Early Activation of a Multilevel Prostate Cancer Screening Model: Pilot Phase Results and Strategic Perspectives in Lombardy Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Program Design and Setting
2.2. Recruitment and Digital Portal Workflow
- Not eligible: If any exclusion criteria were met, the participant was thanked and informed that he was not eligible for the screening program (with reasons, e.g., recent PSA test);
- Eligible, FH-negative: If eligible and with no positive family history, the participant was directed to proceed to blood testing for PSA and informed that further steps would depend on the PSA result (with no additional risk factors);
- Eligible, FH-positive: If eligible and family history was positive, the participant was likewise directed to get a PSA test, but also notified that having a familial risk factor already places him in a higher risk category.
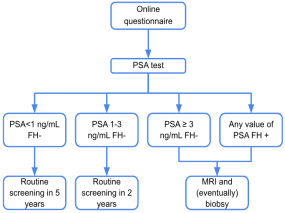
2.3. Diagnostic Criteria and Follow-Up Procedures
2.3.1. PSA Threshold and Risk Categories
- PSA < 1.0 ng/mL (with no FH): This was considered low risk. These men are very unlikely to have significant prostate cancer at age 50, based on evidence from risk modeling. They were scheduled for a long rescreening interval of 5 years (i.e., next screening at age 55), as per EAU recommendations and the pilot protocol. They were effectively exited from the immediate screening cycle and advised that no further action was needed until recall. For men with PSA < 1.0 ng/mL, a 5-year interval before re-testing was chosen based on longitudinal evidence showing very low prostate cancer risk in this group over a decade [13]. This extended interval reduces the burden of testing and minimizes overdiagnosis while maintaining safety.
- PSA 1.0–3.0 ng/mL (with no FH): This range was considered intermediate risk. These men were not referred immediately, but due to their higher PSA (above the age norm of ~1 ng/mL), they were at increased risk for developing detectable cancer in the near future. They were advised to have a shorter-interval recall with PSA testing in 2 years (at age 52). Thus, they remained in the program with closer surveillance.
- PSA > 3.0 ng/mL: This was the primary trigger for referral in PSA-based screening. A threshold of 3 ng/mL was chosen based on its use in major trials and guidelines as a level requiring further evaluation. All participants with PSA > 3.0 ng/mL were classified as screening-positive and were referred to a urology specialist for further evaluation. Importantly, this criterion applied irrespective of family history status. (In the planning, a prevalence of roughly 4–5% of 50-year-old men was expected to have PSA > 3 ng/mL).
- Family history positive (any PSA level): The presence of a first-degree family history of prostate cancer was treated as an independent risk factor, warranting early evaluation. All FH-positive participants were referred to Urology, even if their PSA was modest. This recognizes that men with a significant inherited risk may merit closer examination (the protocol prespecified that these men receive a urologist’s input on further strategy). Notably, FH-positive men still underwent the PSA test; a few of them did indeed have high PSA as well, but even those with low PSA were sent for a urologic check. In practice, many FH-positive men with PSA below 3 were managed by urologists with advice to repeat PSA sooner (e.g., in 1 year) rather than immediate MRI, as per the protocol’s flexibility.
2.3.2. Urologic Assessment
- Low clinical risk: e.g., normal DRE, PSA only mildly elevated, and no concerning features. The urologist in this case could opt to defer invasive workup and advise repeating PSA at a shorter interval (such as 1 year). The participant would then be followed in the screening program with annual PSA checks until risk status changed.
- Intermediate risk: e.g., suspicious DRE or PSA persistently in the 3–10 ng/mL range without clear findings. The urologist would refer the participant for an MRI of the prostate to gather more information. No biopsy would be done at this stage; MRI results would determine the next steps.
- High risk: e.g., very high PSA (>10–15 ng/mL) or highly suspicious DRE. The urologist could directly indicate the need for MRI and a biopsy (anticipating a strong likelihood of significant cancer). Even in high-risk cases, MRI would generally be performed first to allow targeted biopsy of lesions.
2.3.3. MRI and Biopsy
- MRI PI-RADS 4 or 5 (highly suspicious for cancer): Biopsy indicated.
- MRI PI-RADS 3 (indeterminate) with PSA density ≥ 0.10 ng/mL/cc: Biopsy indicated.
- MRI PI-RADS 3 with PSA density < 0.10: Can defer biopsy, recommend close follow-up (e.g., recheck in 1 year).
- MRI PI-RADS 1–2 (no lesion or clearly benign) with PSA density < 0.20: No biopsy; patient returns to surveillance (screening recall at 1 year, given the discordance).
- MRI PI-RADS 1–2 but PSA density ≥ 0.20: Consider biopsy despite a “negative” MRI, as the high PSA density suggests possible diffuse or MRI-occult cancer.
2.3.4. Quality Assurance
2.3.5. Data Collection and Analysis
- Uptake (participation rate): the proportion of the eligible population who initiated the screening (i.e., completed the questionnaire) during the pilot period. The denominator (~108,000) was the estimated number of men in the target birth cohorts residing in Lombardy.
- Eligibility rate: the proportion of participants who were confirmed eligible (i.e., did not have an exclusion) and proceeded to PSA testing.
- Risk factor positivity: the proportion of participants with a positive family history.
- PSA result distribution: percentages of screened men falling into the PSA-based risk categories (<1, 1–3, >3 ng/mL).
- Referral rate: overall percentage of participants who screened positive and were referred for urologic evaluation (this includes those with PSA > 3 ng/mL and/or FH positive).
- Findings at assessment: number of men undergoing urologic exam, and subsequent MRI and biopsy, as available in the data. (Given the short follow-up, cancer detection yield was expected to be very low; nonetheless, any confirmed diagnoses were recorded.)
3. Results
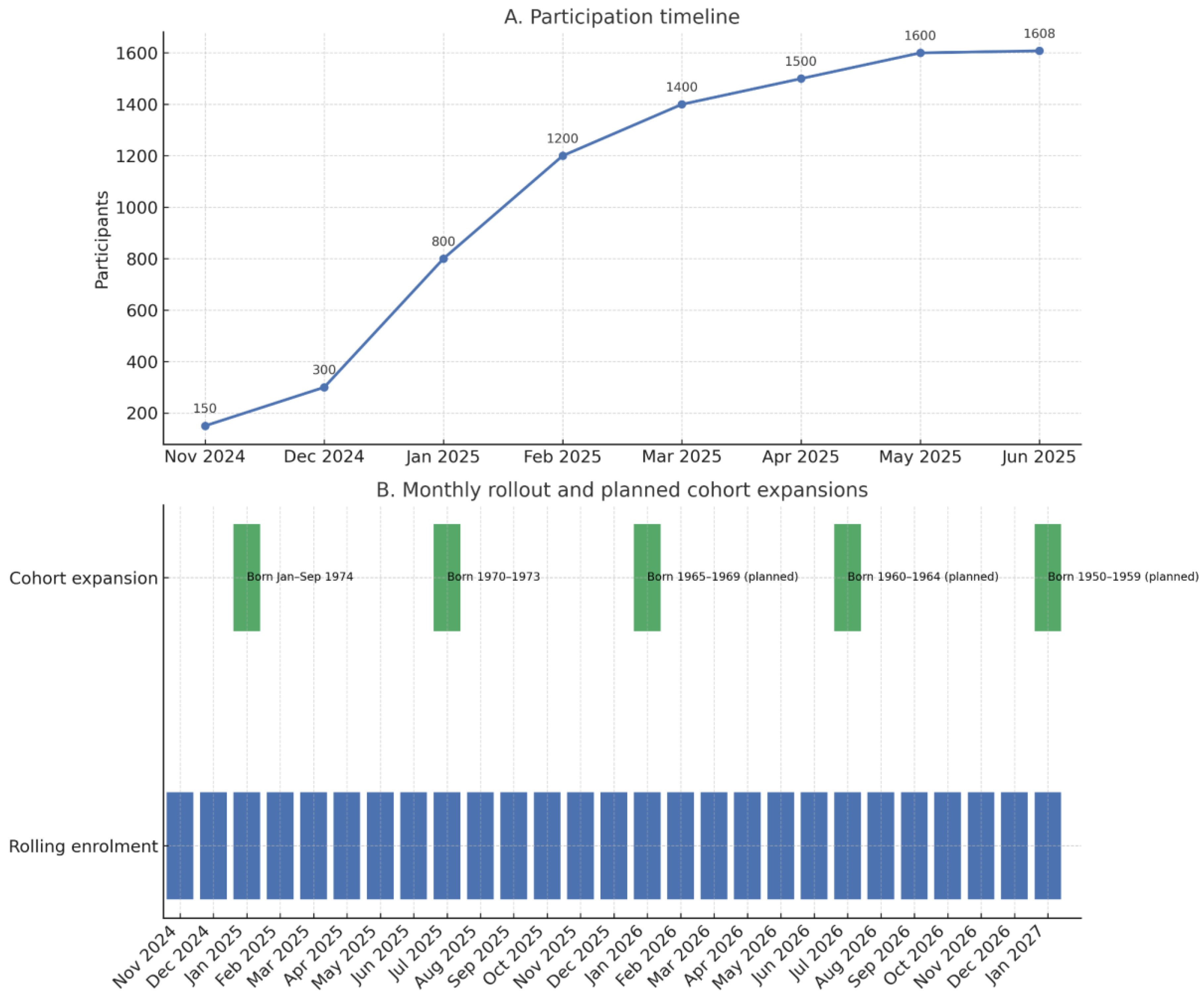
3.1. Participation and Baseline Characteristics
3.1.1. Population Reached
3.1.2. Eligibility
3.2. Screening Test Results and Risk Stratification
3.2.1. PSA Testing Compliance
3.2.2. PSA Level Distribution
- About 824 (58.4%) of screened men have PSA < 1.0 ng/mL, qualifying as low risk (family history negative in these cases). These men were directed to 5-year recall. In the pilot, this low-PSA group constituted roughly two-fifths of the participants, which aligns with international data that 40–50% of men in their late 40s to 50 have PSA below 1 [15,16];
- Approximately 364 (25.8%) men have PSA in the 1.0–3.0 ng/mL range (and FH-negative). This intermediate group—over half of participants—is slated for 2-year recall. The relatively large size of this group is expected, as PSA values between 1 and 3 are common at age 50. Many of these men’s PSAs cluster near the lower end of that range; only a minority were close to 3;
- Around 2.1% (n. 29) of men had PSA > 3.0 ng/mL (FH-negative) on their screening test, exceeding the referral threshold. This figure is consistent with the program’s planning assumption that ~15% of participants would be referred for further workup when combining PSA > 3 and FH risk (since ~13% have FH, ~2% would need to have PSA > 3 to total 15%). Indeed, as more results came in, the cumulative referral rate approached that target (see below);
- By definition, 100% of FH-positive men are considered elevated risk regardless of PSA. In the pilot, 195 (13.8%) participants were FH-positive. Their PSA distribution was variable: some had very low PSA (e.g., 0.4–0.6) but still went to urology due to family history, whereas a few had high PSA as well. The protocol treated FH-positive with PSA >3 equivalently, but practically these were double-flagged (they would have been referred for either criterion).
3.3. Referral and Second-Level Findings
3.3.1. Referral Rate
3.3.2. Urologic Examination
- No concerning findings (low clinical risk): 122 men (91%)—they were advised to continue routine surveillance. In 110 (82.7%) of these cases, the urologist explicitly noted a plan for a repeat PSA in 1 year; in 12 cases, the urologist recommended a follow-up within one year.
- Indication for MRI (intermediate risk): 10 men (7%)—based on DRE or PSA dynamics, the urologist requested an MRI for further evaluation.
- Other outcomes: 1 man declined further workup despite recommendations (recorded as “refused further care”).
3.3.3. MRI Findings
- -
- Five men were considered at low risk (PI-RADS 2 and low-risk PSA density) and were deferred to a PSA test in one year
- -
- Two men were referred for a biopsy
3.3.4. Biopsies and Cancer Diagnoses
3.3.5. Projected Cancer Yield
4. Discussion
4.1. Feasibility of the Multilevel Model
4.2. Low Participation and the Need for Active Invitation
4.3. Equity and Access
4.4. Risk Profile of the Screened Cohort
4.5. Resource Implications and System Capacity
- Urology consultations: We projected that ~16% of the participants would need a urologist visit. At full target population (eventually all men 50–69 years old over the years), this could be a substantial volume. However, the plan is to phase into age cohorts gradually (as we started with 50-year-olds, then 51, etc.), so that each year only one new age group is added. This spreads the influx over two decades. Additionally, if ~40% of men never respond even after invitations (a realistic scenario), the effective referral load is lower. During the pilot, urology clinics easily handled the referrals (which were very few). The true test will come as participation increases. We are mapping out capacity: Lombardy has a robust urology network, but certain areas may need to allocate more clinic slots. One mitigation strategy is that not all referrals occur at once—they will trickle in throughout the year as people get screened in a staggered fashion (birthday-based). The regional coordination is also considering involving office-based urologists and training them in the protocol to expand capacity, as well as telemedicine consults for straightforward cases (e.g., discussing a plan for FH-positive men with low PSA could be done remotely).
- MRI imaging: An MRI was indicated in about half of those seen by urologists in our early data. If 16% see urology and ~7% of those get MRI, that implies ~1% of all screened men ultimately need an MRI. It should be noted, however, that the current sample consists of younger men; therefore, the reported percentages are expected to change over time as older age groups are progressively invited. Lombardy’s radiology services have finite MRI machines and slots, and many are already utilized for other indications. Therefore, MRI capacity is a potential bottleneck if the screening volume grows quickly. The pilot highlighted this issue, albeit on a small scale. One planned response is the adoption of abbreviated MRI protocols (e.g., biparametric MRI without contrast, ~15-min scans) for screening purposes, as suggested by the UK’s IP1-PROSTAGRAM study, which found that a short MRI could detect significant cancers with similar biopsy rates as PSA screening [33,34]. Implementing faster MRI exams dedicated to screening could increase throughput. The region’s technical guidelines (Appendix C) also set minimum requirements so that MRI can be done at more centers (including accredited private clinics) to boost capacity. Funding from the national oncology plan has been earmarked to invest in MRI services specifically for screening.
- Biopsies and downstream treatment: The fraction of men proceeding to biopsy is yet to be seen (likely a subset of those with positive MRI). Our protocol’s restrictive biopsy criteria (only PI-RADS ≥ 4 or PI-RADS 3 with high PSA density) aim to keep biopsy rates low, focusing on likely higher-grade cancers. In the pilot’s first months, biopsy utilization was extremely low (basically one case). Even with higher participation, we anticipate a biopsy rate well below that of historical PSA screening. For example, in ERSPC, many biopsies were done on PSA 3–4 with no MRI filter, whereas here MRI-negative men mostly avoid biopsy. Thus, while treatment facilities must be ready for an uptick in detected cancers, we expect the increase to be gradual and manageable. The absence of any overdiagnosed low-risk cancers so far is encouraging—none of the early detected lesions were low-grade (indeed we have none diagnosed yet; if the pending case is positive, it is likely intermediate or high grade given PI-RADS 4). Over time, we will track detection rates and ensure that any surge in cases can be handled by surgical and radiotherapy centers. The regional cancer network is involved in planning, to avoid treatment delays for newly diagnosed cases.
4.6. Comparison to Other Studies/Programs
- The Swedish and Dutch experiences (ERSPC centers, Göteborg) showed that organized PSA screening can reduce advanced disease and mortality [4,35]. However, they also highlighted overdiagnosis. By incorporating MRI and risk-based intervals, Lombardy’s model represents a modernized screening approach. Early data from the UK (BARCODE, PROSTAGRAM) and German PROBASE trials support this kind of approach [32,33,36]. Our real-world implementation provides practical evidence outside a trial setting.
- Lithuania’s national PSA screening program (cited as the only EU country with routine PSA screening so far) achieved significant coverage but without initial MRI triage [37]. Our results could inform them and others on how to integrate MRI and handle referrals efficiently.
- Notably, some regions/countries remain cautious (e.g., the UK’s NHS has not launched screening yet, though planning research pilots) [38]. The low uptake we saw might be used as an argument by skeptics that men are “not interested” in PSA screening. We interpret it differently: men will participate if properly invited and educated—hence our pivot to direct invitations. Also, men who did come forward in our pilot likely represent the more motivated segment; the challenge will be reaching those less proactively engaged (perhaps by involving GPs more intimately in recommending the program during health visits). This touches on the health equity aspect: we must ensure that all socio-economic groups are informed. Future expansions will include a communication strategy targeting lower-awareness populations, possibly through local public health units.
4.7. Strategic Adjustments and Future Perspective
- Expansion of target ages.
- 2.
- Active invitation and reminders
- 3.
- General practitioner (GP) involvement
- 4.
- Communication and education
- 5.
- Monitoring and evaluation
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DRE | Digital Rectal Examination |
| FH | Family History of prostate cancer |
| GP | General Practitioner |
| MRI | Magnetic Resonance Imaging |
| PSA | Prostate-Specific Antigen |
Appendix A
- Consent to the privacy policy
- Consent to participation in the prostate cancer screening program
- View, enter, or modify phone number (mandatory) and email address
- Request for activation of notification service
- Have you ever been diagnosed with prostate cancer?
- ◦
- YES → conclusion
- ◦
- NO
- Have you had a PSA (Prostate Specific Antigen) blood test in the last 2 years?
- ◦
- YES → conclusion
- ◦
- NO
- ◦
- DON’T KNOW → follow same path as NO
- Have you had any of the following exams in the last 5 years?
- ◦
- a. Prostate biopsy
- ◦
- b. Transrectal ultrasound
- ◦
- c. Abdominal CT scan
- ◦
- d. Full-body CT scan
- ◦
- e. Abdominal MRI
- ◦
- f. Full-body MRI
- ◦
- YES → conclusion
- ◦
- NO
- ◦
- DON’T KNOW → follow same path as NO
- Have you ever been diagnosed with a mutation in one or more of the following genes: BRCA1, BRCA2, CHEK2, ATM?
- ◦
- YES → conclusion
- ◦
- NO
- ◦
- DON’T KNOW → follow same path as NO
- Has your father, at least one son and/or at least one brother ever been diagnosed with prostate cancer?
- ◦
- YES → go to question 6
- ◦
- NO → conclusion
- If yes, which relative? (multiple answers possible)
- ◦
- a. Father → conclusion
- ◦
- b. Son → conclusion
- ◦
- c. Brother → conclusion
- Positive Family History (PDF FH-Positive)
- Negative Family History (PDF FH-Negative)
- Not Eligible Outcome
Appendix B
Appendix C
- 1.
- Minimum Requirements for Participation in Screening
- Hospitals must meet the following technical criteria:
- a.
- MRI machines: 1.5 or 3 Tesla
- b.
- Software capable of biparametric MRI
- c.
- Use of pelvic phased-array coils and quality control procedures
- d.
- Minimum of 100 prostate MRI readings in the past year
- 2.
- Technical Requirements for Image Acquisition and Reporting
- a.
- Acquisition
- Axial diffusion-weighted imaging and axial/sagittal T2 imaging as per PI-RADS guidelines
- b.
- Reporting
- Structured PI-RADS-based report with zone mapping (mandatory)
- Schematic map of prostate (recommended)
- PI-RADS v2.1 scoring (1–5)
- c.
- Validation
- All radiologists must interpret a reference dataset provided by the screening coordination and submit results to Regione Lombardia
- 3.
- Reporting
- Use of Structured Radiological Report (per Ministry of Health, DM 7/9/2023)
- 4.
- Use of Artificial Intelligence (AI)
- Reports must be finalized by experienced radiologists with specific experience and training in the oncological field of the prostate
- CE-certified AI tools capable of supporting diagnosis and offer significant sensitivity and specificity values by using machine learning may be considered
- AI results are statistical aids; clinical responsibility lies with the radiologist
- Centralized or federated AI solutions may be evaluated after quality verification
References
- Raychaudhuri, R.; Lin, D.W.; Montgomery, R.B. Prostate Cancer: A Review. JAMA 2025, 333, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Tidd-Johnson, A.; Sebastian, S.A.; Co, E.L.; Afaq, M.; Kochhar, H.; Sheikh, M.; Mago, A.; Poudel, S.; Fernandez, J.A.; Rodriguez, I.D.; et al. Prostate cancer screening: Continued controversies and novel biomarker advancements. Curr. Urol. 2022, 16, 197–206. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.W.; Cousins, M.M.; Tsodikov, A.; Soni, P.D.; Crook, J.M. Mortality reduction and cumulative excess incidence (CEI) in the prostate-specific antigen (PSA) screening era. Sci. Rep. 2024, 14, 5810. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Månsson, M.; Wallström, J.; Axcrona, U.; Carlsson, S.V.; Egevad, L.; Geterud, K.; Khatami, A.; Kohestani, K.; Pihl, C.G.; et al. Prostate Cancer Screening with PSA and MRI Followed by Targeted Biopsy Only. N. Engl. J. Med. 2022, 387, 2126–2137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gandaglia, G.; Albers, P.; Abrahamsson, P.A.; Briganti, A.; Catto, J.W.F.; Chapple, C.R.; Montorsi, F.; Mottet, N.; Roobol, M.J.; Sønksen, J.; et al. Structured Population-based Prostate-specific Antigen Screening for Prostate Cancer: The European Association of Urology Position in 2019. Eur. Urol. 2019, 76, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. PROMIS study group. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union. Council Recommendation of 9 December 2022 on strengthening prevention through early detection: A new EU approach on cancer screening replacing Council Recommendation 2003/878/EC. Off. J. Eur. Union 2022, C 473, 1–10. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=oj:JOC_2022_473_R_0001 (accessed on 10 August 2025).
- Council of the European Union. Council Recommendation of 2 December 2003 on cancer screening. Off. J. Eur. Union 2003, L 327, 34–38. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:327:0034:0038:EN:PDF (accessed on 15 August 2025).
- Ministero della Salute. Piano Oncologico Nazionale: Documento di Pianificazione e Indirizzo per la Prevenzione e il Contrasto del Cancro 2023–2027; Ministero della Salute: Roma, Italy, 2023; pp. 32–35. Available online: https://www.osservatorionazionalescreening.it/sites/default/files/allegati/PON%202023-2027.pdf (accessed on 15 August 2025).
- Giunta Regione Lombardia. Determinazione in Ordine All’avvio del Programma di Screening Della Prostata. Deliberazione N° XII/2767 del 15 July 2024. Available online: https://www.regione.lombardia.it/wps/wcm/connect/2f578959-2ff6-487b-9f75-d33c6b68f123/DGR2767_2024_all.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-2f578959-2ff6-487b-9f75-d33c6b68f123-pi41-29 (accessed on 10 August 2025).
- Van Poppel, H.; Hogenhout, R.; Albers, P.; van den Bergh, R.C.N.; Barentsz, J.O.; Roobol, M.J. A European Model for an Organised Risk-stratified Early Detection Programme for Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Ulmert, D.; Sjoberg, D.D.; Bennette, C.J.; Björk, T.; Gerdtsson, A.; Manjer, J.; Nilsson, P.M.; Dahlin, A.; Bjartell, A.; et al. Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40–55 and long term risk of metastasis: Case-control study. BMJ 2013, 346, f2023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pylväläinen, J.; Talala, K.; Raitanen, J.; Rannikko, A.; Auvinen, A. Association of prostate-specific antigen density with prostate cancer mortality after a benign systematic prostate biopsy result. BJU Int. 2025, 135, 841–850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ulmert, D.; Cronin, A.M.; Björk, T.; O’Brien, M.F.; Scardino, P.T.; Eastham, J.A.; Becker, C.; Berglund, G.; Vickers, A.J.; Lilja, H. Prostate-specific antigen at or before age 50 as a predictor of advanced prostate cancer diagnosed up to 25 years later: A case-control study. BMC Med. 2008, 6, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weight, C.J.; Kim, S.P.; Jacobson, D.J.; McGree, M.E.; Karnes, R.J.; St Sauver, J. Men (aged 40–49 years) with a single baseline prostate-specific antigen below 1.0 ng/mL have a very low long-term risk of prostate cancer: Results from a prospectively screened population cohort. Urology 2013, 82, 1211–1217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Auvinen, A.; Raitanen, J.; Moss, S.; de Koning, H.J.; Hugosson, J.; Tammela, T.; Roobol, M.; Lilja, H.; Hakama, M. Test sensitivity in the European prostate cancer screening trial: Results from Finland, Sweden, and the Netherlands. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Donovan, J.L.; Turner, E.L.; Metcalfe, C.; Young, G.J.; Walsh, E.I.; Lane, J.A.; Noble, S.; Oliver, S.E.; Evans, S.; et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA 2018, 319, 883–895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Gohagan, J.K.; Prorok, P.C.; Hayes, R.B.; Kramer, B.S. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: History, organization, and status. Control. Clin. Trials 2000, 21 (Suppl. 6), 251S–272S. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.R.; Schoots, I.G.; Bokhorst, L.P.; Drost, F.H.; van Leenders, G.J.; Krestin, G.P.; Dwarkasing, R.S.; Barentsz, J.O.; Schröder, F.H.; Bangma, C.H.; et al. Characteristics of Prostate Cancer Found at Fifth Screening in the European Randomized Study of Screening for Prostate Cancer Rotterdam: Can We Selectively Detect High-grade Prostate Cancer with Upfront Multivariable Risk Stratification and Magnetic Resonance Imaging? Eur. Urol. 2018, 73, 343–350. [Google Scholar] [CrossRef]
- Kohestani, K.; Månsson, M.; Arnsrud Godtman, R.; Stranne, J.; Wallström, J.; Carlsson, S.; Hellström, M.; Hugosson, J. The GÖTEBORG prostate cancer screening 2 trial: A prospective, randomised, population-based prostate cancer screening trial with prostate-specific antigen testing followed by magnetic resonance imaging of the prostate. Scand. J. Urol. 2021, 55, 116–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camilloni, L.; Ferroni, E.; Cendales, B.J.; Pezzarossi, A.; Furnari, G.; Borgia, P.; Guasticchi, G.; Giorgi Rossi, P.; Methods to increase participation Working Group. Methods to increase participation in organised screening programs: A systematic review. BMC Public Health 2013, 13, 464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scaioli, G.; Varì, M.G.; Barbera, G.; Durbano, A.; Pinto, S.; Lo Moro, G.; Bert, F.; Siliquini, R. Invitation strategies for improving uptake in cervical, breast, and colorectal cancer screening: A systematic review. Eur. J. Cancer Prev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Anttila, A.; von Karsa, L.; Aasmaa, A.; Fender, M.; Patnick, J.; Rebolj, M.; Nicula, F.; Vass, L.; Valerianova, Z.; Voti, L.; et al. Cervical cancer screening policies and coverage in Europe. Eur. J. Cancer 2009, 45, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Deandrea, S.; Molina-Barceló, A.; Uluturk, A.; Moreno, J.; Neamtiu, L.; Peiró-Pérez, R.; Saz-Parkinson, Z.; Lopez-Alcalde, J.; Lerda, D.; Salas, D. Presence, characteristics and equity of access to breast cancer screening programmes in 27 European countries in 2010 and 2014. Results from an international survey. Prev. Med. 2016, 91, 250263. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.G.; Murtas, R.; Ballotari, P.; Cavalieri d’Oro, L.; Gambino, M.L.; Fanetti, A.C.; Maifredi, G.; Manzoni, F.; Sampietro, G.; Leoni, O.; et al. Analysis of predictive factors for non-adherence to organized screening for colorectal and breast cancers in the pre-pandemic period (2018–2019) in Lombardy Region (Northern Italy). Epidemiol. Prev. 2024, 48, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Girardi, D.; Vecchio, R.; Tanious, M.; Cacitti, S.; Ancarani, C.; Dalle Carbonare, S.; Perotti, P.; Bonafede, C.; Cavallo, R.; Gentile, L.; et al. Disparities in access to breast, colorectal, and cervical cancer screening programmes have intensified during the pandemic period. Findings of a health equity audit conducted by the Pavia Healthcare Protection Agency (Lombardy Region, Northern Italy). Epidemiol. Prev. 2024, 48, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Cereda, D.; Federici, A.; Guarino, A.; Serantoni, G.; Gruppo PRECEDE-PROCEED; Coppola, L.; Lemma, P.; Rossi, P.G. Development and first application of an audit system for screening programs based on the PRECEDE-PROCEED model: An experience with breast cancer screening in the region of Lombardy (Italy). BMC Public Health 2020, 20, 1778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Odelli, S.; Zeduri, M.; Schivardi, M.R.; Archi, D.; Coppola, L.; Genco Russo, R.; Moscheni, M.; Tettamanzi, E.; Terragni, F.; Viscardi, M.; et al. Impact of PRECEDE-PROCEED Model Audits in Cancer Screening Programs in Lombardy Region: Supporting Equity and Quality Improvement. Curr. Oncol. 2024, 31, 5960–5973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krilaviciute, A.; Kaaks, R.; Seibold, P.; de Vrieze, M.; Lakes, J.; Radtke, J.P.; Kuczyk, M.; Harke, N.N.; Debus, J.; Fink, C.A.; et al. Risk-adjusted Screening for Prostate Cancer-Defining the Low-risk Group by Data from the PROBASE Trial. Eur. Urol. 2024, 86, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Eldred-Evans, D.; Burak, P.; Connor, M.J.; Day, E.; Evans, M.; Fiorentino, F.; Gammon, M.; Hosking-Jervis, F.; Klimowska-Nassar, N.; McGuire, W.; et al. Population-Based Prostate Cancer Screening With Magnetic Resonance Imaging or Ultrasonography: The IP1-PROSTAGRAM Study. JAMA Oncol. 2021, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fazekas, T.; Shim, S.R.; Basile, G.; Baboudjian, M.; Kói, T.; Przydacz, M.; Abufaraj, M.; Ploussard, G.; Kasivisvanathan, V.; Rivas, J.G.; et al. Magnetic Resonance Imaging in Prostate Cancer Screening: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 745–754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eeles, R.A.; Bancroft, E.K.; McHugh, J.K.; Saunders, E.; Brook, M.; McGrowder, E.; Wakerell, S.; James, D.; Page, E.; Osborne, A.; et al. Effect of polygenic risk score for clinically significant prostate cancer in a screening program: The BARCODE 1 study results. JCO 2024, 42, 10500. [Google Scholar] [CrossRef]
- Patasius, A.; Krilaviciute, A.; Smailyte, G. Prostate Cancer Screening with PSA: Ten Years’ Experience of Population Based Early Prostate Cancer Detection Programme in Lithuania. J. Clin. Med 2020, 9, 3826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burki, T. Prostate Cancer UK launches the TRANSFORM trial. Lancet 2024, 403, 1738. [Google Scholar] [CrossRef] [PubMed]
- Daskivich, T.J.; Luu, M.; Heard, J.; Thomas, I.C.; Leppert, J.T. Overtreatment of Prostate Cancer Among Men with Limited Longevity in the Active Surveillance Era. JAMA Intern. Med. 2025, 185, 28–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tesfai, A.; Norori, N.; Harding, T.A.; Wong, Y.H.; Hobbs, M.D. The impact of pre-biopsy MRI and additional testing on prostate cancer screening outcomes: A rapid review. BJUI Compass 2024, 5, 426–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Health Agency (ATS) | Participants (n) | Eligible for PSA (n) | Temporarily Ineligible 1 (n) |
|---|---|---|---|
| ATS Milano | 3401 | 2366 | 1035 |
| ATS Insubria | 1296 | 919 | 377 |
| ATS Montagna | 237 | 169 | 68 |
| ATS Brianza | 1184 | 782 | 402 |
| ATS Bergamo | 624 | 508 | 116 |
| ATS Brescia | 910 | 680 | 230 |
| ATS Val Padana | 595 | 431 | 164 |
| ATS Pavia | 311 | 217 | 94 |
| Total | 8558 | 6072 | 2486 |
| Category (Screening Outcome) | N (% of Participants) | Recommended Action |
|---|---|---|
| FH-negative, PSA < 1.0 ng/mL (low risk) | 824 (58.4) | Routine recall in 5 years |
| FH-negative, PSA 1.0–3.0 ng/mL (intermediate) | ~364 (25.8) | Shorter recall in 2 years |
| FH-negative, PSA > 3.0 ng/mL (elevated PSA) | ~29 (2.1) | Refer to Urology now (DRE) |
| FH-positive (any PSA) (elevated familial risk) | ~195 (13.8) | Refer to Urology now (DRE) |
| Total participants screened | 1412 (100%) 1 | – |
| Cohort (Age) | Eligible Population (No PSA Test in Past 2 Years) | Participation Rate (Scenario) | Number Screened | Expected Incidence (/100,000/Year) * | Interval (Years) | Test Sensitivity | Expected Cases | Expected Yield (/1000) |
|---|---|---|---|---|---|---|---|---|
| 50 years–current situation | 1412 | 1.4 | 21.1 | 2.000 | 0.870 | 0.5 | 0.4 | |
| 50–69 years | 861,163 | 15.0% | 129,174 | 97.600 | 2.000 | 0.870 | 219.4 | 1.7 |
| 50–69 years | 861,163 | 30.0% | 258,349 | 97.600 | 2.000 | 0.870 | 438.7 | 1.7 |
| 50–69 years | 861,163 | 40.0% | 344,465 | 97.600 | 2.000 | 0.870 | 585.0 | 1.7 |
| Outcome Per PSA Test | Standard Care (n/N, %) | Screening Pilot (n/N, %) * | Absolute Difference (%) | 95% CI |
|---|---|---|---|---|
| Urologist visits | 4240/16,933 (25.04%) | 261/1412 (18.48%) | 6.56% | 4.43–8.68% |
| MRI usage | 312/16,933 (1.84%) | 16.8/1412 (1.19%) | 0.65% | 0.05–1.25% |
| Biopsy rate | 57/16,933 (0.34%) | 4.8/1412 (0.34%) | −0.00% | −0.32–0.31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzolini, E.; Cereda, D.; Piccinelli, S.; Viscardi, M.; Deandrea, S. Early Activation of a Multilevel Prostate Cancer Screening Model: Pilot Phase Results and Strategic Perspectives in Lombardy Region. Healthcare 2025, 13, 2041. https://doi.org/10.3390/healthcare13162041
Azzolini E, Cereda D, Piccinelli S, Viscardi M, Deandrea S. Early Activation of a Multilevel Prostate Cancer Screening Model: Pilot Phase Results and Strategic Perspectives in Lombardy Region. Healthcare. 2025; 13(16):2041. https://doi.org/10.3390/healthcare13162041
Chicago/Turabian StyleAzzolini, Elena, Danilo Cereda, Sara Piccinelli, Michela Viscardi, and Silvia Deandrea. 2025. "Early Activation of a Multilevel Prostate Cancer Screening Model: Pilot Phase Results and Strategic Perspectives in Lombardy Region" Healthcare 13, no. 16: 2041. https://doi.org/10.3390/healthcare13162041
APA StyleAzzolini, E., Cereda, D., Piccinelli, S., Viscardi, M., & Deandrea, S. (2025). Early Activation of a Multilevel Prostate Cancer Screening Model: Pilot Phase Results and Strategic Perspectives in Lombardy Region. Healthcare, 13(16), 2041. https://doi.org/10.3390/healthcare13162041






