Pain Neuroscience Education Versus Biomedical Pain Education with Exercise in Primary Dysmenorrhea: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
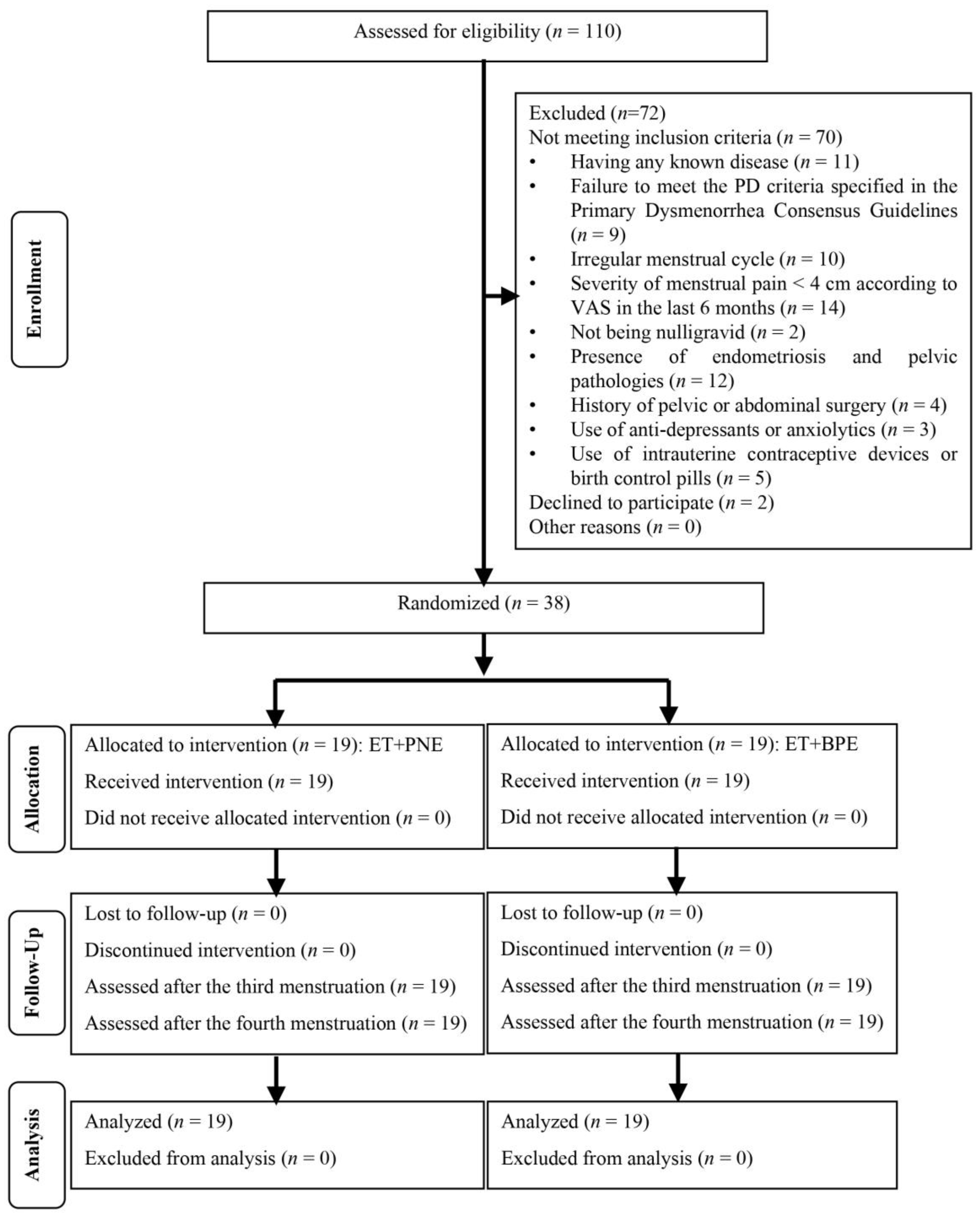
2.2. Participants
2.3. Randomization
2.4. Interventions
2.4.1. Stretching and Relaxation Exercises
2.4.2. Pain Education
- Biomedical Pain Education
- Pain Neuroscience Education
2.5. Outcomes
2.5.1. Descriptive Outcomes
2.5.2. Outcome Measures
- Primary outcome measure
- Secondary outcome measures
2.6. Sample Size
2.7. Statistical Methods
3. Results
3.1. Primary Outcome
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnett, M.; Lemyre, M.N. 345-primary dysmenorrhea consensus guideline. J. Obstet. Gynaecol. Can. 2017, 39, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Soubra, L.; Karout, S.; Rahme, D.; Karout, L.; Khojah, H.M. Primary dysmenorrhea: Pathophysiology, diagnosis, and treatment updates. Korean J. Fam. Med. 2022, 43, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armour, M.; Parry, K.; Manohar, N.; Holmes, K.; Ferfolja, T.; Curry, C.; MacMillan, F.; Smith, C.A. The prevalence and academic impact of dysmenorrhea in 21,573 young women: A systematic review and meta-analysis. J. Women’s Health 2019, 28, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, J.; Avila, M.A.; de Oliveira, R.F.; Dedicação, A.C.; Godoy, A.G.; Rodrigues, J.C.; Driusso, P. Prevalence, pain intensity and symptoms associated with primary dysmenorrhea: A cross-sectional study. BMC Women’s Health 2024, 24, 92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arendt-Nielsen, L.; Morlion, B.; Perrot, S.; Dahan, A.; Dickenson, A.; Kress, H.; Wells, C.; Bouhassira, D.; Drewes, A.M. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur. J. Pain 2018, 22, 216–241. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.A.; Rapkin, A.J.; Seidman, L.C.; Zeltzer, L.K.; Tsao, J.C. Experimental and procedural pain responses in primary dysmenorrhea: A systematic review. J. Pain Res. 2017, 10, 2233–2246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iacovides, S.; Avidon, I.; Baker, F.C. What we know about primary dysmenorrhea today: A critical review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Özgül, S.; Nalan ÇİNAR, G.; Gürşen, C.; Baran, E.; Üzelpasaci, E.; Nakip, G.; Nur Gerlegiz, E.; Çelenay, Ş.T.; Akbayrak, T. The effects of connective tissue manipulation in primary dysmenorrhea: A randomized placebo-controlled study. Reprod. Sci. 2023, 30, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Aparicio, L.; Cuenca-Martínez, F.; Muñoz-Gómez, E.; Mollà-Casanova, S.; Aguilar-Rodríguez, M.; Sempere-Rubio, N. Effects of therapeutic exercise in primary dysmenorrhea: An umbrella and mapping review. Pain Med. 2023, 24, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Allen, M.; Borges, U.; Dosseville, F.; Hosang, T.; Iskra, M.; Mosley, E.; Salvotti, C.; Spolverato, L.; Zammit, N. Effects of voluntary slow breathing on heart rate and heart rate variability: A systematic review and a meta-analysis. Neurosci. Biobehav. Rev. 2022, 138, 104711. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H. Effects of diaphragmatic breathing on health: A narrative review. Medicines 2020, 7, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Upganlawar, D.S.; Patil, S.; Dhage, P.P. Efficacy of Connective Tissue Therapy and Abdominal Stretching Exercises in Individuals with Primary Dysmenorrhea: A Review. Cureus 2023, 15, e46553. [Google Scholar] [CrossRef]
- Tianing, N.W.; Nugraha, M.H.S.; Indrayani, A.W.; Widyadharma, I.P.E. The difference in the effectiveness of warm compress and active stretching exercise in reducing dysmenorrhea pain. Bali Med. J. 2021, 10, 1041–1044. [Google Scholar] [CrossRef]
- Hogans, B.B.; Watt-Watson, J.; Wilkinson, P.; Carr, E.C.; Gordon, D.B. Perspective: Update on pain education. Pain 2018, 159, 1681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dams, L.; Van der Gucht, E.; Haenen, V.; Devoogdt, N.; Smeets, A.; Bernar, K.; Morlion, B.; Moloney, N.; Fieuws, S.; De Groef, A. Effectiveness of pain neuroscience education on somatosensory functioning after surgery for breast cancer: A double-blinded randomized controlled trial. Anat. Rec. 2024, 307, 248–272. [Google Scholar] [CrossRef] [PubMed]
- Manfuku, M.; Nishigami, T.; Mibu, A.; Yamashita, H.; Imai, R.; Tanaka, K.; Kitagaki, K.; Hiroe, K.; Sumiyoshi, K. Effect of perioperative pain neuroscience education in patients with post-mastectomy persistent pain: A retrospective, propensity score-matched study. Support. Care Cancer 2021, 29, 5351–5359. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Butler, D.S. Fifteen years of explaining pain: The past, present, and future. J. Pain 2015, 16, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Clark, J.; Malfliet, A.; Ickmans, K.; Voogt, L.; Don, S.; den Bandt, H.; Goubert, D.; Kregel, J.; Coppieters, I. In the spine or in the brain? Recent advances in pain neuroscience applied in the intervention for low back pain. Clin. Exp. Rheumatol. 2017, 35, 108–115. [Google Scholar]
- Chae, Y.; Park, H.-J.; Lee, I.-S. Pain modalities in the body and brain: Current knowledge and future perspectives. Neurosci. Biobehav. Rev. 2022, 139, 104744. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.; Zimney, K.; Puentedura, E.J.; Diener, I. The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. Physiother. Theory Pract. 2016, 32, 332–355. [Google Scholar] [CrossRef] [PubMed]
- Malfliet, A.; Kregel, J.; Coppieters, I.; De Pauw, R.; Meeus, M.; Roussel, N.; Cagnie, B.; Danneels, L.; Nijs, J. Effect of pain neuroscience education combined with cognition-targeted motor control training on chronic spinal pain: A randomized clinical trial. JAMA Neurol. 2018, 75, 808–817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nijs, J.; Wijma, A.J.; Willaert, W.; Huysmans, E.; Mintken, P.; Smeets, R.; Goossens, M.; van Wilgen, C.P.; Van Bogaert, W.; Louw, A. Integrating motivational interviewing in pain neuroscience education for people with chronic pain: A practical guide for clinicians. Phys. Ther. 2020, 100, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Gutke, A.; Sundfeldt, K.; De Baets, L. Lifestyle and chronic pain in the pelvis: State of the art and future directions. J. Clin. Med. 2021, 10, 5397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barcikowska, Z.; Rajkowska-Labon, E.; Grzybowska, M.E.; Hansdorfer-Korzon, R.; Zorena, K. Inflammatory markers in dysmenorrhea and therapeutic options. Int. J. Environ. Res. Public Health 2020, 17, 1191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, H.; Hong, M.; Duan, J.; Liu, P.; Fan, X.; Shang, E.; Su, S.; Guo, J.; Qian, D.; Tang, Y. Altered cytokine gene expression in peripheral blood monocytes across the menstrual cycle in primary dysmenorrhea: A case-control study. PLoS ONE 2013, 8, e55200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belavy, D.L.; Van Oosterwijck, J.; Clarkson, M.; Dhondt, E.; Mundell, N.L.; Miller, C.T.; Owen, P.J. Pain sensitivity is reduced by exercise training: Evidence from a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Cortés, R.; Salazar-Méndez, J.; Calatayud, J.; Malfliet, A.; Lluch, E.; Mendez-Rebolledo, G.; Guzmán-Muñoz, E.; López-Bueno, R.; Suso-Martí, L. The optimal dose of pain neuroscience education added to an exercise programme for patients with chronic spinal pain: A systematic review and dose–response meta-analysis. Pain 2022, 165, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Siddall, B.; Ram, A.; Jones, M.D.; Booth, J.; Perriman, D.; Summers, S.J. Short-term impact of combining pain neuroscience education with exercise for chronic musculoskeletal pain: A systematic review and meta-analysis. Pain 2022, 163, e20–e30. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.M.; Carmo, A.C.N.; Leal, L.B.L.G.; de Souza, R.P.; Rocha, P.R.S.; Funez, M.I. A systematic review of the added value of perioperative pain neuroscience education. Patient Educ. Couns. 2023, 117, 107984. [Google Scholar] [CrossRef] [PubMed]
- Benedict, T.M.; Nitz, A.J.; Gambrel, M.K.; Louw, A. Pain neuroscience education improves post-traumatic stress disorder, disability, and pain self-efficacy in veterans and service members with chronic low back pain: Preliminary results from a randomized controlled trial with 12-month follow-up. Mil. Psychol. 2023, 36, 376–392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galan-Martin, M.A.; Montero-Cuadrado, F.; Lluch-Girbes, E.; Coca-López, M.C.; Mayo-Iscar, A.; Cuesta-Vargas, A. Pain neuroscience education and physical therapeutic exercise for patients with chronic spinal pain in Spanish physiotherapy primary care: A pragmatic randomized controlled trial. J. Clin. Med. 2020, 9, 1201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huysmans, E.; Goudman, L.; Coppieters, I.; Van Bogaert, W.; Moens, M.; Buyl, R.; Nijs, J.; Louw, A.; Logghe, T.; Putman, K. Effect of perioperative pain neuroscience education in people undergoing surgery for lumbar radiculopathy: A multicentre randomised controlled trial. Br. J. Anaesth. 2023, 131, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Lluch, E.; Dueñas, L.; Falla, D.; Baert, I.; Meeus, M.; Sanchez-Frutos, J.; Nijs, J. Preoperative pain neuroscience education combined with knee joint mobilization for knee osteoarthritis. Clin. J. Pain 2018, 34, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Fuentes, F.; Cuyul-Vasquez, I.; Bustos-Medina, L.; Fuentes, J. Effects of pain neuroscience education and rehabilitation following arthroscopic rotator cuff repair. A randomized clinical trial. Physiother. Theory Pract. 2023, 39, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Dams, L.; Van der Gucht, E.; Devoogdt, N.; Smeets, A.; Bernar, K.; Morlion, B.; Godderis, L.; Haenen, V.; De Vrieze, T.; Fieuws, S. Effect of pain neuroscience education after breast cancer surgery on pain, physical, and emotional functioning: A double-blinded randomized controlled trial (educan trial). Pain 2023, 164, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Núñez-Cortés, R.; Espinoza-Ordóñez, C.; Pommer, P.P.; Horment-Lara, G.; Pérez-Alenda, S.; Cruz-Montecinos, C. A single preoperative pain neuroscience education: Is it an effective strategy for patients with carpal tunnel syndrome? Med. Hypotheses 2019, 126, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; The CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010, 1, 100–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armour, M.; Ee, C.C.; Naidoo, D.; Ayati, Z.; Chalmers, K.J.; Steel, K.A.; de Manincor, M.J.; Delshad, E. Exercise for dysmenorrhoea. Cochrane Database Syst. Rev. 2019, 2019, CD004142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aboushady, R.; El-saidy, T.M.K. Effect of home based stretching exercises and menstrual care on primary dysmenorrhea and premenstrual symptoms among adolescent girls. IOSR J. Nurs. Health Sci. 2016, 5, 10–17. [Google Scholar]
- López-Liria, R.; Torres-Álamo, L.; Vega-Ramírez, F.A.; García-Luengo, A.V.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Rocamora-Pérez, P. Efficacy of physiotherapy treatment in primary dysmenorrhea: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 7832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ortiz, M.I.; Cortés-Márquez, S.K.; Romero-Quezada, L.C.; Murguía-Cánovas, G.; Jaramillo-Díaz, A.P. Effect of a physiotherapy program in women with primary dysmenorrhea. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Soni, P.; Desai, D. Effectiveness of Pilates and Self-Stretching Exercise on Pain and Quality of Life in Primary Dysmenorrhea”—A Comparative Study. Indian J. Physiother. Occup. Ther. 2021, 15, 129–138. [Google Scholar] [CrossRef]
- Anderson, A.W.; Soncini, A.; Lyons, K.; Hanney, W.J. The Effect of Myofascial Stretching on Mechanical Nociception and Contributing Neural Mechanisms. NeuroSci 2024, 5, 158–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hush, J.M.; Nicholas, M.; Dean, C.M. Embedding the IASP pain curriculum into a 3-year pre-licensure physical therapy program: Redesigning pain education for future clinicians. Pain Rep. 2018, 3, e645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orhan, C.; Lenoir, D.; Favoreel, A.; Van Looveren, E.; Yildiz Kabak, V.; Mukhtar, N.B.; Cagnie, B.; Meeus, M. Culture-sensitive and standard pain neuroscience education improves pain, disability, and pain cognitions in first-generation Turkish migrants with chronic low back pain: A pilot randomized controlled trial. Physiother. Theory Pract. 2021, 37, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wickström, K.; Edelstam, G. Minimal clinically important difference for pain on the VAS scale and the relation to quality of life in women with endometriosis. Sex. Reprod. Healthc. 2017, 13, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D.; McGrath, P.A.; Rafii, A.; Buckingham, B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983, 17, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kızılkaya, N. Perimenstrual Şikayetlerin Hafifletilmesinde Hemşirelik Girişimlerinin Etkinliği [Effectiveness of Nursing Interventions in Relieving Perimenstrual Complaints]. Ph.D. Thesis, İstanbul University, İstanbul, Turkey, 1994. [Google Scholar]
- Moos, R.H. The development of a menstrual distress questionnaire. Psychosom. Med. 1968, 30, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Düzce Keleş, E.; Birtane, M.; Ekuklu, G.; Kılınçer, C.; Çalıyurt, O.; Taştekin, N. Validity and reliability of the Turkish version of the central sensitization inventory. Arch. Rheumatol. 2022, 37, 518–526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sullivan, M.J.; Bishop, S.R.; Pivik, J. The pain catastrophizing scale: Development and validation. Psychol. Assess. 1995, 7, 524. [Google Scholar] [CrossRef]
- Süren, M.; Okan, I.; Gökbakan, A.M.; Kaya, Z.; Erkorkmaz, Ü.; Arici, S.; Karaman, S.; Kahveci, M. Factors associated with the pain catastrophizing scale and validation in a sample of the Turkish population. Turk. J. Med. Sci. 2014, 44, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, X.; Liu, J.-H.; Huang, J.-P. Efficacy of non-pharmacological interventions for primary dysmenorrhoea: A systematic review and Bayesian network meta-analysis. BMJ Evid.-Based Med. 2024, 44, 104–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matthewman, G.; Lee, A.; Kaur, J.G.; Daley, A.J. Physical activity for primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2018, 219, 255.e1–255.e20. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Ozturk, N.; Gerçek Öter, E.; Kürek Eken, M. The effect of abdominal massage and stretching exercise on pain and dysmenorrhea symptoms in female university students: A single-blind randomized-controlled clinical trial. Health Care Women Int. 2023, 44, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Kirmizigil, B.; Demiralp, C. Effectiveness of functional exercises on pain and sleep quality in patients with primary dysmenorrhea: A randomized clinical trial. Arch. Gynecol. Obstet. 2020, 302, 153–163. [Google Scholar] [CrossRef]
- Celenay, S.T.; Ozcelikel, G.; Bayrakli, A. Efficacy of progressive muscle relaxation technique in primary dysmenorrhea: A randomized controlled trial. Taiwan J. Obstet. Gynecol. 2024, 63, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; McAuley, J.H.; Huebscher, M.; Kamper, S.J.; Traeger, A.C.; Moseley, G.L. Does changing pain-related knowledge reduce pain and improve function through changes in catastrophizing? Pain 2016, 157, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Traeger, A.C.; Lee, H.; Hübscher, M.; Skinner, I.W.; Moseley, G.L.; Nicholas, M.K.; Henschke, N.; Refshauge, K.M.; Blyth, F.M.; Main, C.J. Effect of intensive patient education vs placebo patient education on outcomes in patients with acute low back pain: A randomized clinical trial. JAMA Neurol. 2019, 76, 161–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amer-Cuenca, J.J.; Pecos-Martín, D.; Martínez-Merinero, P.; Lluch Girbés, E.; Nijs, J.; Meeus, M.; Ferrer Peña, R.; Fernández-Carnero, J. How much is needed? Comparison of the effectiveness of different pain education dosages in patients with fibromyalgia. Pain Med. 2020, 21, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Çinar, G.N.; Akbayrak, T.; Gürşen, C.; Baran, E.; Üzelpasacı, E.; Nakip, G.; Bozdağ, G.; Beksaç, M.S.; Özgül, S. Factors related to primary dysmenorrhea in Turkish women: A multiple multinomial logistic regression analysis. Reprod. Sci. 2021, 28, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, F.; Zeng, F.; Yu, J.; Cui, F.; Yang, B.; Zhang, L. Revealing the mechanism of central pain hypersensitivity in primary dysmenorrhea: Evidence from neuroimaging. Quant. Imaging Med. Surg. 2024, 14, 3075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, S.; Dowding, C.; Olive, L.; Payne, L.A.; Druitt, M.; Seidman, L.C.; Skvarc, D.; Mikocka-Walus, A. Pain catastrophizing, but not mental health or social support, is associated with menstrual pain severity in women with dysmenorrhea: A cross-sectional survey. Psychol. Health Med. 2022, 27, 1410–1420. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | PNE+ET (n = 19) | BPE+ET (n = 19) | p-Value |
|---|---|---|---|
| Demographic and physical characteristics | |||
| Age, y | 20.89 ± 3.51 | 20.37 ± 2.36 | 0.591 a |
| Education | |||
| ˂High school | - | - | 1.000 b |
| ≥High school | 19 (100%) | 19 (100%) | |
| Marital status | |||
| Single | 19 (100%) | 19 (100%) | 1.000 b |
| Married | - | - | |
| Working status | |||
| Employed | 3 (15.8%) | 2 (10.5%) | 0.500 b |
| Unemployed | 16 (84.2%) | 17 (89.5%) | |
| BMI, kg/m2 | 22.50 ± 3.20 | 22.56 ± 3.60 | 0.956 a |
| Lifestyle characteristics | |||
| Smoking | |||
| No | 16.0 (84.2%) | 14.0 (73.7%) | 0.426 b |
| Yes | 3.0 (15.8%) | 5.0 (26.3%) | |
| Smoking exposure, package-year | 3.09 ± 3.43 | 0.92 ± 1.21 | 0.180 a |
| Alcohol consumption | |||
| No | 18.0 (94.7%) | 18.0 (94.7%) | 1.000 b |
| Yes | 1.0 (5.3%) | 1.0 (5.3%) | |
| Regular exercise | |||
| No | 19.0 (100%) | 19.0 (100%) | 1.000 b |
| Yes | - | - | |
| Menstrual characteristics | |||
| Age at menarche, y | |||
| <12 y | 1 (5.3%) | - | 0.311 b |
| ≥ 12 y | 18 (94.7%) | 19 (100%) | |
| Menstrual cycle duration, d | 27.11 ± 3.25 | 28.68 ± 3.23 | 0.234 c |
| Menstruation duration, d | |||
| 3–7 d | 19 (100%) | 19 (100%) | 1.000 b |
| >7 d | - | - | |
| Average menstrual pain severity in the last 6 months | 6.71 ± 1.66 | 6.35 ± 1.64 | 0.593 c |
| Menstrual pain duration, hours/cycle | 13.84 ± 15.81 | 16.74 ± 17.49 | 0.525 c |
| Use of medication (analgesic, NSAI) for PD | |||
| No | 10 (52.6%) | 12 (63.2%) | 0.511 b |
| Yes | 9 (47.4%) | 7 (36.8%) | |
| Use of analgesic/NSAI during last menstruation | |||
| No | 10 (52.6%) | 13 (68.4%) | 0.319 b |
| Yes | 9 (47.4%) | 6 (31.6%) |
| Outcome Measure | Time Point | Within-Group Effect | Between-Group Effect | Between-Group Effect Size | Cohen’s d 95% CI | ||
|---|---|---|---|---|---|---|---|
| PNE+ET (n = 19) | BPE+ET (n = 19) | p a | dCohen | Lower | Upper | ||
| Primary Outcome Measures | |||||||
| Mean pain intensity, cm | Baseline | 4.74 ± 1.36 x | 4.64 ± 1.95 x | 0.863 | |||
| After | 1.70 ±0.96 y | 2.93 ± 1.82 y | 0.023 | 0.847 | −1.507 | −0.177 | |
| Follow-up | 1.85 ± 1.07 y | 3.23 ± 1.28 y | 0.001 | 1.174 | −1.858 | −0.477 | |
| p b | <0.001 | 0.002 | |||||
| Maximum pain intensity, cm | Baseline | 7.74 ± 1.29 x | 7.09 ± 1.5 x | 0.339 | |||
| After | 3.91 ± 1.72 y | 5.24 ± 1.67 y | 0.030 | 0.786 | −1.442 | −0.120 | |
| Follow-up | 4.18 ± 2.24 y | 5.48 ± 1.48 y | 0.046 | 0.683 | −1.333 | −0.023 | |
| p b | <0.001 | 0.003 | |||||
| Secondary Outcome Measures | |||||||
| Menstrual stress | Baseline | 77.89 ± 25.10 x | 80.37 ± 30.73 x | 0.751 | |||
| After | 40.05 ± 23.02 y | 46.21 ± 27.09 y | 0.525 | 0.245 | −0.882 | 0.395 | |
| Follow-up | 36.21 ± 26.39 y | 44.11 ± 30.90 y | 0.325 | 0.275 | −0.912 | 0.366 | |
| p b | <0.001 | <0.001 | |||||
| Central sensitization symptoms | Baseline | 43.05 ± 19.05 x | 36.37 ± 20.29 x | 0.418 | |||
| After | 18.16 ± 10.38 y | 31.00 ± 20.07 y | 0.043 | 0.804 | −1.461 | −0.136 | |
| Follow-up | 16.95 ± 10.34 y | 29.11 ± 20.15 y | 0.043 | 0.759 | −1.414 | −0.095 | |
| p b | <0.001 | 0.046 | |||||
| Menstrual pain catastrophizing | Baseline | 25.26 ± 12.80 x | 24.05 ± 15.46 x | 0.773 | |||
| After | 7.37 ± 5.19 y | 15.53 ± 13.51 y | 0.043 | 0.797 | −1.454 | −0.130 | |
| Follow-up | 7.42 ± 7.46 y | 15.53 ± 13.94 y | 0.025 | 0.725 | −1.378 | −0.063 | |
| p b | <0.001 | <0.001 | |||||
| Exercise Compliance | PNE + ET (n = 19) | BPE + ET (n = 19) | p |
|---|---|---|---|
| Exercise compliance (%) | 97.70 ± 4.27 87.50–100% | 97.04 ± 5.65 81.25–100% | 0.895 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erol, B.N.; Gürşen, C.; Mümüşoğlu, S.; Özgül, S. Pain Neuroscience Education Versus Biomedical Pain Education with Exercise in Primary Dysmenorrhea: A Randomized Controlled Trial. Healthcare 2025, 13, 1954. https://doi.org/10.3390/healthcare13161954
Erol BN, Gürşen C, Mümüşoğlu S, Özgül S. Pain Neuroscience Education Versus Biomedical Pain Education with Exercise in Primary Dysmenorrhea: A Randomized Controlled Trial. Healthcare. 2025; 13(16):1954. https://doi.org/10.3390/healthcare13161954
Chicago/Turabian StyleErol, Büşra N., Ceren Gürşen, Sezcan Mümüşoğlu, and Serap Özgül. 2025. "Pain Neuroscience Education Versus Biomedical Pain Education with Exercise in Primary Dysmenorrhea: A Randomized Controlled Trial" Healthcare 13, no. 16: 1954. https://doi.org/10.3390/healthcare13161954
APA StyleErol, B. N., Gürşen, C., Mümüşoğlu, S., & Özgül, S. (2025). Pain Neuroscience Education Versus Biomedical Pain Education with Exercise in Primary Dysmenorrhea: A Randomized Controlled Trial. Healthcare, 13(16), 1954. https://doi.org/10.3390/healthcare13161954


_MD__MPH_PhD.png)



