Spine 2021, 37, 826–832. [Google Scholar] [CrossRef]
- Bazancir, Z.; Talu, B.; Korkmaz, M.F. Postoperative rehabilitation versus early mobilization following scoliosis surgery: A single-blind randomized clinical trial. J. Orthop. Sci. 2023, 28, 308–314. [Google Scholar] [CrossRef]
- Shen, J.; Lin, Y.; Luo, J.; Xiao, Y. Cardiopulmonary exercise testing in patients with idiopathic scoliosis. J. Bone Jt. Surg. Am. 2016, 98, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, E.F.; Alexandre, A.S.; Liu, C.Y.; Poletto, P.R.; Gotfryd, A.O.; Vidotto, M.C.; Dourado, V.Z. Functional aerobic exercise capacity limitation in adolescent idiopathic scoliosis. Spine J. 2014, 14, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, A.J.; Nachemson, A.L. Back pain and function 23 years after fusion for adolescent idiopathic scoliosis: A case-control study—Part II. Spine 2003, 28, E373–E383. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.R.; Turnbull, D.; Tournavitis, N.; Borysov, M. Treatment of scoliosis—Evidence and management (review of the literature). Middle East J. Rehabil. Health Stud. 2016, 3, 35377. [Google Scholar] [CrossRef]
- Ferland, C.E.; Saran, N.; Valois, T.; Bote, S.; Chorney, J.M.; Stone, L.S.; Ouellet, J.A. Preoperative distress factors predicting postoperative pain in adolescents undergoing surgery: A preliminary study. J. Pediatr. Health Care 2017, 31, 5–15. [Google Scholar] [CrossRef]
- Koch, K.D.; Buchanan, R.; Birch, J.G.; Morton, A.A.; Gatchel, R.J.; Browne, R.H. Adolescents undergoing surgery for idiopathic scoliosis: How physical and psychological characteristics relate to patient satisfaction with the cosmetic result. Spine 2001, 26, 2119–2124. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ch’ng, P.Y.; Wong, T.S.; Link, X.W.; Chung, W.H.; Chiu, C.K.; Chan, C.Y.W.; Lean, M.L.; Kwan, M.K. Patients’ perception and satisfaction on neck and shoulder imbalance in adolescent idiopathic scoliosis. Global Spine J. 2023, 13, 752–763. [Google Scholar] [CrossRef]
- Nikouei, F.; Ghandhari, H.; Ameri, E.; Mokarami, F. Shoulder imbalance in adolescent idiopathic scoliosis: A systematic review of the current state of the art. Arch. Bone Jt. Surg. 2022, 10, 992–1003. [Google Scholar] [CrossRef]
- Fällström, K.; Cochran, T.; Nachemson, A. Long-term effects on personality development in patients with adolescent idiopathic scoliosis: Influence of type of treatment. Spine 1986, 11, 756–758. [Google Scholar] [CrossRef]
- Savvides, P.; Gerdhem, P.; Grauers, A.; Danielsson, A.; Diarbakerli, E. Self-experienced trunk appearance in individuals with and without idiopathic scoliosis. Spine 2020, 45, 522–527. [Google Scholar] [CrossRef]
- Tsutsui, S.; Pawelek, J.; Bastrom, T.; Lenke, L.; Lowe, T.; Betz, R.; Clements, D.; Newton, P.O. Dissecting the effects of spinal fusion and deformity magnitude on quality of life in patients with adolescent idiopathic scoliosis. Spine 2009, 34, E653–E658. [Google Scholar] [CrossRef] [PubMed]
- Helenius, L.; Diarbakerli, E.; Grauers, A.; Lastikka, M.; Oksanen, H.; Pajulo, O.; Löyttyniemi, E.; Manner, T.; Gerdhem, P.; Helenius, I. Back pain and quality of life after surgical treatment for adolescent idiopathic scoliosis at 5-year follow-up: Comparison with healthy controls and patients with untreated idiopathic scoliosis. JBJS 2019, 101, 1460–1466. [Google Scholar] [CrossRef]
- Charalampidis, A.; Rundberg, L.; Möller, H.; Gerdhem, P. Predictors of persistent postoperative pain after surgery for idiopathic scoliosis. J. Child. Orthop. 2021, 15, 458–463. [Google Scholar] [CrossRef]
- Pellegrino, L.N.; Avanzi, O. Prospective evaluation of quality of life in adolescent idiopathic scoliosis before and after surgery. J. Spinal Disord. Tech. 2014, 27, 409–414. [Google Scholar] [CrossRef]
- Alzakri, A.; AlMuhid, F.; Almousa, N.; Aljehani, M.; Alhalabi, H. Saudi patients outcomes after surgical treatment of adolescent idiopathic scoliosis. J. Orthop. Surg. Res. 2023, 18, 450. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Tsirikos, A.I. Current concepts and controversies on adolescent idiopathic scoliosis: Part II. Indian J. Orthop. 2013, 47, 219–229. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | TG (n = 16) | CG (n = 16) | p |
|---|---|---|---|
| Age (years, mean ± SD) | 17.38 ± 2.36 | 17.44 ± 2.07 | 0.937 a |
| Gender (female/male, %) | 13/3, (81.3/18.8) | 10/6, 62.5/37.5 | 0.433 b |
| Height (m, mean ± SD) | 166.75 ± 9.46 | 169.38 ± 8.85 | 0.424 a |
| Weight (kg, mean ± SD) | 56.81 ± 8.04 | 56.19 ± 8.69 | 0.834 a |
| BMI (kg/m2, mean ± SD) | 20.47 ± 2.5 | 19.64 ± 2.85 | 0.383 a |
| PTR history (yes/no, %) | 5/11, (31.3/68.8) | 4/12, (25/75) | 1.000 b |
| Sports habit (yes/no, %) | 1/15 (6.3/93.8) | 2/14 (12.5/87.5) | 1.000 b |
| Scoliosis classification (T, TL, L, %) | 9/4/3, 56.3/25/18.8 | 8/6/2 (50/37.5/12.5) | 0.796 b |
| Risser stage (mean ± SD) | 4.44 ± 0.81 | 4.69 ± 0.6 | 0.299 c |
| Preop. Cobb angle (°, mean ± SD) | 55 ± 14.22 | 55.25 ± 9.28 | 0.953 a |
| Postop. Cobb angle (°, mean ± SD) | 12.31 ± 5.85 | 9.88 ± 4.97 | 0.273 c |
| Number of fused vertebrae (mean ± SD) | 11.69 ± 2.5 | 11.63 ± 2.91 | 0.924 c |
| Time since surgery (months, mean ± SD) | 16.81 ± 6.12 | 16 ± 5.89 | 0.592 c |
| Post-surgical brace duration (months, mean ± SD) | 3.25 ± 2.08 | 2.69 ± 1.7 | 0.469 c |
| TG (n = 16) Mean ± SD/Median (Min.–Max.) | CG (n = 16) Mean ± SD/Median (Min.–Max.) | p (Between-Group) | d/r [95% CI] (Between-Group) | ||
|---|---|---|---|---|---|
| Kraus–Weber test (sec) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 42.88 ± 35.16/31 (6–124) 67 ± 34.89/59 (27–148) <0.001 c −0.88 [−0.96−(−0.68)] | 35.75 ± 28.36/30.5 (6–105) 41.06 ± 35.32/26.5 (12–96) 0.103 c −0.41 [−0.75–0.11] | 0.546 b 0.013 b | −0.44 [−0.68–(−0.11)] |
| Biering–Sørensen test (sec) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 12.13 ± 14.72/7 (1–55) 41.44 ± 30.13/26.5 (12–96) <0.001 c −0.87 [0.95−(−0.68)] | 15.25 ± 14.51/12 (1–52) 15.81 ± 13.31/13 (1–43) 0.529 c −0.16 [−0.61–0.37] | 0.427 b 0.005 b | −0.49 [−0.72–(−0.18] |
| Lateral Bridge test–left (sec) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 16.5 ± 14.02/15 (1–45) 31.94 ± 18.16/28 (9–62) <0.001 d −1.48 [−20.1−(−9.88)] | 16.19 ± 14.99/13.5 (1–62) 18.94 ± 16.92/16 (1–67) 0.074 c −0.45 [−0.77–0.06] | 0.94 b 0.035 b | −0.37 [−0.64–(−0.03)] |
| Lateral Bridge test–right (sec) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 14.94 ± 12.32/12 (1–36) 31.75 ± 15.17/31 (10–57) <0.001 c −0.88 [−0.96−(−0.68)] | 16.13 ± 12.14/15 (1–47) 16.31 ± 12.74/15 (1–50) 0.864 d −0.04 [−2.48–2.1] | 0.691 b 0.004 a | 1,1 [5.31–25.57] |
| Sit-and-Reach test (cm) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | −22.06 ± 11.67/−23 (−38−[−1]) −17.88 ± 11.67/−16.5 (−35–5) <0.001 d −1.91 [−5.36−(−3.02)] | −25.75 ± 9.87/−25 (−46−[−1]) −25.88 ± 9.34/−26 (−44−[−1]) 0.827 d −0.06 [−1.07–1.32] | 0.342 a 0.041 a | 0.76 [0.35–15.65] |
| Lateral Bending test–left (cm) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 9.88 ± 3.4/10 (5–15) 11.88 ± 3.44/11.5 (6–17) <0.001 d −1.73 [−2.61−(−1.39)] | 8.94 ± 2.2/8.5 (6–13) 9.25 ± 2.52/8.5 (6–14) 0.173 d −0.36 [−0.78–0.15] | 0.362 a 0.02 a | 0.87 [0.44–4.81] |
| Lateral Bending test–right (cm) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 9.75 ± 3.66/8.5 (5–15) 11.63 ± 3.78/11.5 (7–18) 0.001 c −0.83 [−0.94−(−0.57)] | 8.5 ± 2.39/9 (4–12) 8.81 ± 2.43/8.5 (5–13) 0.333 d −0.24 [−0.98–0.35] | 0.414 b 0.018 a | 0.89 [0.5–5.12] |
| Six-Minute Walk Test (m) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 544.75 ± 64.86/531.5 (465–690) 565.38 ± 58.56/553.5 (495–685) <0.001 c −0.79 [−0.92−(−0.49)] | 557.56 ± 55.19/527.5 (502–675) 556.13 ± 56.59/533.5 (483–665) 0.842 c −0.05 [−0.53–0.45] | 0.365 b 0.598 b | −0.09 [−0.43–0.26] |
| Numeric Rating Scale | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 4.69 ± 1.96/5 (1–7) 1.94 ± 0.998/2 (1–4) 0.001 c −0.86 [−0.95−(−0.64)] | 3.94 ± 1.95/4 (1–7) 4.13 ± 2.13/5 (1–7) 0.594 d −0.13 [−0.92–0.55] | 0.286 a 0.004 b | −0.51 [−0.73−(−0.19)] |
| SAQ (total) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 30 ± 8.59/30 (16–47) 25.31 ± 5.84/26 (15–38) <0.001 d 1.24 [2.67–6.71] | 28.19 ± 9.04/31 (15–40) 28.94 ± 9.18/29 (17–47) 0.583 d −0.14 [−3.6–2.1] | 0.565 a 0.193 a | −0.47 [−9.18–1.93] |
| SAQ (appearance) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 17.63 ± 4.43/17 (12–28) 15.13 ± 2.96/15 (11–22) <0.001 d 1.13 [1.32–3.69] | 16.69 ± 4.62/16.5 (10–24) 17.13± 5.37/16.5 (10–30) 0.578 d −0.14 [−2.08–1.2] | 0.562 a 0.202 a | −0.46 [−5.13–1.13] |
| SAQ (expectation) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 12.38 ± 6.58/15.5 (4–20) 10.19 ± 4.45/10.5 (4–16) 0.01 c −0.64 [−0.86−(−0.22)] | 11.5 ± 5.42/12.5 (4–20) 11.81 ± 5.33/13 (5–20) 0.739 d −0.08 [−2.28–1.65] | 0.531 b 0.357 a | −0.33 [−5.17–1.92] |
| SRS-30 (total) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 19.08 ± 2.44 /19 (15–23.8) 21.68 ± 1.41/ 21.4 (19.1–24.1) <0.001 d −1.73 [−3.4−(−1.8)] | 20.54 ± 2.07/20.6 (17.5–24.1) 19.99 ± 2.02/20 (16.5–23.2) 0.021 d −0.64 [0.09–0.99] | 0.077 b 0.01 a | 0.97 [0.42–2.94] |
| SRS-30 (pain) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 3.63 ± 0.75/ 3.5 (2.3–5) 4.3 ± 0.34/ 4.3 (3.8–4.8) <0.001 d −1.19 [−0.96−(−0.37)] | 3.99 ± 0.69/ 4.2 (2.8–5) 3.83 ± 0.76/ 3.9 (2.6–5) 0.033 d −0.59 [0.01–0.3] | 0.179 a 0.032 a | 0.8 [0.04–0.9] |
| SRS-30 (function) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 3.74 ± 0.66/ 3.9 (2.4–4.7) 4.38 ± 0.45/ 4.4 (3.7–5) <0.001 d −1.56 [−0.86−(−0.43)] | 4.11 ± 0.54/4 (3.3–5) 4.05 ± 0.42/4 (3.4–4.9) 0.485 d −0.18 [−0.11–0.22] | 0.093 a 0.004 a | 0.76 [0.02–0.65] |
| SRS-30 (self-image) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 4.06 ± 0.53/4 (3–5) 4.4 ± 0.47/4.5 (3.4–5) 0.015 d −0.68 [−0.59−(−0.07)] | 4.21 ± 0.49/4.2 (3.4–5) 4.06 ± 0.5/4.1 (3.3–5) 0.163 d −0.37 [−0.07–0.38] | 0.438 a 0.54 a | 0.71 [−0.06–0.69] |
| SRS-30 (mental health) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 3.29 ± 0.58/3.1 (2.4–4.4) 3.7 ± 0.41/3.6 (3–4.4) 0.004 d −0.85 [−0.67−(−0.15)] | 3.65 ± 0.44/3.6 (3–4.4) 3.65 ± 0.6/3.7 (2.2–4.4) 1.000 d 0.0 [−0.23–0.23] | 0.055 a 0.786 a | 0.1 [−0.32–0.42] |
| SRS-30 (satisfaction) | Pre-treatment Post-treatment p (within-group) d/r [95% CI] | 4.33 ± 0.76/4.7 (2–5) 4.89 ± 0.21/5 (4.3–5) 0.002 c −0.77 [−0.92−(−0.45)] | 4.58 ± 0.53/4.7 (3.7–5) 4.63 ± 0.42/4.7 (4–5) 0.865 c −0.04 [−0.53–0.46] | 0.205 b 0.046 b | −0.35 [−0.63−(−0.005] |
| TG (n = 16) Mean ± SD/Median (Min.–Max.) | CG (n = 16) Mean ± SD/Median (Min.–Max.) | p | d/r [95% CI] | ||
|---|---|---|---|---|---|
| Kraus–Weber test (sec) | ∆ pre-post | 24.13 ± 12.73/21 (9–66) | 5.31 ± 10.24/3 (−6–29) | <0.001 b | −0.67 [−0.83−(−0.43)] |
| Biering–Sørensen test (sec) | ∆ pre-post | 29.31 ± 19.61/24 (9–68) | 0.56 ± 7.02/1.5 (−17–14) | <0.001 b | −0.82 [−0.91−(−0.66)] |
| Lateral Bridge test–left (sec) | ∆ pre-post | 15.44 ± 10.4/10.5 (2–41) | 2.75 ± 5.5/2.5 (−6–17) | <0.001 a | 1.522 [6.59–18.79] |
| Lateral Bridge test–right (sec) | ∆ pre-post | 16.81 ± 8.76/15 (4–32) | 0.19 ± 4.29/0 (−7–9) | <0.001 a | 2.411 [11.57–21.68] |
| Sit-and-Reach test (cm) | ∆ pre-post | 4.06 ± 2.32/3.5 (1–9) | −0.13 ± 2.25/0 (−5–4) | <0.001 a | 1.94 [2.71–5.92] |
| Lateral Bending test–left (cm) | ∆ pre-post | 2 ± 1.16/2 (0–4) | 0.31 ± 0.87/0.5 (−2–1) | <0.001 b | −0.65 [−0.81−(−0.39)] |
| Lateral Bending test–right (cm) | ∆ pre-post | 1.88 ± 1.36/1.5 (0–4) | 0.31 ± 1.25/0 (−2–3) | 0.003 b | −0.52 [−0.74−(−0.21)] |
| Six-Minute Walk Test (m) | ∆ pre-post | 20.63 ± 17.55/19 (−8–50) | −1.44 ± 14.77/−2.5 (−42–17) | <0.001 b | −0.6 [−0.79−(−0.32)] |
| Numeric Rating Scale | ∆ pre-post | −2.75 ± 1.39/−3 (−5–0) | 0.19 ± 1.38/0 (−3–3) | <0.001 b | −0.76 [−0.87−(−0.55)] |
| SAQ (total) | ∆ pre-post | −4.69 ± 3.79/−4 (−12–1) | 0.75 ± 5.35/2 (−12–9) | 0.002 a | −1.173 [−8.78–2.09] |
| SAQ (appearance) | ∆ pre-post | −2.5 ± 2.22/−1.5 (−7–0) | 0.44 ± 3.08/0 (−4–10) | 0.001 b | −0.61 [−0.79−(−0.33)] |
| SAQ (expectation) | ∆ pre-post | −2.19 ± 3.08/−2 (−9–2) | 0.31 ± 3.68/1 (−8–8) | 0.046 a | −0.736 [−4.95–0.05] |
| SRS-30 (total) | ∆ pre-post | 2.6 ± 1.5/2.7 (−0.7–5.2) | −0.5 ± 0.85/−0.35 (−1.8–0.7) | <0.001 a | 2.575 [2.26–4.03] |
| SRS-30 (pain) | ∆ pre-post | 0.66 ± 0.56/0.8 (−0.3–1.5) | −0.16 ± 0.3/−0.2 (−0.7–0.5) | <0.001 a | 1.876 [0.5–1.13] |
| SRS-30 (function) | ∆ pre-post | 0.64 ± 0.4/0.65 (0.1–1.6) | −0.06 ± 0.3/−0.05 (−0.7–0.5) | <0.001 a | 1.937 [0.44–0.96] |
| SRS-30 (self-image) | ∆ pre-post | 0.33 ± 0.48/0.4 (−0.8–1.1) | −0.15 ± 0.43/−0.15 (−0.8–0.7) | 0.005 a | 1.069 [0.16–0.81] |
| SRS-30 (mental health) | ∆ pre-post | 0.41 ± 0.49/0.5 (−0.4–1.6) | 0.0 ± 0.43/−0.1 (−0.8–0.8) | 0.017 a | 0.896 [0.08–0.74] |
| SRS-30 (satisfaction) | ∆ pre-post | 0.57 ± 0.61/0.3 (0–2.3) | 0.44 ± 0.59/0 (−0.7–1.8) | 0.001 b | −0.57 [−0.76−(−0.27)] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çetinkaya, İ.; Kuru Çolak, T.; Korkmaz, M.F.; Aydoğan, M. Telerehabilitation After Surgery in Adolescent Idiopathic Scoliosis: A Randomized Controlled Trial. Healthcare 2025, 13, 2063. https://doi.org/10.3390/healthcare13162063
Çetinkaya İ, Kuru Çolak T, Korkmaz MF, Aydoğan M. Telerehabilitation After Surgery in Adolescent Idiopathic Scoliosis: A Randomized Controlled Trial. Healthcare. 2025; 13(16):2063. https://doi.org/10.3390/healthcare13162063
Chicago/Turabian StyleÇetinkaya, İrem, Tuğba Kuru Çolak, Mehmet Fatih Korkmaz, and Mehmet Aydoğan. 2025. "Telerehabilitation After Surgery in Adolescent Idiopathic Scoliosis: A Randomized Controlled Trial" Healthcare 13, no. 16: 2063. https://doi.org/10.3390/healthcare13162063
APA StyleÇetinkaya, İ., Kuru Çolak, T., Korkmaz, M. F., & Aydoğan, M. (2025). Telerehabilitation After Surgery in Adolescent Idiopathic Scoliosis: A Randomized Controlled Trial. Healthcare, 13(16), 2063. https://doi.org/10.3390/healthcare13162063






