Cost-Effectiveness of Endoscopic Stricturotomy Versus Resection Surgery for Crohn’s Disease Strictures
Abstract
1. Introduction
2. Methods
2.1. Base Model
2.2. Outcomes
2.3. Costs
2.4. Assumptions
2.5. Analysis
3. Results
3.1. Base Case Analysis
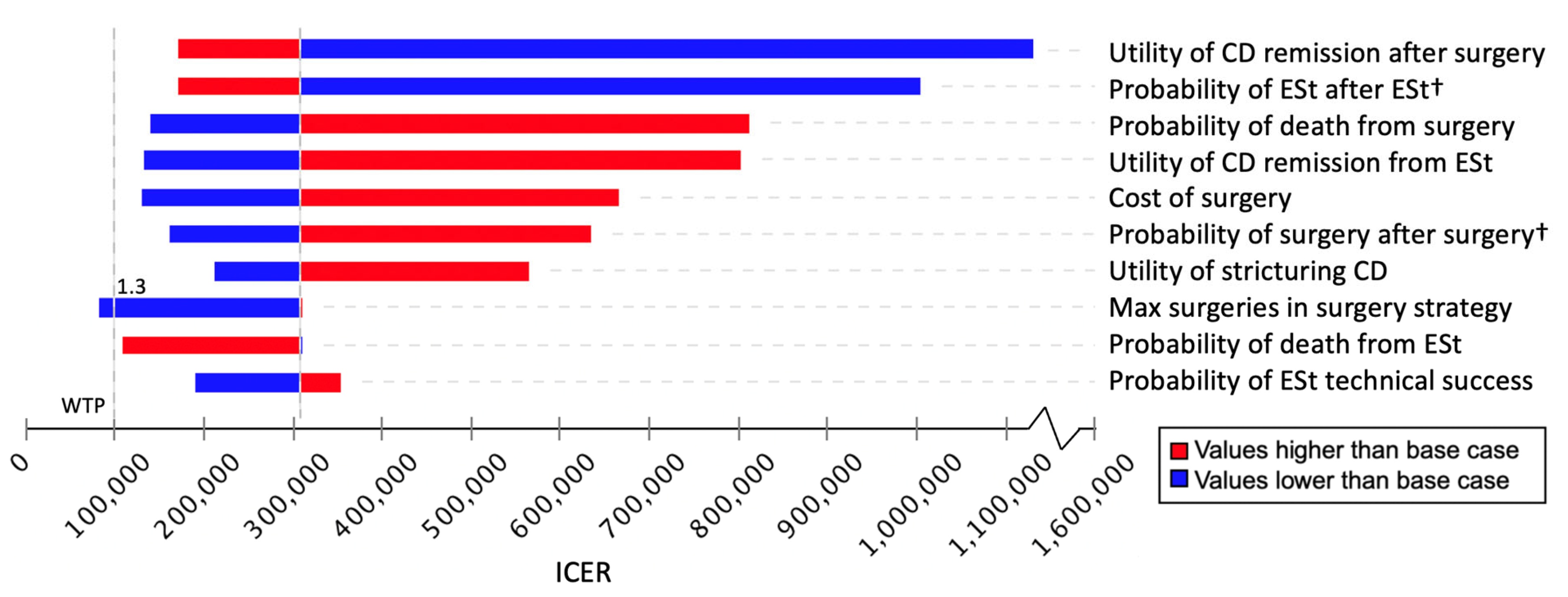
3.2. Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Crohn’s Disease |
| EBD | Endoscopic Balloon Dilation |
| ESt | Endoscopic Stricturotomy |
| IBD | Inflammatory Bowel Disease |
| ICER | Incremental Cost-Effectiveness Ratio |
| PSA | Probabilistic Sensitivity Analysis |
| QALY | Quality-Adjusted Life Year |
| US | United States |
| WTP | Willingness To Pay |
References
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Fleshner, P.; Shen, B. Therapeutic Armamentarium for Stricturing Crohn’s Disease: Medical Versus Endoscopic Versus Surgical Approaches. Inflamm. Bowel Dis. 2015, 21, 2194–2213. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shen, B. Endoscopic Therapy in Crohn’s Disease: Principle, Preparation, and Technique. Inflamm. Bowel Dis. 2015, 21, 2222–2240. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Cattan, S.; Blain, A.; Beaugerie, L.; Carbonnel, F.; Parc, R.; Gendre, J.P. Long-term evolution of disease behavior of Crohn’s disease. Inflamm. Bowel Dis. 2002, 8, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Kochhar, G.; Navaneethan, U.; Farraye, F.A.; Schwartz, D.A.; Iacucci, M.; Bernstein, C.N.; Dryden, G.; Cross, R.; Bruining, D.H.; et al. Practical guidelines on endoscopic treatment for Crohn’s disease strictures: A consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol. Hepatol. 2020, 5, 393–405. [Google Scholar] [CrossRef]
- Thia, K.T.; Sandborn, W.J.; Harmsen, W.S.; Zinsmeister, A.R.; Loftus, E.V., Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010, 139, 1147–1155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henriksen, M.; Jahnsen, J.; Lygren, I.; Aadland, E.; Schulz, T.; Vatn, M.H.; Moum, B.; Ibsen Study Group. Clinical course in Crohn’s disease: Results of a five-year population-based follow-up study (the IBSEN study). Scand. J. Gastroenterol. 2007, 42, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.B.; Click, B.H.; Koutroubakis, I.E.; Ramos Rivers, C.; Regueiro, M.; Swoger, J.; Schwartz, M.; Hashash, J.; Barrie, A.; Dunn, M.A.; et al. The Cost of Crohn’s Disease: Varied Health Care Expenditure Patterns Across Distinct Disease Trajectories. Inflamm. Bowel Dis. 2017, 23, 107–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, R.D.; Larson, L.R.; Roth, J.M.; Becker, R.V.; Mummert, L.L. The cost of hospitalization in Crohn’s disease. Am. J. Gastroenterol. 2000, 95, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. Endoscopic management of inflammatory bowel disease-associated complications. Curr. Opin. Gastroenterol. 2020, 36, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Katsinelos, P.; Mimidis, K.; Paroutoglou, G.; Christodoulou, K.; Pilpilidis, I.; Katsiba, D.; Kalomenopoulou, M.; Papagiannis, A.; Tsolkas, P.; Kapitsinis, I.; et al. Needle-knife papillotomy: A safe and effective technique in experienced hands. Hepatogastroenterology 2004, 51, 349–352. [Google Scholar] [PubMed]
- Lan, N.; Hull, T.L.; Shen, B. Endoscopic stricturotomy and ileo-colonic resection in patients with primary Crohn’s disease-related distal ileum strictures. Gastroenterol. Rep. 2020, 8, 312–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, N.; Stocchi, L.; Delaney, C.P.; Hull, T.L.; Shen, B. Endoscopic stricturotomy versus ileocolonic resection in the treatment of ileocolonic anastomotic strictures in Crohn’s disease. Gastrointest. Endosc. 2019, 90, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Shen, B. Endoscopic Stricturotomy Versus Balloon Dilation in the Treatment of Anastomotic Strictures in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Lan, N.; Wu, X.R.; Shen, B. Endoscopic stricturotomy in the treatment of anastomotic strictures in inflammatory bowel disease (IBD) and non-IBD patients. Gastroenterol. Rep. 2020, 8, 143–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, N.; Shen, B. Endoscopic Stricturotomy with Needle Knife in the Treatment of Strictures from Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Crespi, M.; Dulbecco, P.; De Ceglie, A.; Conio, M. Strictures in Crohn’s Disease: From Pathophysiology to Treatment. Dig. Dis. Sci. 2020, 65, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Schulberg, J.D.; Wright, E.K.; Holt, B.A.; Wilding, H.E.; Hamilton, A.L.; Ross, A.L.; Kamm, M.A. Efficacy of drug and endoscopic treatment of Crohn’s disease strictures: A systematic review. J. Gastroenterol. Hepatol. 2021, 36, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Lim, F.; Faye, A.S.; Shen, B.; Hur, C. Endoscopic Balloon Dilation Is Cost-Effective for Crohn’s Disease Strictures. Dig. Dis. Sci. 2022, 67, 5462–5471. [Google Scholar] [CrossRef]
- Lan, N.; Wu, J.J.; Wu, X.R.; Hull, T.L.; Shen, B. Endoscopic treatment of pouch inlet and afferent limb strictures: Stricturotomy vs. balloon dilation. Surg. Endosc. 2021, 35, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Moran, G.W.; Benchimol, E.I.; Targownik, L.E.; Heitman, S.J.; Hubbard, J.N.; Seow, C.H.; Novak, K.L.; Ghosh, S.; Panaccione, R.; et al. Surgical Rates for Crohn’s Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study. Am. J. Gastroenterol. 2017, 112, 1840–1848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rungoe, C.; Langholz, E.; Andersson, M.; Basit, S.; Nielsen, N.M.; Wohlfahrt, J.; Jess, T. Changes in medical treatment and surgery rates in inflammatory bowel disease: A nationwide cohort study 1979–2011. Gut 2014, 63, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hebuterne, X.; Abitbol, V.; Nachury, M.; Brixi, H.; Bourreille, A.; Picon, L.; et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vuyyuru, S.K.; Kante, B.; Kumar, P.; Sahu, P.; Kedia, S.; Ranjan, M.K.; Sharma, R.; Panwar, R.; Makharia, G.; Ahuja, V. Real world analysis on the efficacy and safety of anti-tumor necrosis factor therapy in patients with stricturing Crohn’s disease. Sci. Rep. 2021, 11, 11740. [Google Scholar] [CrossRef] [PubMed]
- Arias, E. United States Life Tables, 2017. Natl. Vital Stat. Rep. 2019, 68, 1–66. [Google Scholar] [PubMed]
- U.S. Center for Medicare and Medicaid Services. Procedure Price Lookup. Available online: https://www.medicare.gov/procedure-price-lookup/ (accessed on 18 June 2022).
- U.S. Center for Medicare and Medicaid Services. Inpatient Charge Data FY 2018. Available online: https://data.cms.gov/provider-summary-by-type-of-service/medicare-inpatient-hospitals/medicare-inpatient-hospitals-by-provider-and-service (accessed on 18 June 2022).
- U.S. Bureau of Labor Statistics. May 2021 National Occupational Employment and Wage Estimates United States 2021 [Updated 31 March 2022]. Available online: https://www.bls.gov/oes/current/oes_nat.htm (accessed on 18 June 2022).
- Magnuson, E.A.; Chinnakondepalli, K.; Vilain, K.; Kearon, C.; Julian, J.A.; Kahn, S.R.; Goldhaber, S.Z.; Jaff, M.R.; Kindzelski, A.L.; Herman, K.; et al. Cost-Effectiveness of Pharmacomechanical Catheter-Directed Thrombolysis Versus Standard Anticoagulation in Patients with Proximal Deep Vein Thrombosis: Results from the ATTRACT Trial. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tillinger, W.; Mittermaier, C.; Lochs, H.; Moser, G. Health-related quality of life in patients with Crohn’s disease: Influence of surgical operation—A prospective trial. Dig. Dis. Sci. 1999, 44, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Liberato, N.L. Biological therapies in Crohn’s disease: Are they cost-effective? A critical appraisal of model-based analyses. Expert Rev. Pharmacoecon. Outcomes Res. 2014, 14, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Gregor, J.C.; McDonald, J.W.; Klar, N.; Wall, R.; Atkinson, K.; Lamba, B.; Feagan, B.G. An evaluation of utility measurement in Crohn’s disease. Inflamm. Bowel Dis. 1997, 3, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.J.; Corey, K.E.; Samur, S.; Choi, J.G.; Kaplan, L.M.; Chhatwal, J.; Hur, C. Cost-effectiveness Analysis of Bariatric Surgery for Patients With Nonalcoholic Steatohepatitis Cirrhosis. JAMA Netw. Open 2019, 2, e190047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omidvari, A.H.; Ali, A.; Hazelton, W.D.; Kroep, S.; Lee, M.; Naber, S.K.; Lauren, B.N.; Ostvar, S.; Richmond, E.; Kong, C.Y.; et al. Optimizing Management of Patients With Barrett’s Esophagus and Low-Grade or No Dysplasia Based on Comparative Modeling. Clin. Gastroenterol. Hepatol. 2020, 18, 1961–1969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campbell, J.; McGarry, L.A.; Shikora, S.A.; Hale, B.C.; Lee, J.T.; Weinstein, M.C. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am. J. Manag. Care. 2010, 16, e174–e187. [Google Scholar] [PubMed]
- U.S. Bureau of Labor Statistics. Consumer Price Index Inflation Calculator. Available online: https://www.bls.gov/data/inflation_calculator.htm (accessed on 18 June 2022).
- Gutierrez, A.; Rivero, M.; Martín-Arranz, M.D.; García Sánchez, V.; Castro, M.; Barrio, J.; de Francisco, R.; Barreiro-de Acosta, M.; Julia, B.; Cea-Calvo, L.; et al. Perioperative management and early complications after intestinal resection with ileocolonic anastomosis in Crohn’s disease: Analysis from the PRACTICROHN study. Gastroenterol. Rep. 2019, 7, 168–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pokala, A.; Shen, B. Update of endoscopic management of Crohn’s disease strictures. Intest. Res. 2020, 18, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, A.; Efron, D.T.; Stevens, K.; Manukyan, M.C.; Joseph, B.; Sakran, J.V. Hospital variation in mortality after emergent bowel resections: The role of failure-to-rescue. J. Trauma Acute Care Surg. 2018, 84, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Pillai, N.; Dusheiko, M.; Burnand, B.; Pittet, V. A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS ONE 2017, 12, e0185500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taleban, S.; Van Oijen, M.G.; Vasiliauskas, E.A.; Fleshner, P.R.; Shen, B.; Ippoliti, A.F.; Targan, S.R.; Melmed, G.Y. Colectomy with Permanent End Ileostomy Is More Cost-Effective than Ileal Pouch-Anal Anastomosis for Crohn’s Colitis. Dig. Dis. Sci. 2016, 61, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Nabi, Z.; Ramchandani, M.; Pooja, K.; Gupta, R.; Tandan, M.; Reddy, D.N. Endoscopic stricturotomy (standalone, hybrid and graded) for refractory inflammatory bowel disease strictures: Case series with technical review (with videos). Indian J. Gastroenterol. 2025, 44, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Nabi, Z.; Ramchandani, M.; Pooja, K.; Gupta, R.; Tandan, M.; Reddy, D.N. Endoscopic stricturotomy for inflammatory bowel disease strictures in anatomically challenging locations (deep small bowel, duodenum, anal canal and pouch): A case series with technical review (with videos). Indian J. Gastroenterol. 2025, 44, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Liu, Z.; Wang, B.; Ke, J.; Guo, Q. Surgery combined endoscopic stricturotomy for deep small bowel strictures from Crohn’ disease: A prospective, single-center cohort study of a novel approach. Surg. Endosc. 2025, 39, 4292–4299. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.B.; Yang, H.; Li, B.; Zhang, Y.; Huang, S.; Peng, B.; Lin, H.; Kurban, M.; Li, M.; Guo, Q. Balloon-assisted enteroscopy-based endoscopic stricturotomy for deep small bowel strictures from Crohn’s disease: First cohort study of a novel approach. Dig. Liver Dis. 2023, 55, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kurban, M.; Li, M.; Huang, Z.; Lin, H.; Hu, P.; Gao, X.; Shen, B.; Guo, Q. Device-assisted enteroscopy-based stricturotomy for small bowel strictures associated with Crohn’s disease (with video). Gastroenterol. Rep. 2022, 10, goac073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Parameter | Base Case | Sensitivity Ranges | Distribution | References | |
|---|---|---|---|---|---|
| Low | High | ||||
| Probabilities (%) | |||||
| Technical success of endoscopic stricturotomy | 0.95 | 0.80 | 1.00 | Beta | [14] |
| Complications from endoscopic stricturotomy | 0.06 | 0.00 | 0.20 | Beta | [14] |
| Complications from endoscopic stricturotomy, perforation | 0.083 | 0.00 | 1.00 | Beta | [14,15] |
| Bleeding from endoscopic stricturotomy leading to death | 0.000070 | 0.00 | 0.01 | Beta | [19] |
| Death from endoscopic stricturotomy procedure | 0.000029 | 0.00 | 0.01 | Beta | [19] |
| Surgery after endoscopic stricturotomy with no technical success | 0.70 | 0.00 | 1.00 | Beta | [19] |
| Complications from surgery | 0.21 | 0.088 | 0.43 | Beta | [19] |
| Factor by which complications from surgery increase due to salvage surgery | 1.38 | 1.18 | 1.59 | Normal | [19] |
| Death from surgery | 0.012 | 0.005 | 0.025 | Beta | [19] |
| Death from emergency surgery | 0.068 | 0.014 | 0.15 | Beta | [19] |
| Costs ($) | |||||
| Endoscopic stricturotomy | 1796.77 | 898.39 | 3593.54 | Gamma | [19,26] |
| Surgery | 23,684.73 | 11,842.37 | 47,369.46 | Gamma | [19,27] |
| Bleeding from endoscopic stricturotomy | 2338.52 | 1169.26 | 4677.04 | Gamma | [19] |
| Productivity cost of surgery | 1505.84 | 752.92 | 3011.68 | Gamma | [19,28] |
| Productivity cost of surgical complications | 1170.86 | 585.43 | 2341.72 | Gamma | [19,28] |
| Productivity cost of endoscopic stricturotomy appointment | 167.27 | 83.64 | 334.54 | Gamma | [19,28] |
| Utilities | |||||
| Remission from endoscopic stricturotomy | 0.86 | 0.84 | 0.88 | Beta | [19] |
| Remission from surgery | 0.89 | 0.88 | 0.91 | Beta | [19] |
| Severe Crohn’s | 0.62 | 0.50 | 0.73 | Beta | [19] |
| Disutilities | |||||
| Endoscopic stricturotomy, daily x 1 day | −0.30 | −0.35 | −0.26 | Beta | [19] |
| Surgery, daily, to be applied × 4 weeks | −0.22 | −0.25 | −0.19 | Beta | [19] |
| Bleeding from endoscopic stricturotomy | −0.10 | −0.12 | −0.085 | Beta | [19] |
| Complications from surgery | −0.24 | −0.27 | −0.20 | Beta | [19] |
| Cost ($) | Incremental Cost ($) | Effectiveness (QALY) | Incremental Effectiveness | ICER ($/QALY) | |
|---|---|---|---|---|---|
| Endoscopic Stricturotomy | 16,748 | Reference | 6.28 | Reference | Reference |
| Resection Surgery | 45,135 | 28,388 | 6.37 | 0.09 QALYs per person | 308,787.03 |
| ESt | Surgery | |
|---|---|---|
| Median ESts (±IQR) | 4 (±2) | 0 (±0) |
| Median surgeries (±IQR) | 0 (±1) | 2 (±1) |
| Maximum ESts (not capped) | 13 | 10 |
| Maximum surgeries (cap at 5) | 5 | 5 |
| Median ESt perforations with emergency surgery (±IQR) | 0 (±0) | 0 (±0) |
| Median failed ESts (±IQR) | 0 (±0) | 0 (±0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlin, K.L.; Kim, G.; Lim, F.; Faye, A.S.; Hur, C.; Shen, B. Cost-Effectiveness of Endoscopic Stricturotomy Versus Resection Surgery for Crohn’s Disease Strictures. Healthcare 2025, 13, 1801. https://doi.org/10.3390/healthcare13151801
Karlin KL, Kim G, Lim F, Faye AS, Hur C, Shen B. Cost-Effectiveness of Endoscopic Stricturotomy Versus Resection Surgery for Crohn’s Disease Strictures. Healthcare. 2025; 13(15):1801. https://doi.org/10.3390/healthcare13151801
Chicago/Turabian StyleKarlin, Kate Lee, Grace Kim, Francesca Lim, Adam S. Faye, Chin Hur, and Bo Shen. 2025. "Cost-Effectiveness of Endoscopic Stricturotomy Versus Resection Surgery for Crohn’s Disease Strictures" Healthcare 13, no. 15: 1801. https://doi.org/10.3390/healthcare13151801
APA StyleKarlin, K. L., Kim, G., Lim, F., Faye, A. S., Hur, C., & Shen, B. (2025). Cost-Effectiveness of Endoscopic Stricturotomy Versus Resection Surgery for Crohn’s Disease Strictures. Healthcare, 13(15), 1801. https://doi.org/10.3390/healthcare13151801






