Semantic Processing Deficits and Their Use as Early Biomarkers in Schizophrenia
Abstract
1. Introduction
2. Methodology
2.1. Sample
2.2. Study Design
2.3. Procedure
2.4. Instruments
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Baseline Result
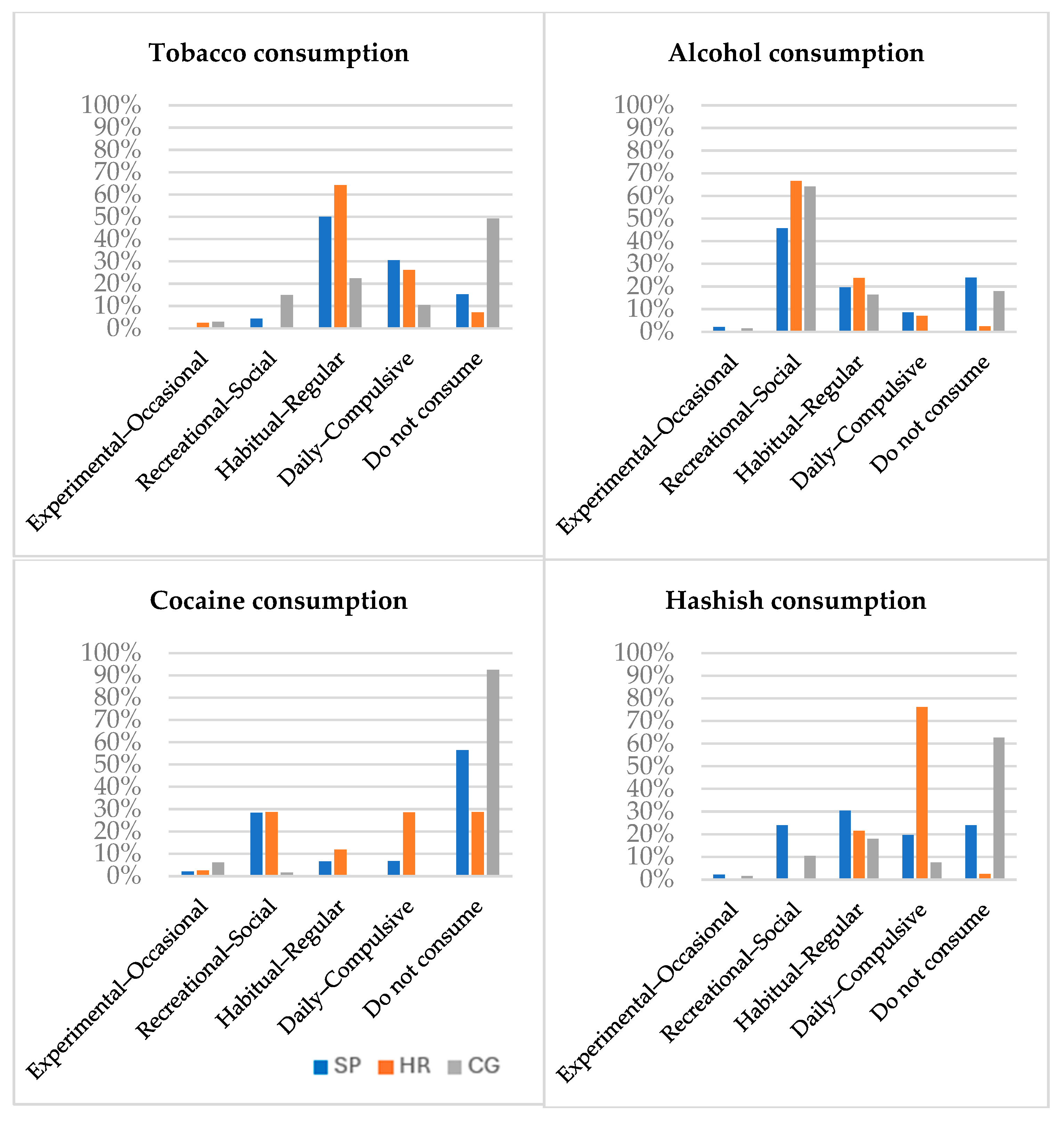
3.2.1. Substance Use
3.2.2. Cognitive Capacity
3.3. Psychopathology and Symptomatology
3.4. Semantic Associations
3.5. Correlations Between Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Meier, S.M.; Kähler, A.K.; Bergen, S.E.; Sullivan, P.F.; Hultman, C.M.; Mattheisen, M. Chronicity and Sex Affect Genetic Risk Prediction in Schizophrenia. Front. Psychiatry 2020, 11, 313. Available online: https://www.frontiersin.org/journals/psychiatry/articles/10.3389/fpsyt.2020.00313/full (accessed on 4 November 2024). [CrossRef]
- Bleuler, E. Dementia praecox oder Gruppe der Schizophrenien, 2008th ed.; Verlag P: Munich, Germany, 1911. [Google Scholar]
- Kraepelin, E. Dementia Praecox and Paraphrenia; Chicago Medical Book: Chicago, IL, USA, 1919. [Google Scholar]
- Chhabra, S.; Badcock, J.C.; Maybery, M.T.; Leung, D. Voice identity discrimination in schizophrenia. Neuropsychologia 2012, 50, 2730–2735. [Google Scholar] [CrossRef]
- Morice, R.D.; Ingram, J.C.L. Language analysis in schizophrenia: Diagnostic implications. Aust. N. Z. J. Psychiatry 1982, 16, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Walenski, M.; Weickert, T.W.; Maloof, C.J.; Ullman, M.T. Grammatical processing in schizophrenia: Evidence from morphology. Neuropsychologia 2010, 48, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, G.; Bambini, V.; Bechi, M.; Buonocore, M.; Spangaro, M.; Repaci, F.; Cocchi, F.; Bianchi, L.; Guglielmino, C.; Sapienza, J.; et al. Communicative-pragmatic abilities mediate the relationship between cognition and daily functioning in schizophrenia. Neuropsychology 2021, 35, 42–56. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Bhattacharya, K.; Chatterjee, G.; Biswas, A.; Ghosal, M. Pragmatic deficits in patients with schizophrenia and right hemisphere damage: A pilot study. Int. J. Lang. Commun. Disord. 2023, 58, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Kuperberg, G.R. Language in schizophrenia Part 1: An Introduction. Lang Linguist. Compass 2010, 4, 576–589. [Google Scholar] [CrossRef]
- Collins, A.M.; Loftus, E.F. A spreading-activation theory of semantic processing. Psychol. Rev. 1975, 82, 407–428. [Google Scholar] [CrossRef]
- Lundin, N.B.; Jones, M.N.; Myers, E.J.; Breier, A.; Minor, K.S. Semantic and phonetic similarity of verbal fluency responses in early-stage psychosis. Psychiatry Res. 2022, 309, 114404. [Google Scholar] [CrossRef]
- Doughty, O.J.; Done, D.J. Is semantic memory impaired in schizophrenia? A systematic review and meta-analysis of 91 studies. Cogn. Neuropsychiatry 2009, 14, 473–509. [Google Scholar] [CrossRef]
- Almeida, V.N.; Radanovic, M. Semantic priming and neurobiology in schizophrenia: A theoretical review. Neuropsychologia 2021, 163, 108058. [Google Scholar] [CrossRef]
- Tan, E.J.; Rossell, S.L.; Subotnik, K.L.; Ventura, J.; Nuechterlein, K.H. Cognitive heterogeneity in first-episode psychosis and its relationship with premorbid developmental adjustment. Psychol. Med. 2022, 52, 3885–3894. [Google Scholar] [CrossRef] [PubMed]
- Kambanaros, M.; Messinis, L.; Georgiou, V.; Papathanassopoulos, P. Action and object naming in schizophrenia. J. Clin. Exp. Neuropsychol. 2010, 32, 1083–1094. [Google Scholar] [CrossRef]
- Andrease, N.C. Scale for the Assessment of Positive Symptoms. University of Lowa. 1986. Available online: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/GetPdf.cgi?id=phd000837.1 (accessed on 6 August 2025).
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br. J. Psychiatry 1989, 155, 49–52. [Google Scholar] [CrossRef]
- Almeida, V.N. Neurophysiological basis of the N400 deflection, from Mismatch Negativity to Semantic Prediction Potentials and late positive components. Int. J. Psychophysiol. 2021, 166, 134–150. [Google Scholar] [CrossRef]
- Crow, T.J. Positive and Negative Schizophrenic Symptoms and the Role of Dopamine. Br. J. Psychiatry 1980, 137, 383–386. [Google Scholar] [CrossRef]
- Buchanan, R.W.; Javitt, D.C.; Marder, S.R.; Schooler, N.R.; Gold, J.M.; McMahon, R.P.; Heresco-Levy, U.; Carpenter, W.T. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The Efficacy of Glutamatergic Agents for Negative Symptoms and Cognitive Impairments. Am. J. Psychiatry 2007, 164, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; McGhie, A.A. Comparative Study of Disordered Attention in Schizophrenia. J. Ment. Sci. 1962, 108, 487–500. [Google Scholar] [CrossRef]
- Graux, J.; Courtine, J.B.; Bruneau, N.; Camus, V.; El-Hage, W. Higher fundamental voice frequency is related to extrapyramidal symptoms in schizophrenia. Schizophr. Res. 2015, 161, 517–518. [Google Scholar] [CrossRef]
- de Boer, J.N.; van Hoogdalem, M.; Mandl, R.C.W.; Brummelman, J.; Voppel, A.E.; Begemann, M.J.H.; van Dellen, E.; Wijnen, F.N.K.; Sommer, I.E.C. Language in schizophrenia: Relation with diagnosis, symptomatology and white matter tracts. Npj Schizophr. 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.A.S.; Michel, C.; Cruzado, L. Estados clínicos de alto riesgo para esquizofrenia y otras formas de psicosis: Una breve revisión. High risk clinical states for schizophrenia and other forms of psychosis: A brief review. Rev. Neuropsiquiatr. 2017, 80, 1. [Google Scholar] [CrossRef]
- Bukenaite, A.; Stochl, J.; Mossaheb, N.; Schäfer, M.R.; Klier, C.M.; Becker, J.; Schloegelhofer, M.; Papageorgiou, K.; Montejo, A.L.; Russo, D.A.; et al. Usefulness of the CAPE-P15 for detecting people at ultra-high risk for psychosis: Psychometric properties and cut-off values. Schizophr. Res. 2017, 189, 69–74. [Google Scholar] [CrossRef]
- Simon, A.E.; Velthorst, E.; Nieman, D.H.; Linszen, D.; Umbricht, D.; de Haan, L. Ultra high-risk state for psychosis and non-transition: A systematic review. Schizophr. Res. 2011, 132, 8–17. [Google Scholar] [CrossRef]
- Miret, S.; Fatjó-Vilas, M.; Peralta, V.; Fañanás, L. Basic symptoms in schizophrenia, their clinical study and relevance in research. Rev. Psiquiatr. Salud Ment. 2016, 9, 111–122. [Google Scholar] [CrossRef]
- Schultze-Lutter, F. Subjective symptoms of schizophrenia in research and the clinic: The basic symptom concept. Schizophr. Bull. 2009, 35, 5–8. [Google Scholar] [CrossRef]
- Schultze-Lutter, F.; Michel, C.; Schmidt, S.J.; Schimmelmann, B.G.; Maric, N.P.; Salokangas, R.K.R.; Riecher-Rössler, A.; van der Gaag, M.; Nordentoft, M.; Raballo, A. EPA guidance on the early detection of clinical high risk states of psychoses. Eur. Psychiatr. 2015, 30, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.E.; Dvorsky, D.N.; Boesch, J.; Roth, B.; Isler, E.; Schueler, P.; Petralli, C.; Umbricht, D. Defining subjects at risk for psychosis: A comparison of two approaches. Schizophr. Res. 2006, 81, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yung, A.R.; Nelson, B.; Stanford, C.; Simmons, M.B.; Cosgrave, E.M.; Killackey, E.; Phillips, L.J.; Bechdolf, A.; Buckby, J.; McGorry, P.D. Validation of ‘prodromal’ criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr. Res. 2008, 105, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Catalan, A.; Salazar De Pablo, G.; Aymerich, C.; Damiani, S.; Sordi, V.; Radua, J.; Oliver, D.; McGuire, P.; Giuliano, A.J.; Stone, W.S. Neurocognitive Functioning in Individuals at Clinical High Risk for Psychosis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 859–867. [Google Scholar] [CrossRef]
- Swendsen, J.; Conway, K.P.; Degenhardt, L.; Glantz, M.; Jin, R.; Merikangas, K.R.; Sampson, N.; Kessler, R.C. Mental disorders as risk factors for substance use, abuse and dependence: Results from the 10-year follow-up of the National Comorbidity Survey. Addiction 2010, 105, 1117–1128. [Google Scholar] [CrossRef]
- Khantzian, E.J. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. Am. J. Psychiatry 1985, 142, 1259–1264. [Google Scholar] [CrossRef]
- Potvin, S.; Stip, E.; Roy, J.Y. Schizophrenia and addiction: An evaluation of the self-medication hypothesis. Encephale 2003, 29, 193–203. [Google Scholar] [PubMed]
- Carnap, R. Meaning And Necessity [Internet]; The University Chicago Press: Chicago, IL, USA, 1947; Available online: http://archive.org/details/meaningandnecess033225mbp (accessed on 29 May 2025).
- Fernando, C.V.; González-Nosti, M. BETA: Batería para la Evaluación de los Trastornos Afásicos: Manual; Instituto de Orientación Psicológica EOS: Madrid, Spain, 2009; Available online: https://digibuo.uniovi.es/dspace/handle/10651/54802 (accessed on 7 February 2024).
- Kay, S.R.; Flszbeln, A.; Qpjer, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia [Internet]. Schizophr. Bull. 1987, 13, 261–376. Available online: https://academic.oup.com/schizophreniabulletin/article/13/2/261/1919795 (accessed on 11 February 2024). [CrossRef] [PubMed]
- American Psychiatric Association. Text Revision Dsm-5-tr. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022; p. 1142. [Google Scholar]
- Riecher-Rössler, A.; Studerus, E. Prediction of conversion to psychosis in individuals with an at-risk mental state: A brief update on recent developments. Curr. Opin. Psychiatry 2017, 30, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Mossaheb, N.; Becker, J.; Schaefer, M.R.; Klier, C.M.; Schloegelhofer, M.; Papageorgiou, K.; Amminger, G.P. The Community Assessment of Psychic Experience (CAPE) questionnaire as a screening-instrument in the detection of individuals at ultra-high risk for psychosis. Schizophr. Res. 2012, 141, 210–214. [Google Scholar] [CrossRef]
- Yung, A.; Phillips, L.; McGorry, P.; Ward, J.; Donovan, K.; Thompson, K. Comprehensive assessment of at-risk mental states (CAARMS). Aust. N. Z. J. Psychiatry 2005, 39, 964–971. Available online: https://vizdom.cz/wp-content/uploads/2021/12/CAARMS_FINAL.pdf (accessed on 4 May 2025). [CrossRef]
- Whitehorn, D.D.; Kopala, D.L. The Recognition and Management of Early Psychosis: A Preventive Approach. Can. Child Adolesc. Psychiatry Rev. 2003, 12, 28. [Google Scholar]
- Fabbro, F. The Bilingual Brain: Cerebral Representation of Languages. Brain Lang. 2001, 79, 211–222. [Google Scholar] [CrossRef]
- Skinner, H.A. The drug abuse screening test. Addict. Behav. 1982, 7, 363–371. [Google Scholar] [CrossRef]
- Yudko, E.; Lozhkina, O.; Fouts, A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J. Subst. Abus. Treat. 2007, 32, 189–198. [Google Scholar] [CrossRef]
- Peralta, V.; Cuesta, M.J. Psychometric properties of the Positive and Negative Syndrome Scale (PANSS) in schizophrenia. Psychiatry Res. 1994, 53, 31–40. [Google Scholar] [CrossRef]
- Geck, S.; Roithmeier, M.; Bühner, M.; Wehr, S.; Weigel, L.; Priller, J.; Davis, J.M.; Leucht, S. COSMIN systematic review and meta-analysis of the measurement properties of the Positive and Negative Syndrome Scale (PANSS). EclinicalMedicine 2025, 82, 1–18. [Google Scholar] [CrossRef]
- Leucht, S.; Samara, M.; Heres, S.; Patel, M.X.; Woods, S.W.; Davis, J.M. Dose equivalents for second-generation antipsychotics: The minimum effective dose method. Schizophr. Bull. 2014, 40, 314–326. [Google Scholar] [CrossRef]
- Leucht, S.; Crippa, A.; Siafis, S.; Patel, M.X.; Orsini, N.; Davis, J.M. Dose-Response Meta-Analysis of Antipsychotic Drugs for Acute Schizophrenia. Am. J. Psychiatry 2020, 177, 342–353. [Google Scholar] [CrossRef]
- Adams, C.E.; Rathbone, J.; Thornley, B.; Clarke, M.; Borrill, J.; Wahlbeck, K.; Awad, A.G. Chlorpromazine for schizophrenia: A Cochrane systematic review of 50 years of randomised controlled trials. BMC Med. 2005, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Konings, M.; Bak, M.; Hanssen, M.; Van Os, J.; Krabbendam, L. Validity and reliability of the CAPE: A self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr. Scand. 2006, 114, 55–61. [Google Scholar] [CrossRef]
- Ros-Morente, A.; Vilagrà-Ruiz, R.; Rodriguez-Hansen, G.; Wigman, J.H.; Barrantes-Vidal, N. Process of adaptation to Spanish of the Community Assessment of Psychic Experiences (CAPE). Actas Esp. Psiquiatr. 2011, 39, 95–105. [Google Scholar] [PubMed]
- Pino, O.; Guilera, G.; Rojo, J.; Gómez-Benito, J.; Purdon, S. SCIP-S. Screening del Deterioro Cognitivo en Psiquiatría; TEA Ediciones [Internet]: Madrid, Spain, 2014. Available online: https://web.teaediciones.com/Ejemplos/Manual_SCIP-S_web.pdf (accessed on 1 March 2024).
- Pino, O.; Guilera, G.; Rojo, J.; Gómez-Benito, B.M.; Crespo-Facorro, B.; Cuesta, M.J.; Franco, M.; Martinez-Aran, A.; Segarra, N.; Tabarés-Seisdedos, R.; et al. Spanish version of the Screen for Cognitive Impairment in Psychiatry (SCIP-S): Psychometric properties of a brief scale for cognitive evaluation in schizophrenia. Schizophr. Res. 2007, 99, 139–148. [Google Scholar] [CrossRef]
- Pino, Ó.; Guilera, G.; Gómez, J.; Rojo, J.E. Escala breve para evaluar el deterioro cognitivo en pacientes psiquiátricos. Psicothema 2006, 18, 447–452. [Google Scholar] [PubMed]
- Sachs, G.; Lasser, I.; Pudon, E.; Erfurth, A. Screening for cognitive impairment in schizophrenia: Psychometric properties of the German version of the Screen for Cognitive Impairment in Psychiatry (SCIP-G). Schizophr. Res. 2021, 25, 100197. [Google Scholar] [CrossRef] [PubMed]
- Baldomero, E.B.; Cercós, C.L.; Varela, C.; Riesgo, Y.; Roca, M. Diagnóstico y manejo de la esquizofrenia en España: El proyecto ACEE. Actas Esp. Psiquiatr. 2006, 34, 224–230. [Google Scholar]
- Arias, F. Abuse or dependence on cannabis and other psychiatric disorders. Madrid study on dual pathology prevalence. Actas Esp. Psiquiatr. 2013, 41, 122–131. [Google Scholar]
- Arseneault, L.; Cannon, M.; Witton, J.; Murray, R.M. Causal association between cannabis and psychosis: Examination of the evidence. Br. J. Psychiatry 2004, 184, 110–117. [Google Scholar] [CrossRef]
- Kølbæk, P.; Blicher, A.B.; Buus, C.W.; Feller, S.G.; Holm, T.; Dines, D.; O’Leary, K.M.; Sørensen, R.S.; Opler, M.; Correll, C.U. Inter-rater reliability of ratings on the six-item Positive and Negative Syndrome Scale (PANSS-6) obtained using the Simplified Negative and Positive Symptoms Interview (SNAPSI). Nord. J. Psychiatry 2018, 72, 431–436. [Google Scholar] [CrossRef]
- Shafer, A.; Dazzi, F. Meta-analysis of the positive and Negative Syndrome Scale (PANSS) factor structure. J. Psychiatr. Res. 2019, 115, 113–120. [Google Scholar] [CrossRef]
- Ahmed, A.O.; Strauss, G.P.; Buchanan, R.W.; Kirkpatrick, B.; Carpenter, W.T. Schizophrenia heterogeneity revisited: Clinical, cognitive, and psychosocial correlates of statistically-derived negative symptoms subgroups. J. Psychiatr. Res. 2018, 97, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Pedrero, E.; Ortuño-Sierra, J.; Paino, M.; Muñiz, J. Experiencias psicóticas atenuadas y consumo de sustancias en universitarios. Adicciones 2016, 28, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Brenner, K.; Schmitz, N.; Pawliuk, N.; Fathalli, F.; Joober, R.; Ciampi, A.; King, S. Validation of the English and French versions of the Community Assessment of Psychic Experiences (CAPE) with a Montreal community sample. Schizophr. Res. 2007, 95, 86–95. [Google Scholar] [CrossRef]
- Mark, W.; Toulopoulou, T. Validation of the Chinese version of Community Assessment of Psychic Experiences (CAPE) in an adolescent general population. Asian J. Psychiatry 2017, 26, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Siddi, S.; Preti, A.; Bhatia, T.; Deshpande, S.N. Subclinical psychotic symptoms in Indian adults: Application of the Community Assessment of Psychic Experiences (CAPE). Asian J. Psychiatr. 2023, 81, 103451. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Bonoldi, I.; Yung, A.R.; Borgwardt, S.; Kempton, M.J.; Valmaggia, L.; Barale, F.; Caverzasi, E.; McGuire, P. Predicting Psychosis Meta-analysis of Transition Outcomes in Individuals at High Clinical Risk. Arch. Gen. Psychiatry 2012, 69, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Borgwardt, S.; Bechdolf, A.; Addington, J.; Riecher-Rössler, A.; Schultze-Lutter, F.; Keshavan, M.; Wood, S.; Ruhrmann, S.; Seidman, L.J. The psychosis high-risk state: A comprehensive state-of-the-art review. JAMA Psychiatry 2013, 70, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Ramey, T.; Regier, P.S. Cognitive impairment in substance use disorders. CNS Spectr. 2019, 24, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Starin, A.; Mulder, C.; Duivenvoorden, J.; De Hann, L.; Van der Gaag, M. Fewer symptoms vs. more side-effects in schizophrenia? Opposing pathways between antipsychotic medication compliance and quality of life. Schizophr. Res. 2009, 113, 27–33. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Dodge, M.; Aloia, M.; Egan, M.F.; Weinberger, D.R. Effects of neuroleptic medications on speech disorganization in schizophrenia: Biasing associative networks towards meaning. Psychol. Med. 2000, 30, 1123–1130. [Google Scholar] [CrossRef]
- Barattieri di San Pietro, C.; Luzzatti, C.; Ferrari, E.; de Girolamo, G.; Marelli, M. Automated clustering and switching algorithms applied to semantic verbal fluency data in schizophrenia spectrum disorders. Lang. Cogn. Neurosci. 2023, 38, 950–965. [Google Scholar] [CrossRef]
- Salavera, C.; Puyuelo, M.; Antoňanzas, J.L.; Teruel, P. Semantics, pragmatics, and formal thought disorders in people with schizophrenia. Neuropsychiatr. Dis. Treat. 2013, 9, 177–183. [Google Scholar] [CrossRef]
- Agurto, C.; Norel, R.; Wen, B.; Wei, Y.; Zhang, D.; Bilgrami, Z.; Hsi, X.; Zhang, T.; Pasternak, O.; Li, H.; et al. Are language features associated with psychosis risk universal? A study in Mandarin-speaking youths at clinical high risk for psychosis. World Psychiatry 2023, 22, 157–158. [Google Scholar] [CrossRef]
- Gudayol-Ferré, E.; Lara, J.P.; Herrera-Guzman, I.; Böhm, P.; Rodés, E.; Ansaldo, A.I.; Peña-Casanova, J. Semantic memory as assessed by the Pyramids and Palm Trees Test: The impact of sociodemographic factors in a Spanish-speaking population. J. Int. Neuropsychol. Soc. 2008, 14, 148–151. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Ferrera, S.; Mccarthy, R.A.; Mckenna, P.J. Language in schizophrenia and its relationship to formal thought disorder. Psychol. Med. 2001, 31, 197–205. [Google Scholar] [CrossRef]
- Dell, G.S.; Burger, L.K.; Svec, W.R. Language production and serial order: A functional analysis and a model. Psychol. Rev. 1997, 104, 123–147. [Google Scholar] [CrossRef]
- Gordon, J.; Dell, G. Learning to divide the labor between syntax and semantics: A connectionist account of deficits in light and heavy verb production. Brain Cogn. 2002, 48, 376–381. [Google Scholar] [CrossRef]
- Koukouli, F.; Rooy, M.; Tziotis, D.; Sailor, K.A.; O’Neill, H.C.; Levenga, J.; Witte, M.; Nilges, M.; Changeux, J.-P.; Hoeffer, C.A.; et al. Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nat. Med. 2017, 23, 347–354. [Google Scholar] [CrossRef]
- Howard, D.; Nickels, L.; Coltheart, M.; Cole-Virtue, J. Cumulative semantic inhibition in picture naming: Experimental and computational studies. Cognition 2006, 100, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, G.M.; Dell, G.S.; Schwartz, M.F. The dark side of incremental learning: A model of cumulative semantic interference during lexical access in speech production. Cognition 2010, 114, 227–252. [Google Scholar] [CrossRef]
- Wienrich, C.; Heße, U.; Müller-Plath, G. Eye movements and attention in visual feature search with graded target-distractor-similarity. J. Eye Mov. Res. 2009, 3, 1–19. Available online: https://bop.unibe.ch/JEMR/article/view/2288 (accessed on 10 November 2024). [CrossRef]
- Alherz, F.; Alherz, M.; Almusawi, H. NMDAR hypofunction and somatostatin-expressing GABAergic interneurons and receptors: A newly identified correlation and its effects in schizophrenia. Schizophr. Res. Cogn. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Lewis, D.A.; Curley, A.A.; Glausier, J.; Volk, D.W. Cortical Parvalbumin Interneurons and Cognitive Dysfunction in Schizophrenia. Trends Neurosci. 2012, 35, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A.; Mousavi, S.Z.; Kamali, M.; Maroufizadeh, S. Normative data for the Pyramids and Palm Trees Test in literate Persian adults. Iran. J. Neurol. 2018, 17, 18–23. [Google Scholar] [PubMed]
- Agurto, C.; Pietrowicz, M.; Norel, R.; Eyigoz, E.K.; Stanislawski, E.; Cecchi, G.; Corcoran, C. Analyzing acoustic and prosodic fluctuations in free speech to predict psychosis onset in high-risk youths. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada; 2020; pp. 5575–5579. Available online: https://ieeexplore.ieee.org/document/9176841/ (accessed on 14 June 2024).
- Bora, E.; Yalincetin, B.; Akdede, B.B.; Alptekin, K. Neurocognitive and linguistic correlates of positive and negative formal thought disorder: A meta-analysis. Schizophr. Res. 2019, 209, 2–11. [Google Scholar] [CrossRef]
- Brisch, R.; Saniotis, A.; Wolf, R.; Bielau, H.; Bernstein, H.G.; Steiner, J.; Bogerts, B.; Braun, K.; Jankowski, Z.; Kumaratilake, J.; et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Front. Psychiatry 2014, 5, 47. [Google Scholar] [PubMed]
- Takeuchi, H.; MacKenzie, N.E.; Samaroo, D.; Agid, O.; Remington, G.; Leucht, S. Antipsychotic Dose in Acute Schizophrenia: A Meta-analysis. Schizophr. Bull. 2020, 46, 1439–1458. [Google Scholar] [CrossRef] [PubMed]
| TS | SP | HR | CG | ||
|---|---|---|---|---|---|
| Age | Variables | 146 | 46 | 40 | 60 |
| ℜ | 28.82 | 45.17 | 22.57 | 20.45 | |
| SD | 13.20 | 11.58 | 4.71 | 2.25 | |
| Minimum | 15 | 24 | 15 | 15 | |
| Maximum | 68 | 68 | 33 | 33 | |
| Gender | Male | 90 (61.6%) | 30 (65.2%) | 30 (75%) | 30 (50%) |
| Female | 56 (38.4%) | 16 (34.8%) | 10 (25%) | 30 (50%) | |
| Level of education | Primary | 12 (7.7%) | 10 (21.7%) | 1 (2.5%) | 1 (1.7%) |
| Secondary | 124 (84.9%) | 34 (73.9%) | 35 (87.5%) | 55 (91.7%) | |
| Higher Ed. | 10 (6.8%) | 2 (4.3%) | 4 (10%) | 4 (6.7%) | |
| History of mental disorder in first-degree relatives | Yes | 76 (52.1%) | 36 (78.3%) | 22 (55%) | 18 (30%) |
| No | 70 (47.9%) | 10 (21.7%) | 18 (45%) | 42 (70%) | |
| Group | ℜ | SD | Min | Mx | F | DF | p | ηp2 | |
|---|---|---|---|---|---|---|---|---|---|
| Total SCIP-S | PS | 42.58 | 10.64 | 27 | 66 | 3.007 | 2 | 0.052 | 0.038 |
| HR | 40.33 | 10.74 | 27 | 73 | |||||
| CG | 45.01 | 8.52 | 27 | 61 | |||||
| Immediate memory | PS | 34.97 | 9.19 | 27 | 69 | 4.219 | 2 | 0.016 | 0.053 |
| HR | 37.35 | 9.29 | 27 | 61 | |||||
| CG | 39.74 | 7.72 | 27 | 61 | |||||
| Working memory | SP | 47.15 | 9.33 | 27 | 69 | 0.531 | 2 | 0.589 | 0.007 |
| HR | 48.14 | 10.22 | 32 | 68 | |||||
| CG | 46.28 | 8.42 | 32 | 71 | |||||
| Verbal fluency | PS | 52.60 | 11.97 | 27 | 71 | 1.977 | 2 | 0.142 | 0.025 |
| HR | 50.45 | 10.96 | 27 | 71 | |||||
| CG | 54.49 | 8.66 | 35 | 73 | |||||
| Delayed memory | PS | 42.56 | 9.19 | 27 | 60 | 4.528 | 2 | 0.012 | 0.056 |
| HR | 38.69 | 8.02 | 27 | 58 | |||||
| CG | 44.11 | 9.90 | 27 | 62 | |||||
| Processing speed | PS | 44.84 | 9.48 | 27 | 60 | 0.269 | 2 | 0.765 | 0.004 |
| HR | 45.11 | 8 | 27 | 69 | |||||
| CG | 45.91 | 6.91 | 27 | 61 |
| PS (N = 46) | |||
|---|---|---|---|
| ℜ (SD) | Min. | Max. | |
| PANSS Negative | 19.13 (9.98) | 8 | 48 |
| PANSS Positive | 19.67 (7.29) | 8 | 44 |
| PANSS Overall | 49.73(12.30) | 30 | 86 |
| Chlorpromazine | 433.71(258.28) | 10.23 | 1040.67 |
| Chlorpromazine PS with positive symptoms | 421.27 (260.27) | 10.23 | 1040.67 |
| Chlorpromazine PS with negative symptoms | 451.38 (261.47) | 10.23 | 950 |
| CAPE 42 | HR (N = 40) | CG (N= 60) | Student’s t | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ℜ (SD) | Min. | Max. | ℜ (SD) | Min. | Max. | F | Sig | ||
| Positive dimension | Positive symptoms | 35.7 (8.12) | 23 | 66 | 25.3 (3.61) | 15 | 32 | 16.936 | <0.001 |
| Weighted positive symptoms | 1.78 (0.40) | 1.6 | 3.30 | 1.26 (0.18) | 0.75 | 1.6 | 16.936 | <0.001 | |
| Positive distress | 21.67 (9.8) | 10 | 50 | 7.76 (4.42) | 2 | 23 | 41.959 | <0.001 | |
| Weighted positive distress | 1.78 (0.40) | 1.50 | 2.50 | 0.38 (0.22) | 0.10 | 1.15 | 41.959 | <0.001 | |
| Total weighted positive score | 2.87 (0.82) | 2.8 | 5.30 | 1.65 (0.35) | 1.10 | 2.60 | 32.494 | <0.001 | |
| Negative dimension | Negative symptoms | 26.9 (7.08) | 19 | 48 | 21.3 (4.03) | 13 | 27 | 6.430 | 0.013 |
| Weighted negative symptoms | 1.92 (0.50) | 1.36 | 3.43 | 1.52 (0.28) | 0.93 | 1.8 | 6.430 | 0.013 | |
| Negative distress | 20.97 (8.34) | 13 | 53 | 10.68 (5.75) | 0 | 23 | 1.372 | 0.244 | |
| Weighted negative distress | 1.49 (0.59) | 1.2 | 3.79 | 0.76 (0.41) | 0 | 1.64 | 1.372 | 0.244 | |
| Total weighted negative score | 3.42 (1.02) | 2.43 | 7 | 2.28 (0.67) | 1 | 3.36 | 2.269 | 0.136 | |
| Depressive dimension | Depressive symptoms | 17.97 (5.2) | 10 | 30 | 14 (2.97) | 9 | 19 | 18.315 | <0.001 |
| Weighted depressive symptoms | 2.24 (0.65) | 1.25 | 3.75 | 1.75 (0.37) | 1.13 | 2.2 | 18.315 | <0.001 | |
| Depressive distress | 15.2 (5.02) | 9 | 28 | 9.66 (4.42) | 1 | 22 | 1.747 | 0.189 | |
| Weighted depressive distress | 1.90 (0.62) | 1.13 | 3.50 | 1.20 (0.55) | 0.13 | 2.4 | 1.747 | 0.189 | |
| Total weighted depressive score | 4.15 (1.14) | 2.50 | 6.75 | 2.95 (0.86) | 1.25 | 4.5 | 5.604 | 0.020 | |
| Total dimension | Total symptoms | 81.55(18.44) | 62 | 143 | 60.5 (7.3) | 47 | 62 | 32.819 | <0.001 |
| Weighted total symptoms | 1.94 (0.43) | 1.45 | 3.40 | 1.44 (0.17) | 1.12 | 1.69 | 32.819 | <0.001 | |
| Total distress | 59.05(19.7) | 35 | 117 | 28.28 (11.1) | 5 | 52 | 9.687 | 0.002 | |
| Weighted total distress | 1.40 (0.46) | 1.3 | 2.79 | 0.67 (0.26) | 0.12 | 1.24 | 9.687 | 0.002 | |
| Total CAPE-42 weighted score | 3.34 (0.85) | 2.45 | 5.55 | 2.11 (0.41) | 1.31 | 2.4 | 21.388 | <0.001 | |
| Intergroups Comparison | Difference in Means | CI 95% | p | ||
|---|---|---|---|---|---|
| Low. | High. | ||||
| BETA test Groups | HR VS PS | 2.59 * | 1.19 | 3.99 | <0.001 |
| HR VS CG | −2.20 * | −3.49 | −0.91 | <0.001 | |
| PS VS CG | −4.79 * | −6.05 | −3.54 | <0.001 | |
| PS | HR | CG | X | p | ||||
|---|---|---|---|---|---|---|---|---|
| Failed | success | Failed | success | Failed | success | |||
| Item 3 | 11 (23.9%) | 35 (76.1%) | 12 (30%) | 28 (70%) | 7 (11.7%) | 53 (88.3%) | 6304 | 0.043 |
| Item 4 | 5 (10.9%) | 41 (89.1%) | 1 (2.5%) | 39 (97.5%) | 1 (1.7%) | 59 (98.3%) | 6.171 | 0.046 |
| Item 5 | 4 (8.7%) | 42 (91.3%) | 0 | 40 (100%) | 0 | 60 (100%) | 9.729 | 0.008 |
| Item 8 | 9 (19.6%) | 37 (80.4%) | 4 (10%) | 38 (90%) | 1 (1.7%) | 59 (98.3%) | 8.579 | 0.014 |
| Item 9 | 18 (39.1%) | 28 (60.9%) | 18 (45%) | 24 (55%) | 7 (11.7%) | 53 (88.3%) | 14.086 | <0.001 |
| Item 10 | 5 (10.9%) | 41 (89.1%) | 0 | 40 (100%) | 0 | 60 (100%) | 6.657 | 0.036 |
| Item 11 | 27 (58.7%) | 19 (41.3%) | 18 (45%) | 22 (55%) | 10 (16.7%) | 50 (83.3%) | 21.475 | <0.001 |
| Item 13 | 10 (21.7%) | 36 (78.3%) | 2 (5%) | 38 (95%) | 3 (5%) | 57 (95%) | 9.251 | 0.010 |
| Item 14 | 4 (8.7%) | 42 (91.3%) | 0 | 40 (100%) | 0 | 60 (100%) | 9.729 | 0.008 |
| Item 16 | 3 (6.5%) | 43 (93.5%) | 1 (2.5%) | 39 (97.5%) | 0 | 60 (100%) | 12.512 | 0.002 |
| Item 17 | 6 (13%) | 40 (87%) | 1 (2.5%) | 39 (97.5%) | 0 | 60 (100%) | 11.370 | 0.003 |
| Item 18 | 5 (10.9%) | 41 (89.1%) | 3 (7.5%) | 37 (92.5%) | 0 | 60 (100%) | 7.045 | 0.030 |
| Item 19 | 4 (8.7%) | 42 (91.3%) | 0 | 40 (100%) | 0 | 60 (100%) | 9.729 | 0.008 |
| Item 20 | 3 (6.5%) | 43 (93.5%) | 0 | 40 (100%) | 0 | 60 (100%) | 7.249 | 0.027 |
| Item 21 | 9 (19.6%) | 37 (80.4%) | 0 | 40 (100%) | 0 | 60 (100%) | 22.641 | <0.001 |
| Item 22 | 17 (37%) | 29 (63%) | 6 (15%) | 34 (85%) | 3 (5%) | 57 (95%) | 20.023 | <0.001 |
| Item 23 | 4 (8.7%) | 42 (91.3%) | 0 | 40 (100%) | 0 | 60 (100%) | 9.729 | 0.008 |
| Item 24 | 3 (6.5%) | 43 (93.5%) | 0 | 40 (100%) | 0 | 60 (100%) | 7.249 | 0.027 |
| Item 25 | 7 (15.2%) | 39 (84.8%) | 1 (2.5%) | 39 (97.5%) | 1 (1.7%) | 59 (98.3%) | 8.344 | 0.015 |
| Item 26 | 16 (34.8%) | 30 (65.2%) | 12 (30%) | 30 (70%) | 1 (1.7%) | 59 (98.3%) | 20,803 | <0.001 |
| Item 27 | 16 (34.8%) | 30 (65.2%) | 3 (7.5%) | 39 (92.5%) | 0 | 60 (100%) | 32.081 | <0.001 |
| Item 28 | 36 (78.3%) | 10 (21.7%) | 25 (62.5%) | 15 (37.5%) | 11 (18.3%) | 49 (81.7%) | 41.007 | <0.001 |
| Item 29 | 16 (34.8%) | 30 (65.2%) | 3 (7.5%) | 39 (92.5%) | 3 (5%) | 57 (95%) | 22.919 | <0.001 |
| Item 30 | 10 (21.7%) | 36 (78.3%) | 5 (12.5%) | 37 (87.5%) | 1 (1.7%) | 59 (98.3%) | 12.234 | 0.002 |
| Amount of Chlorpromazine PS | PANSS General PS | CAPE-42 Total HR Persons and CG | CAPE 42 Distress HR Persons and CG | SCIP Cognitive Results PS HR and CG | ||
|---|---|---|---|---|---|---|
| BETA | Pearson’s correlation | −0.342 * | −0.643 ** | −0.566 ** | −0.579 ** | 0.259 ** |
| Sig. (Bilateral) | 0.020 | <0.001 | <0.001 | <0.001 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cano, A.; Polonio-López, B.; Bernal-Jiménez, J.J.; Martín-Conty, J.L.; Mordillo-Mateos, L.; Martinez-Lorca, M. Semantic Processing Deficits and Their Use as Early Biomarkers in Schizophrenia. Healthcare 2025, 13, 1958. https://doi.org/10.3390/healthcare13161958
Martínez-Cano A, Polonio-López B, Bernal-Jiménez JJ, Martín-Conty JL, Mordillo-Mateos L, Martinez-Lorca M. Semantic Processing Deficits and Their Use as Early Biomarkers in Schizophrenia. Healthcare. 2025; 13(16):1958. https://doi.org/10.3390/healthcare13161958
Chicago/Turabian StyleMartínez-Cano, Alfonso, Begoña Polonio-López, Juan José Bernal-Jiménez, José L. Martín-Conty, Laura Mordillo-Mateos, and Manuela Martinez-Lorca. 2025. "Semantic Processing Deficits and Their Use as Early Biomarkers in Schizophrenia" Healthcare 13, no. 16: 1958. https://doi.org/10.3390/healthcare13161958
APA StyleMartínez-Cano, A., Polonio-López, B., Bernal-Jiménez, J. J., Martín-Conty, J. L., Mordillo-Mateos, L., & Martinez-Lorca, M. (2025). Semantic Processing Deficits and Their Use as Early Biomarkers in Schizophrenia. Healthcare, 13(16), 1958. https://doi.org/10.3390/healthcare13161958







