Mobile Health Applications for Secondary Prevention After Myocardial Infarction or PCI: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Study Design and Protocol Development
2.2. Search Strategy
2.3. Eligibility Criteria
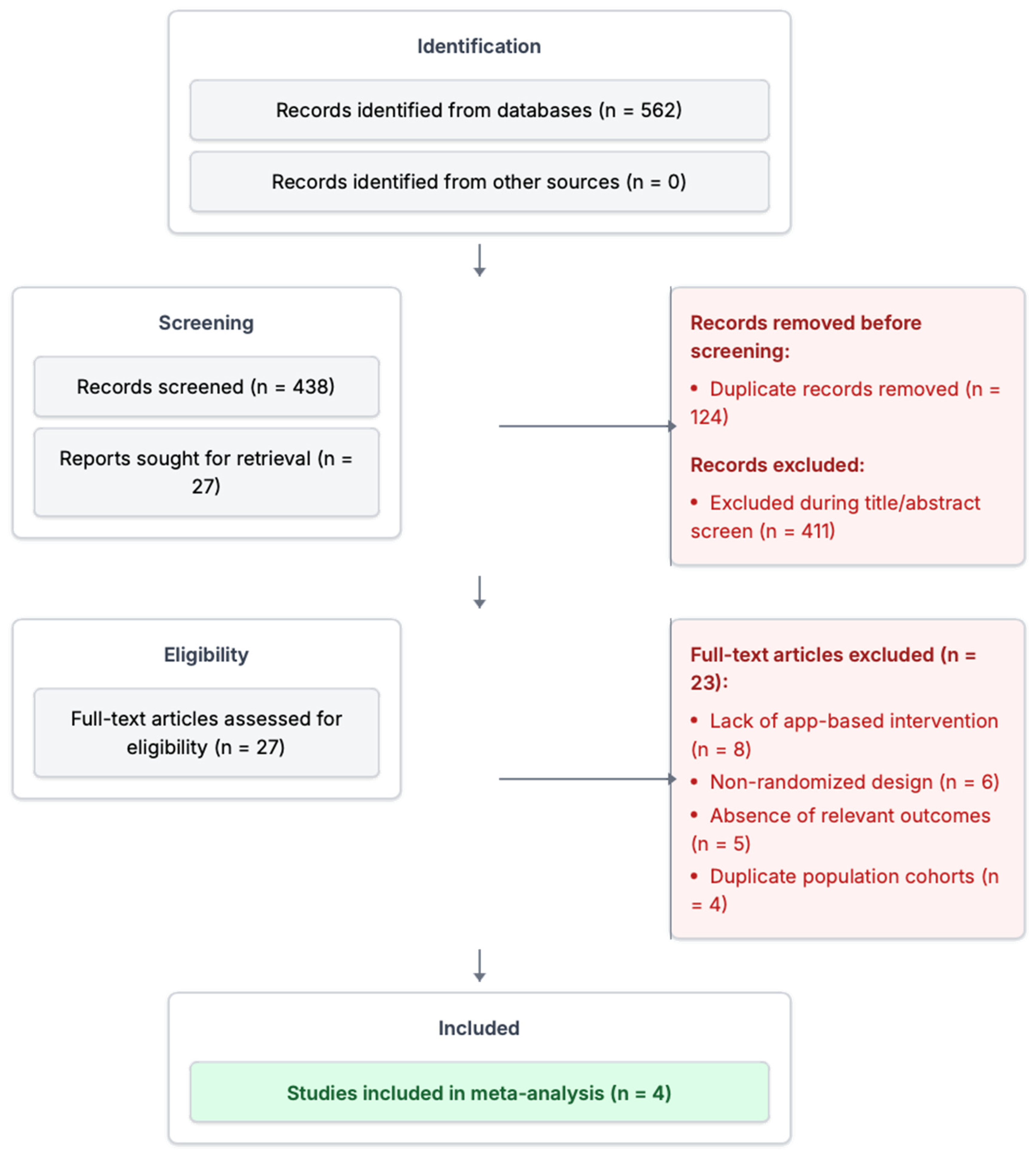
2.4. Study Selection and Screening Process
2.5. Data Extraction and Outcomes of Interest
2.6. Risk of Bias Assessment and Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Population Characteristics
3.4. Intervention Features and App Adherence
3.5. Primary Outcome: Unplanned Hospital Readmissions
3.6. Secondary Outcomes: Patient-Reported Outcomes
3.7. Subgroup and Sensitivity Analyses
4. Discussion
5. Limitations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| ARR | Absolute Risk Reduction |
| CI | Confidence Interval |
| ED | Emergency Department |
| HRQoL | Health-Related Quality of Life |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| MACEs | Major Adverse Cardiovascular Events |
| MI | Myocardial Infarction |
| mHealth | Mobile Health |
| NNT | Number Needed to Treat |
| PCI | Percutaneous Coronary Intervention |
| QoL | Quality of Life |
| RCT | Randomized Controlled Trial |
| RR | Risk Ratio |
References
- Tüner, H.; Polat, F.; Alıç, E.; Kaya, A.N.; Çakmak, Ç.B.; Coşkun, F.; Özbek, E. Effectiveness of Cardiac Rehabilitation in Enhancing Adherence and Improving Clinical Outcomes Post-Acute Coronary Syndrome: A Randomized Controlled Trial. Clin. Cardiol. 2025, 48, e70160. [Google Scholar] [CrossRef] [PubMed]
- Nkonde-Price, C.; Reynolds, K.; Najem, M.; Yang, S.-J.; Batiste, C.; Cotter, T.; Lahti, D.; Gin, N.; Funahashi, T. Comparison of Home-Based vs Center-Based Cardiac Rehabilitation in Hospitalization, Medication Adherence, and Risk Factor Control Among Patients with Cardiovascular Disease. JAMA Netw. Open 2022, 5, e2228720. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Allison, T.G.; Lennon, R.; Lopez-Jimenez, F.; Lerman, L.O.; Lerman, A. Digital health intervention during cardiac rehabilitation: A randomized controlled trial. Am. Heart J. 2017, 188, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cobo, C.; Bernal-Jiménez, M.Á.; Calle, G.; Gheorghe, L.L.; Gutiérrez-Barrios, A.; Cañadas, D.; A Tur, J.; Vázquez-García, R.; Santi-Cano, M.J. Efficacy of a Mobile Health App (eMOTIVA) Regarding Compliance with Cardiac Rehabilitation Guidelines in Patients with Coronary Artery Disease: Randomized Controlled Clinical Trial. JMIR mHealth uHealth 2024, 12, e55421. [Google Scholar] [CrossRef] [PubMed]
- Arian, M.; Valinejadi, A.; Soleimani, M. Reviews Evaluating Information Technology-Based Cardiac Rehabilitation Programs and Support: A Systematic Review. Iran. J. Public Health 2022, 51, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Wongvibulsin, S.; E Habeos, E.; Huynh, P.P.; Xun, H.; Shan, R.; Rodriguez, K.A.P.; Wang, J.; Gandapur, Y.K.; Osuji, N.; Shah, L.M.; et al. Digital Health Interventions for Cardiac Rehabilitation: Systematic Literature Review. J. Med. Internet Res. 2021, 23, e18773. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, N.S.; Hartley, A.; Howard, J.; Hajhosseiny, R.; Khawaja, S.; Seligman, H.; Akbari, T.; Alharbi, B.A.; Bassett, P.; Al-Lamee, R.; et al. Randomized Trial of Remote Assessment of Patients After an Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2024, 83, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Krzowski, B.; Boszko, M.; Peller, M.; Hoffman, P.; Żurawska, N.; Skoczylas, K.; Osak, G.; Kołtowski, Ł.; Grabowski, M.; Opolski, G.; et al. Mobile App and Digital System for Patients after Myocardial Infarction (afterAMI): Results from a Randomized Trial. J. Clin. Med. 2023, 12, 2886. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Davey, R.; Keegan, R.; Niyonsenga, T.; Mohanty, I.; Bowen, S.; Regan, E.; Lander, M.; van Berlo, S.; Freene, N. Testing the Effect of a Smartphone App on Hospital Admissions and Sedentary Behavior in Cardiac Rehabilitation Participants: ToDo-CR Randomized Controlled Trial. JMIR mHealth uHealth 2023, 11, e48229. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ge, C.; Shi, Y.; Xu, Y.; Zhao, C.; Gao, L.; Wen, D.; Li, T.; Wang, J.; Yan, S.; et al. Chinese Home-Based Cardiac Rehabilitation Model Delivered by Smartphone Interaction Improves Clinical Outcomes in Patients with Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 731557. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, I.; Maurizi, N.; Dangas, G.; Chatzizisis, Y.S. Answering the Call: Enhancing Telemedicine Reach with Artificial Intelligence Integration. J. Am. Coll. Cardiol. 2024, 84, e311. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gu, X.; Jiang, J.; Li, H.; Duan, R.; Zhang, Y.; Sun, L.; Bao, Z.; Shen, J.; Chen, F. A Hospital-Community-Family-Based Telehealth Program for Patients with Chronic Heart Failure: Single-Arm, Prospective Feasibility Study. JMIR mHealth uHealth 2019, 7, e13229. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, I.; Koutromanos, I.; Chatzidaki, E.; Kachrimanidis, I.; Maurizi, N. Beyond reality: Using the Metaverse to enhance mental health in heart failure patients. Hell. J. Cardiol. 2024, 75, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Beatty, A.L.; Beckie, T.M.; Brewer, L.C.; Brown, T.M.; Forman, D.E.; Franklin, B.A.; Keteyian, S.J.; Kitzman, D.W.; Regensteiner, J.G.; et al. Home-Based Cardiac Rehabilitation: A Scientific Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J. Am. Coll. Cardiol. 2019, 74, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Skalidis, I.; Maurizi, N.; Kaldasch, M.; El Idrissi, B.; Briante, N.; Fournier, S.; Muller, O.; Skalidis, E. Feasibility of clinical and electrocardiographic follow-up of prinzmetal angina using the metaverse. Eur. Heart J. Digit. Health 2022, 3, ztac076-2817. [Google Scholar]
- Skalidis, I.; Cagnina, A.; Fournier, S. Use of large language models for evidence-based cardiovascular medicine. Eur. Heart J. Digit. Health 2023, 4, 368–369. [Google Scholar] [CrossRef] [PubMed]

| Study | Year | Country | Sample Size (N) | Follow-Up Duration | Population | App Features | Control Group | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| TELE-ACS | 2022 | United Kingdom | 337 | 12 months | Post-MI/PCI | Education, reminders, clinician messaging, symptom tracking | Usual care without app | Unplanned readmissions | QoL, medication adherence, emergency department visits |
| afterAMI | 2023 | Poland | 100 | 6 months | Post-MI | Daily notifications, secure messaging, medication reminders | Usual care without app | Unplanned readmissions, urgent visits and MACEs | QoL, satisfaction, adherence |
| ToDo-CR | 2023 | Australia | 120 | 3 months | Post-PCI | Behavioral activation, step tracking, lifestyle coaching | Usual care without app | Unplanned readmissions | QoL, physical activity, behavior change |
| WeChat HBCR | 2024 | China | 270 | 42 months | Post-MI/PCI | Cardiac rehab modules, peer support, real-time physician feedback | Usual care without app | MACEs, unscheduled readmission | QoL, physical activity, LDL-C, blood pressure |
| Study | Mean Age (Years) | Male (%) | HTN (%) | DM (%) | Smoking (%) | Hyperlipidemia (%) | Previous MI (%) | DAPT (%) | Statin Use (%) |
|---|---|---|---|---|---|---|---|---|---|
| TELE-ACS | 61.4 | 68 | 76 | 25 | 21 | 85 | 29 | 94 | 98 |
| afterAMI | 58.3 | 62 | 63 | 12 | 17 | 72 | 22 | 96 | 97 |
| ToDo-CR | 60.2 | 65 | 58 | 22 | 42 | 61 | 24 | 91 | 94 |
| WeChat HBCR | 67.4 | 71 | 49 | 36 | 35 | 58 | 32 | 93 | 96 |
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result |
|---|---|---|---|---|---|
| TELE-ACS | Low risk—Computer-generated sequence and balanced groups | Low risk—Minimal deviation, high adherence, monitored use | Low risk—High retention and complete follow-up | Low risk—Objective outcomes (readmissions), possible blinding | Low risk—Trial registered, prespecified outcomes reported |
| afterAMI | Low risk—Adequate randomization and allocation concealment | Some concerns—Open-label with unclear handling of protocol deviations | Low risk—Follow-up > 95%, well-balanced | Low risk—Outcomes objectively verified | Low risk—Registered protocol followed |
| ToDo-CR | Low risk—Random sequence generation with no baseline imbalance | Low risk—Good adherence and protocol fidelity | Low risk—Attrition < 5%, similar between groups | High risk—Patient-reported outcomes without blinding | Low risk—No selective reporting identified |
| WeChat HBCR | Low risk—Randomization method adequately described | Low risk—No major deviations reported | High risk—>10% missing data, no imputation analysis | Low risk—Objective outcomes with automated logs and tracking | Low risk—Outcomes matched trial registration |
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result |
|---|---|---|---|---|---|
| TELE-ACS | Low risk—Computer-generated sequence and balanced groups | Low risk—Minimal deviation, high adherence, monitored use | Low risk—High retention and complete follow-up | Low risk—Objective outcomes (readmissions), possible blinding | Low risk—Trial registered, prespecified outcomes reported |
| afterAMI | Low risk—Adequate randomization and allocation concealment | Some concerns—Open-label with unclear handling of protocol deviations | Low risk—Follow-up > 95%, well-balanced | Low risk—Outcomes objectively verified | Low risk—Registered protocol followed |
| ToDo-CR | Low risk—Random sequence generation with no baseline imbalance | Low risk—Good adherence and protocol fidelity | Low risk—Attrition < 5%, similar between groups | High risk—Patient-reported outcomes without blinding | Low risk—No selective reporting identified |
| WeChat HBCR | Low risk—Randomization method adequately described | Low risk—No major deviations reported | High risk—>10% missing data, no imputation analysis | Low risk—Objective outcomes with automated logs and tracking | Low risk—Outcomes matched trial registration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalidis, I.; Lu, H.; Maurizi, N.; Fournier, S.; Tsigkas, G.; Apostolos, A.; Cook, S.; Iglesias, J.F.; Garot, P.; Hovasse, T.; et al. Mobile Health Applications for Secondary Prevention After Myocardial Infarction or PCI: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare 2025, 13, 1881. https://doi.org/10.3390/healthcare13151881
Skalidis I, Lu H, Maurizi N, Fournier S, Tsigkas G, Apostolos A, Cook S, Iglesias JF, Garot P, Hovasse T, et al. Mobile Health Applications for Secondary Prevention After Myocardial Infarction or PCI: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare. 2025; 13(15):1881. https://doi.org/10.3390/healthcare13151881
Chicago/Turabian StyleSkalidis, Ioannis, Henri Lu, Niccolo Maurizi, Stephane Fournier, Grigorios Tsigkas, Anastasios Apostolos, Stephane Cook, Juan F. Iglesias, Philippe Garot, Thomas Hovasse, and et al. 2025. "Mobile Health Applications for Secondary Prevention After Myocardial Infarction or PCI: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Healthcare 13, no. 15: 1881. https://doi.org/10.3390/healthcare13151881
APA StyleSkalidis, I., Lu, H., Maurizi, N., Fournier, S., Tsigkas, G., Apostolos, A., Cook, S., Iglesias, J. F., Garot, P., Hovasse, T., Neylon, A., Unterseeh, T., Garot, J., Amabile, N., Sayah, N., Sanguineti, F., Akodad, M., & Antiochos, P. (2025). Mobile Health Applications for Secondary Prevention After Myocardial Infarction or PCI: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare, 13(15), 1881. https://doi.org/10.3390/healthcare13151881









