Clinical Outcomes in Patients with Schizophrenia Treated with Long-Acting Injectable vs. Oral Antipsychotics: A Naturalistic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Assessment Tools
2.4. Statistical Analyses
3. Results
3.1. Demographics
3.2. Between-Group Comparisons of Clinical Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP(s) | Antipsychotic(s) |
| BMI | Body mass index |
| C-SSRS | Columbia Suicide Severity Rating Scale |
| DAI-10 | 10-item Drug Attitude Inventory |
| FDA | US Food and Drug Administration |
| FGA(s) | First-Generation Antipsychotic agent(s) |
| LAI | Long-Acting Injectable |
| QoL | Quality of Life |
| PANSS | Positive And Negative Syndrome Scale |
| SCZ | Schizophrenia |
| SGA(s) | Second-Generation Antipsychotic agent(s) |
References
- Harrison, P.J. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 1999, 122 Pt 4, 593–624. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr. Bull. 2013, 39, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Woerner, M.G.; Alvir, J.M.; Bilder, R.; Goldman, R.; Geisler, S.; Koreen, A.; Sheitman, B.; Chakos, M.; Mayerhoff, D.; et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch. Gen. Psychiatry 1999, 56, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.; Watt, D.; Falloon, I.; Smeeton, N. The natural history of schizophrenia: A five-year follow-up study of outcome and prediction in a representative sample of schizophrenics. Psychol. Med. Monogr. 1989, 15, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Rubio, J.M.; Kane, J.M. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry 2018, 17, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, H.; Curtin, S.C.; Warner, M. Increase in Suicide Mortality in the United States, 1999–2018; NCHS Data Brief; National Center for Health Statistics: Hyattsville, MD, USA, 2020; p. 362.
- Heilä, H.; Haukka, J.; Suvisaari, J.; Lönnqvist, J. Mortality among patients with schizophrenia and reduced psychiatric hospital care. Psychol. Med. 2005, 35, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Barnes, T.R.; Kissling, W.; Engel, R.R.; Correll, C.; Kane, J.M. Relapse prevention in schizophrenia with new-generation antipsychotics: A systematic review and exploratory meta-analysis of randomized, controlled trials. Am. J. Psychiatry 2003, 160, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Thoma, J.; Efthimiou, O.; Huhn, M.; Krause, M.; Reichelt, L.; Röder, H.; Davis, J.M.; Salanti, G.; Leucht, S. Second-generation antipsychotic drugs and short-term mortality: A systematic review and meta-analysis of placebo-controlled randomised controlled trials. Lancet Psychiatry. 2018, 5, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Arbter, D.; Engel, R.R.; Kissling, W.; Davis, J.M. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol. Psychiatry 2009, 14, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Taipale, H.; Tanskanen, A.; Mehtälä, J.; Vattulainen, P.; Correll, C.U.; Tiihonen, J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 2020, 19, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Tardy, M.; Komossa, K.; Heres, S.; Kissling, W.; Salanti, G.; Davis, J.M. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: A systematic review and meta-analysis. Lancet 2012, 379, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Haukka, J.; Taylor, M.; Haddad, P.M.; Patel, M.X.; Korhonen, P. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am. J. Psychiatry 2011, 168, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Hagi, K.; Kurokawa, S.; Kane, J.M.; Correll, C.U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: A systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry 2021, 8, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.; van Rooijen, G.; Doedens, P.; Numminen, E.; van Tricht, M.; de Haan, L. Antipsychotic medication and long-term mortality risk in patients with schizophrenia; a systematic review and meta-analysis. Psychol. Med. 2017, 47, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Torniainen, M.; Mittendorfer-Rutz, E.; Tanskanen, A.; Björkenstam, C.; Suvisaari, J.; Alexanderson, K.; Tiihonen, J. Antipsychotic treatment and mortality in schizophrenia. Schizophr. Bull. 2015, 41, 656–663, Erratum in Schizophr. Bull. 2016, 42, 528. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Tanskanen, A.; Taipale, H. 20-Year Nationwide Follow-Up Study on Discontinuation of Antipsychotic Treatment in First-Episode Schizophrenia. Am. J. Psychiatry 2018, 175, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Kishimoto, T.; Correll, C.U. Non-adherence to medication in patients with psychotic disorders: Epidemiology, contributing factors and management strategies. World Psychiatry 2013, 12, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Heres, S. Long-acting injectable antipsychotics: An underutilized treatment option. J. Clin. Psychiatry 2014, 75, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Fleischhacker, W.W.; Boter, H.; Davidson, M.; Vergouwe, Y.; Keet, I.P.; Gheorghe, M.D.; Rybakowski, J.K.; Galderisi, S.; Libiger, J.; et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: An open randomised clinical trial. Lancet 2008, 371, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, A.; Aadamsoo, K.; Altamura, A.C.; Franco, M.; Gorwood, P.; Neznanov, N.G.; Schronen, J.; Ucok, A.; Zink, M.; Janik, A.; et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr. Res. 2015, 169, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Subotnik, K.L.; Casaus, L.R.; Ventura, J.; Luo, J.S.; Hellemann, G.S.; Gretchen-Doorly, D.; Marder, S.; Nuechterlein, K.H. Long-Acting Injectable Risperidone for Relapse Prevention and Control of Breakthrough Symptoms After a Recent First Episode of Schizophrenia. A Randomized Clinical Trial. JAMA Psychiatry 2015, 72, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Malla, A.; Chue, P.; Jordan, G.; Stip, E.; Koczerginski, D.; Milliken, H.; Joseph, A.; Williams, R.; Adams, B.; Manchanda, R.; et al. An Exploratory, Open-Label, Randomized Trial Comparing Risperidone Long-Acting Injectable with Oral Antipsychotic Medication in the Treatment of Early Psychosis. Clin. Schizophr. Relat. Psychoses 2016, 9, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Medrano, S.; Abdel-Baki, A.; Stip, E.; Potvin, S. Three-Year Naturalistic Study On Early Use Of Long-Acting Injectable Antipsychotics In First Episode Psychosis. Psychopharmacol. Bull. 2018, 48, 25–61. [Google Scholar] [PubMed]
- Winter-van Rossum, I.; Weiser, M.; Galderisi, S.; Leucht, S.; Bitter, I.; Glenthøj, B.; Hasan, A.; Luykx, J.; Kupchik, M.; Psota, G.; et al. Efficacy of oral versus long-acting antipsychotic treatment in patients with early-phase schizophrenia in Europe and Israel: A large-scale, open-label, randomised trial (EULAST). Lancet Psychiatry 2023, 10, 197–208, Erratum in Lancet Psychiatry 2023, 10, e10. [Google Scholar] [CrossRef]
- Lian, L.; Kim, D.D.; Procyshyn, R.M.; Fredrikson, D.H.; Cázares, D.; Honer, W.G.; Barr, A.M. Efficacy of long-acting injectable versus oral antipsychotic drugs in early psychosis: A systematic review and meta-analysis. Early Interv. Psychiatry 2022, 16, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Kim, D.D.; Procyshyn, R.M.; Cázares, D.; Honer, W.G.; Barr, A.M. Long-acting injectable antipsychotics for early psychosis: A comprehensive systematic review. PLoS ONE 2022, 17, e0267808. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Schooler, N.R.; Marcy, P.; Correll, C.U.; Achtyes, E.D.; Gibbons, R.D.; Robinson, D.G. Effect of Long-Acting Injectable Antipsychotics vs. Usual Care on Time to First Hospitalization in Early-Phase Schizophrenia: A Randomized Clinical Trial. JAMA Psychiatry 2020, 77, 1217–1224, Erratum in JAMA Psychiatry 2020, 77, 1310. [Google Scholar] [CrossRef] [PubMed]
- Kotzalidis, G.D.; Rapinesi, C.; Chetoni, C.; De Filippis, S. Aripiprazole IM depot as an option for the treatment of bipolar disorder. Expert Opin. Pharmacother. 2021, 22, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.S.; Goa, K.L. Long-acting risperidone: A review of its use in schizophrenia. CNS Drugs 2004, 18, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Svensson, M.; Shao, H.; Vouri, S.M.; Park, H. Cost-effectiveness analysis of monthly, 3-monthly, and 6-monthly long-acting injectable and oral paliperidone in adults with schizophrenia. J. Manag. Care Spec. Pharm. 2023, 29, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Seebaluck, J.; Downes, M.A.; Brown, J.; Harris, K.; Isoardi, K.Z.; Chan, B.S. Case series profile of olanzapine post-injection delirium/sedation syndrome. Br. J. Clin. Pharmacol. 2023, 89, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, J.-P. Long-acting injectable antipsychotics: Focus on olanzapine pamoate. Neuropsychiatr. Dis. Treat. 2010, 6, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Detke, H.C.; Weiden, P.J.; Llorca, P.M.; Choukour, M.; Watson, S.B.; Brunner, E.; Ascher-Svanum, H. Comparison of olanzapine long-acting injection and oral olanzapine: A 2-year, randomized, open-label study in outpatients with schizophrenia. J. Clin. Psychopharmacol. 2014, 34, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. FDA Investigates Deaths Related to Use of Olanzapine Pamoate. JAMA 2013, 310, 361. [Google Scholar] [CrossRef]
- Rapinesi, C.; Kotzalidis, G.D.; Mazzarini, L.; Brugnoli, R.; Ferracuti, S.; De Filippis, S.; Cuomo, I.; Giordano, G.; Del Casale, A.; Angeletti, G.; et al. Long-Acting Injectable (LAI) Aripiprazole Formulations in the Treatment of Schizophrenia and Bipolar Disorder: A Systematic Review. Clin. Drug Investig. 2019, 39, 713–735. [Google Scholar] [CrossRef]
- Faden, J.; Ramirez, C.; Martinez, V.; Citrome, L. An overview of the currently available and emerging long-acting formulations of risperidone for schizophrenia and bipolar disorder. Expert Rev. Neurother. 2024, 24, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Harary, E.; Eshet, R.; Tohami, O.; Weiser, M.; Leucht, S.; Merenlender-Wagner, A.; Sharon, N.; Davis, G.L., III; Suett, M.; et al. Efficacy and safety of TV-46000, a long-acting, subcutaneous, injectable formulation of risperidone, for schizophrenia: A randomised clinical trial in the USA and Bulgaria. Lancet Psychiatry 2023, 10, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Poloni, N.; Ielmini, M.; Caselli, I.; Lucca, G.; Gasparini, A.; Lorenzoli, G.; Callegari, C. Oral Antipsychotic Versus Long-Acting Injections Antipsychotic in Schizophrenia Spectrum Disorder: A Mirror Analysis in a Real-World Clinical Setting. Psychopharmacol. Bull. 2019, 49, 17–27. [Google Scholar] [PubMed]
- Kaur, R.; Sidana, A.; Malhotra, N.; Tyagi, S. Oral versus long-acting injectable antipsychotic in first episode schizophrenia: A 12 weeks interventional study. Indian J. Psychiatry 2023, 65, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Schneider-Thoma, J.; Siafis, S.; Qin, M.; Wu, H.; Zhu, Y.; Davis, J.M.; Priller, J.; Leucht, S. Efficacy, acceptability and side-effects of oral versus long-acting- injectables antipsychotics: Systematic review and network meta-analysis. Eur. Neuropsychopharmacol. 2024, 83, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Schneider-Thoma, J.; Siafis, S.; Burschinski, A.; Dong, S.; Wu, H.; Zhu, Y.; Davis, J.M.; Priller, J.; Leucht, S. Long-Acting Injectable Second-Generation Antipsychotics vs Placebo and Their Oral Formulations in Acute Schizophrenia: A Systematic Review and Meta-Analysis of Randomized-Controlled-Trials. Schizophr. Bull. 2024, 50, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Choi, M.Y.; Choi, J.; Park, E.; Tchoe, H.J.; Suh, J.K.; Kim, Y.H.; Won, S.H.; Chung, Y.-C.; Bae, K.-Y.; et al. Comparative Efficacy and Safety of Long-acting Injectable and Oral Second-generation Antipsychotics for the Treatment of Schizophrenia: A Systematic Review and Meta-analysis. Clin. Psychopharmacol. Neurosci. 2018, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, C.; Salazar de Pablo, G.; Pacho, M.; Pérez-Rodríguez, V.; Bilbao, A.; Andrés, L.; Pedruzo, B.; Castillo-Sintes, I.; Aranguren, N.; Fusar-Poli, P.; et al. All-cause mortality risk in long-acting injectable versus oral antipsychotics in schizophrenia: A systematic review and meta-analysis. Mol. Psychiatry 2025, 30, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, Q.; Li, A.-N.; Sun, J.; Wu, B.; Wang, L.; Zhang, H.; Zhang, R.; Li, K.; Xu, X.; et al. Efficacy and safety of aripiprazole once-monthly versus oral aripiprazole in Chinese patients with acute schizophrenia: A multicenter, randomized, double-blind, non-inferiority study. Psychopharmacology (Berlin) 2022, 239, 243–251. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Publishing: Washington, DC, USA, 2013; pp. 87–122. [Google Scholar]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. User’s Guide for the Structured Clinical Interview for DSM-5 Disorders, Research Version (SCID-5-RV); American Psychiatric Association: Arlington, VA, USA, 2015. [Google Scholar]
- First, M.B.; Williams, J.B.W.; Benjamin, L.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5 Personality Disorders SCID-5-PD; American Psychiatric Association: Arlington, VA, USA, 2016. [Google Scholar]
- Posner, K.; Brown, G.K.; Stanley, B.; Brent, D.A.; Yershova, K.V.; Oquendo, M.A.; Currier, G.W.; Melvin, G.A.; Greenhill, L.; Shen, S.; et al. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 2011, 168, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive And Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Siafis, S.; Brandt, L.; McCutcheon, R.A.; Gutwinski, S.; Schneider-Thoma, J.; Bighelli, I.; Kane, J.M.; Arango, C.; Kahn, R.S.; Fleischhacker, W.W.; et al. Relapse in clinically stable adult patients with schizophrenia or schizoaffective disorder: Evidence-based criteria derived by equipercentile linking and diagnostic test accuracy meta-analysis. Lancet Psychiatry 2024, 11, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Hogan, T.P.; Awad, A.G.; Eastwood, R. A self-report scale predictive of drug compliance in schizophrenics: Reliability and discriminative validity. Psychol. Med. 1983, 13, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Gipson, P.Y.; Agarwala, P.; Opperman, K.J.; Horwitz, A.; King, C.A. Columbia Suicide Severity Rating Scale. Pediatr. Emerg. Care 2015, 31, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Austria-Corrales, F.; Jiménez-Tapia, A.; Astudillo-García, C.I.; Arenas-Landgrave, P.; Xochihua-Tlecuitl, T.; Cruz-Cruz, C.; Rivera-Rivera, L.; Gómez-García, J.A.; Palacios-Hernández, B.; Pérez-Amezcua, B.; et al. The Columbia-suicide severity rating scale: Validity and psychometric properties of an online Spanish-language version in a Mexican population sample. Front. Public Health 2023, 11, 1157581. [Google Scholar] [CrossRef] [PubMed]
- Lançon, C.; Reine, G.; Llorca, P.M.; Auquier, P. Validity and reliability of the French-language version of the Positive and Negative Syndrome Scale (PANSS). Acta Psychiatr. Scand. 1999, 100, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, R.; Tarsitani, L.; Mandarelli, G.; Fini, C.; Pancheri, P. Schizofrenia: Il problema delle dimensioni psicopatologiche [Schizophrenia: The issue of psychopathological dimensions]. Giorn. Ital. Psicopatol. 2008, 14, 36–50. (In Italian) [Google Scholar]
- Nielsen, R.E.; Lindström, E.; Nielsen, J.; Levander, S. DAI-10 is as good as DAI-30 in schizophrenia. Eur. Neuropsychopharmacol. 2012, 22, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Arduini, L.; De Cataldo, S.; Stratta, P. Gli aspetti soggettivi del trattamento con farmaci antipsicotici: Studio di validazione della versione italiana della Drug Attitude Inventory (DAI) [Subjective response to neuroleptic medication: A validation study of the Italian version of the Drug Attitude Inventory (DAI)]. Epidemiol. Psichiatr. Soc. 2001, 10, 107–114. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- JASP Team. JASP (Version 0.19.3) [Computer Software]; JASP Team: Amsterdam, NL, USA, 2024. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 14 March 2025).
- Kishimoto, T.; Robenzadeh, A.; Leucht, C.; Leucht, S.; Watanabe, K.; Mimura, M.; Borenstein, M.; Kane, J.M.; Correll, C.U. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: A meta-analysis of randomized trials. Schizophr. Bull. 2014, 40, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Kishimoto, T.; Correll, C.U. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J. Clin. Epidemiol. 2013, 66 (Suppl. S8), S37–S41. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.C.; Zummo, J.; Pettit, A.R.; Stoddard, J.; Doshi, J.A. Antipsychotic Adherence and Rehospitalization in Schizophrenia Patients Receiving Oral Versus Long-Acting Injectable Antipsychotics Following Hospital Discharge. J. Manag. Care Spec. Pharm. 2015, 21, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Citrome, L.; Haddad, P.M.; Lauriello, J.; Olfson, M.; Calloway, S.M.; Kane, J.M. The Use of Long-Acting Injectable Antipsychotics in Schizophrenia: Evaluating the Evidence. J. Clin. Psychiatry 2016, 77 (Suppl. S3), 21984. [Google Scholar] [CrossRef] [PubMed]
- Moccia, L.; Bardi, F.; Anesini, M.B.; Barbonetti, S.; Kotzalidis, G.D.; Rossi, S.; Caso, R.; Grisoni, F.; Mandracchia, G.; Margoni, S.; et al. Pharmacological Interventions for Negative Symptoms in Schizophrenia: A Systematic Review of Randomised Control Trials. Biomedicines 2025, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Fleischhacker, W.W. The Use of Long-Acting Injectable Antipsychotics in Schizophrenia. Curr. Treat. Options Psychiatry 2017, 4, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Mittendorfer-Rutz, E.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; Tanskanen, A.; et al. Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29 823 Patients With Schizophrenia. JAMA Psychiatry 2017, 74, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Long-acting injectable antipsychotics: Shall the last be first? CNS Spectr. 2014, 19, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Emsley, R.; Chiliza, B.; Asmal, L.; Mashile, M.; Fusar-Poli, P. Long-Acting Injectable Antipsychotics in Early Psychosis: A Literature Review. Early Interv. Psychiatry 2013, 7, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Taipale, H.; Mittendorfer-Rutz, E.; Alexanderson, K.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res. 2018, 197, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Fang, S.C.; Shao, Y.J. Comparison of Long-Acting Injectable Antipsychotics With Oral Antipsychotics and Suicide and All-Cause Mortality in Patients With Newly Diagnosed Schizophrenia. JAMA Netw. Open 2021, 4, e218810, Erratum in JAMA Netw. Open 2022, 5, e2210829. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S. The environment and susceptibility to schizophrenia. Prog. Neurobiol. 2011, 93, 23–58. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-C.; Liao, D.-L.; Huang, C.-Y.; Hsu, C.-C.; Cheng, S.-L.; Shao, Y.-J. The effectiveness of long-acting injectable antipsychotics versus oral antipsychotics in the maintenance treatment of outpatients with chronic schizophrenia. Hum. Psychopharmacol. 2020, 35, e2729. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Orsolini, L.; Lamis, D.A.; Goldsmith, D.R.; Nardella, A.; Falcone, G.; Corigliano, V.; Luciano, M.; Fiorillo, A. Suicide Prevention in Schizophrenia: Do Long-Acting Injectable Antipsychotics (LAIs) have a Role? CNS Neurol. Disord. Drug Targets 2017, 16, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Pompili, M.; Amador, X.F.; Girardi, P.; Harkavy-Friedman, J.; Harrow, M.; Kaplan, K.; Krausz, M.; Lester, D.; Meltzer, H.Y.; Modestin, J.; et al. Suicide risk in schizophrenia: Learning from the past to change the future. Ann. Gen. Psychiatry 2007, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Pepe, M.; Montanari, S.; Spera, M.C.; Panaccione, I.; Simonetti, A.; Sani, G. Vortioxetine improves physical and cognitive symptoms in patients with post-COVID-19 major depressive episodes. Eur. Neuropsychopharmacol. 2023, 70, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Bardi, F.; Margoni, S.; Grisoni, F.; Mandracchia, G.; Mazza, M.; Moccia, L.; Kotzalidis, G.D.; Janiri, D.; Tosato, M.; et al. Affective temperament modulates the relationship between physical and psychiatric symptoms during long-COVID: Results from the Gemelli against COVID-19 post-acute care service. J. Affect. Disord. 2025, 383, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.; Chapman, S.C.E.; Parham, R.; Freemantle, N.; Forbes, A.; Cooper, V. Understanding Patients’ Adherence-Related Beliefs About Medicines Prescribed for Long-Term Conditions: A Meta-Analytic Review of the Necessity-Concerns Framework. PLoS ONE 2013, 8, e80633. [Google Scholar] [CrossRef] [PubMed]
- El Abdellati, K.; De Picker, L.; Morrens, M. Antipsychotic Treatment Failure: A Systematic Review on Risk Factors and Interventions for Treatment Adherence in Psychosis. Front. Neurosci. 2020, 14, 531763. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Thompson-Leduc, P.; Ghelerter, I.; Nguyen, H.; Lafeuille, M.H.; Benson, C.; Mavros, P.; Lefebvre, P. Real-World Evidence of the Clinical and Economic Impact of Long-Acting Injectable Versus Oral Antipsychotics Among Patients with Schizophrenia in the United States: A Systematic Review and Meta-Analysis. CNS Drugs 2021, 35, 469–481, Erratum in CNS Drugs 2021, 35, 923. [Google Scholar] [CrossRef] [PubMed]
- Titus-Lay, E.N.; Ansara, E.D.; Isaacs, A.N.; Ott, C.A. Evaluation of Adherence and Persistence with Oral Versus Long-Acting Injectable Antipsychotics in Patients with Early Psychosis. Ment. Health Clin. 2018, 8, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, M.; Rossler, W. Attitudes Towards Long-Acting Depot Antipsychotics: A Survey of Patients, Relatives and Psychiatrists. Psychiatry Res. 2010, 175, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Heres, S.; Reichhart, T.; Hamann, J.; Mendel, R.; Leucht, S.; Kissling, W. Psychiatrists’ Attitude to Antipsychotic Depot Treatment in Patients with First-Episode Schizophrenia. Eur. Psychiatry 2011, 26, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Parellada, E.; Bioque, M. Barriers to the Use of Long-Acting Injectable Antipsychotics in the Management of Schizophrenia. CNS Drugs 2016, 30, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, S.-H.; Yang, Y.K.; Park, J.-I.; Chung, Y.-C. Long-Acting Injectable Antipsychotics for First-Episode Schizophrenia: The Pros and Cons. Schizophr. Res. Treat. 2012, 2012, 560836. [Google Scholar] [CrossRef] [PubMed]
- Llorca, P.M.; Abbar, M.; Courtet, P.; Guillaume, S.; Lancrenon, S.; Samalin, L. Guidelines for the Use and Management of Long-Acting Injectable Antipsychotics in Serious Mental Illness. BMC Psychiatry 2013, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Lennox, L.; Bell, D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013, 3, e001570. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.W.; Fulchino, L.; Rogers, J.; Mogun, H.; Polinski, J.; Henderson, D.C.; Schneeweiss, S.; Fischer, M.A. Impact of drug-reimbursement policies on prescribing: A case-study of a newly marketed long-acting injectable antipsychotic among relapsed schizophrenia patients. Pharmacoepidemiol. Drug Saf. 2018, 27, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A.; Gentile, A.; Stella, E.; Bellomo, A. Metabolic Issues in Patients Affected by Schizophrenia: Clinical Characteristics and Medical Management. Front. Neurosci. 2015, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, V.-A.; Pingali, S.M.; Chouinard, G.; Henderson, D.C.; Mallya, S.G.; Cypess, A.M.; Cohen, B.M.; Öngür, D. Factors Associated with Overweight and Obesity in Schizophrenia, Schizoaffective and Bipolar Disorders. Psychiatry Res. 2016, 237, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Gentile, S. Safety concerns associated with second-generation antipsychotic long-acting injection treatment. A systematic update. Horm. Mol. Biol. Clin. Investig. 2018, 36, 20170004. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martinez, V.; Romero-Rubio, D.; Abad-Perez, M.J.; Descalzo-Cabades, M.A.; Alonso-Gutierrez, S.; Salazar-Fraile, J.; Montagud, V.; Facila, L. Metabolic Syndrome and Cardiovascular Risk in People Treated with Long-Acting Injectable Antipsychotics. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A.; Baldessarini, R.J.; Vitrani, G.; Bonfitto, I.; Cecere, A.C.; Rinaldi, A.; Petito, A.; Bellomo, A. Metabolic Syndrome in Psychotic Disorder Patients Treated with Oral and Long-Acting Injected Antipsychotics. Front. Psychiatry 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Dayabandara, M.; Hanwella, R.; Ratnatunga, S.; Seneviratne, S.; Suraweera, C.; de Silva, V.A. Antipsychotic-Associated Weight Gain: Management Strategies and Impact on Treatment Adherence. Neuropsychiatr. Dis. Treat. 2017, 13, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Dekker, J.M.; Wood, D.; Kahl, K.G.; Holt, R.I.G.; Möller, H.-J. Cardiovascular Disease and Diabetes in People with Severe Mental Illness: Position Statement from the European Psychiatric Association (EPA), Supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur. Psychiatry 2009, 24, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Musil, R.; Obermeier, M.; Russ, P.; Hamerle, M. Weight Gain and Antipsychotics: A Drug Safety Review. Expert Opin. Drug Saf. 2015, 14, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative Effects of 18 Antipsychotics on Metabolic Function in Patients with Schizophrenia, Predictors of Metabolic Dysregulation, and Association with Psychopathology: A Systematic Review and Network Meta-Analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Fransen, A.; Janssen, J.; van Os, J.; Drukker, M. Almost All Antipsychotics Result in Weight Gain: A Meta-Analysis. PLoS ONE 2014, 9, e94112. [Google Scholar] [CrossRef] [PubMed]
- Ishigooka, J.; Nakamura, J.; Fujii, Y.; Iwata, N.; Kishimoto, T.; Iyo, M.; Uchimura, N.; Nishimura, R.; Shimizu, N.; ALPHA Study Group. Efficacy and Safety of Aripiprazole Once-Monthly in Asian Patients with Schizophrenia: A Multicenter, Randomized, Double-Blind, Non-Inferiority Study versus Oral Aripiprazole. Schizophr. Res. 2015, 161, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, W.W.; Sanchez, R.; Perry, P.P.; Jin, N.; Peters-Strickland, T.; Johnson, B.R.; Baker, R.A.; Eramo, A.; McQuade, R.D.; Carson, W.H.; et al. Aripiprazole Once-Monthly for Treatment of Schizophrenia: Double-Blind, Randomised, Non-Inferiority Study. Br. J. Psychiatry 2014, 205, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Shymko, G.; Dobson, L.; Acacio, M.C.; Grace, T.; Tadier, S.; Waters, F. Weight Changes in People with Early Psychosis Treated with Oral or Long-Acting Injectable Aripiprazole. Schizophr. Res. 2023, 251, 74–81. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Smith, E.C.C.; Navagnanavel, J.; Au, E.; Maksyutynska, K.; Papoulias, M.; Singh, R.; Panganiban, K.J.; Humber, B.; Mohr, G.H.; et al. The Impact of Weight Gain on Antipsychotic Nonadherence or Discontinuation: A Systematic Review and Meta-Analysis. Acta Psychiatr. Scand. 2025, 151, 109–126. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Kwan, A.T.H.; Rosenblat, J.D.; Teopiz, K.M.; Mansur, R.B. Psychotropic Drug-Related Weight Gain and Its Treatment. Am. J. Psychiatry 2024, 181, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Lijffijt, M.; Kahlon, R.S.; Gandy, K.; Arvind, R.P.; Amin, P.; Arciniegas, D.B.; Swann, A.C.; Soares, J.C.; Saxena, K. Early and late cortical reactivity to passively viewed emotional faces in pediatric bipolar disorder. J. Affect. Disord. 2019, 253, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Bernardi, E.; Kurian, S.; Restaino, A.; Calderoni, C.; De Chiara, E.; Bardi, F.; Sani, G.; Soares, J.C.; Saxena, K. Understanding Pediatric Bipolar Disorder Through the Investigation of Clinical, Neuroanatomic, Neurophysiological and Neurocognitive Dimensions: A Pilot Study. Brain Sci. 2025, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Odsbu, I.; Hamina, A.; Hjellvik, V.; Handal, M.; Haram, M.; Tesli, M.; Tanskanen, A.; Taipale, H. Initiation of Antipsychotics During the First Year After First-Episode Psychosis: A Population-Based Study. Acta Psychiatr Scand. 2025, 151, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.L.; Dawson, G.; Zummo, J. Clinical benefits and impact of early use of long-acting injectable antipsychotics for schizophrenia. Early Interv. Psychiatry 2016, 10, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Brugnoli, R.; Rapinesi, C.; Kotzalidis, G.D.; Marcellusi, A.; Mennini, F.S.; De Filippis, S.; Carrus, D.; Ballerini, A.; Francomano, A.; Ducci, G.; et al. Model of Management (Mo.Ma) for the patient with schizophrenia: Crisis control, maintenance, relapse prevention, and recovery with long-acting injectable antipsychotics (LAIs). Riv. Psichiatr. 2016, 51, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.J.; Henter, I.D. The effects of early and sustained intervention on the long-term morbidity of schizophrenia. J. Psychiatr. Res. 1998, 32, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Falkai, P.; Fleischhacker, W.W.; Girgis, R.R.; Kahn, R.M.; Uchida, H.; Zhao, J.; Lieberman, J.A. The Long-Term Effects of Antipsychotic Medication on Clinical Course in Schizophrenia. Am. J. Psychiatry 2017, 174, 840–849, Erratum in Am. J. Psychiatry 2017, 174, 805. [Google Scholar] [CrossRef] [PubMed]
- Arango, C.; Buitelaar, J.K.; Correll, C.U.; Díaz-Caneja, C.M.; Figueira, M.L.; Fleischhacker, W.W.; Marcotulli, D.; Parellada, M.; Vitiello, B. The transition from adolescence to adulthood in patients with schizophrenia: Challenges, opportunities and recommendations. Eur. Neuropsychopharmacol. 2022, 59, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.F.; Achtyes, E.D.; Correll, C.U.; Sajatovic, M.; Saklad, S.R. Optimizing treatment with aripiprazole monohydrate: Pharmacokinetic advantages of long-acting injectable formulations, a Consensus Panel Report. J. Clin. Psychiatry 2025, 86, plunlai2424ah1. [Google Scholar] [CrossRef] [PubMed]
- Trovini, G.; Lombardozzi, G.; Kotzalidis, G.D.; Lionetto, L.; Russo, F.; Sabatino, A.; Serra, E.; Castorina, S.; Civita, G.; Frezza, S.; et al. Optimising aripiprazole long-acting injectable: A comparative study of one- and two-injection start regimens in schizophrenia with and without substance use disorders and relationship to early serum levels. Int. J. Mol. Sci. 2025, 26, 1394. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Oral Antipsychotic (n = 35) | LAI (n = 25) | p-Value |
|---|---|---|---|
| Demographic characteristic | |||
| Age (y), mean ± SD | 36.86 ± 13.58 | 43.04 ± 12.60 | 0.075 |
| Age at onset (y), mean ± SD | 24.23 ± 10.42 | 22.84 ± 5.84 | 0.514 |
| Gender, No. (%) | |||
| Female | 17 (48.6%) | 12 (48.0%) | 0.965 |
| Male | 18 (51.4%) | 13 (52.0%) | |
| Education (y), mean ± SD | 11.51 ± 2.90 | 13.16 ± 3.97 | 0.085 |
| Marital status, No. (%) | |||
| Not married | 31 (87.5%) | 21 (84.0%) | 0.706 |
| Married | 4 (12.5%) | 4 (16.0%) | |
| Living status, No. (%) | |||
| Living alone | 1 (3.0%) | 3 (12.0%) | 0.182 |
| Living with someone | 34 (97.0%) | 22 (88.0%) | |
| Clinical characteristic | |||
| BMI, mean ± SD | 29.35 ± 5.45 | 26.78 ± 5.54 | 0.080 |
| Smoking, No. (%) | |||
| Yes | 21 (61.0%) | 10 (39%) | 0.239 |
| No | 14 (39.0%) | 15 (61.0%) | |
| Substance use, No. (%) | |||
| Yes | 16 (45.5%) | 14 (56.0%) | 0.426 |
| No | 19 (54.5%) | 11 (44.0%) | |
| Chlorpromazine equivalent (mg/d), mean ± SD | 307.14 ± 124.94 | 268.00 ± 96.70 | 0.177 |
| Lifetime suicidal ideation, No. (%) | |||
| Yes | 15 (42.9%) | 5 (20.0%) | 0.064 |
| No | 20 (57.1%) | 20 (80.0%) | |
| PANSS General, mean ± SD | 47.16 ± 15.85 | 49.96 ± 13.79 | 0.479 |
| PANSS Positive, mean ± SD | 19.03 ± 12.32 | 24.48 ± 10.02 | 0.064 |
| PANSS Negative, mean ± SD | 20.46 ± 10.70 | 15.64 ± 8.29 | 0.054 |
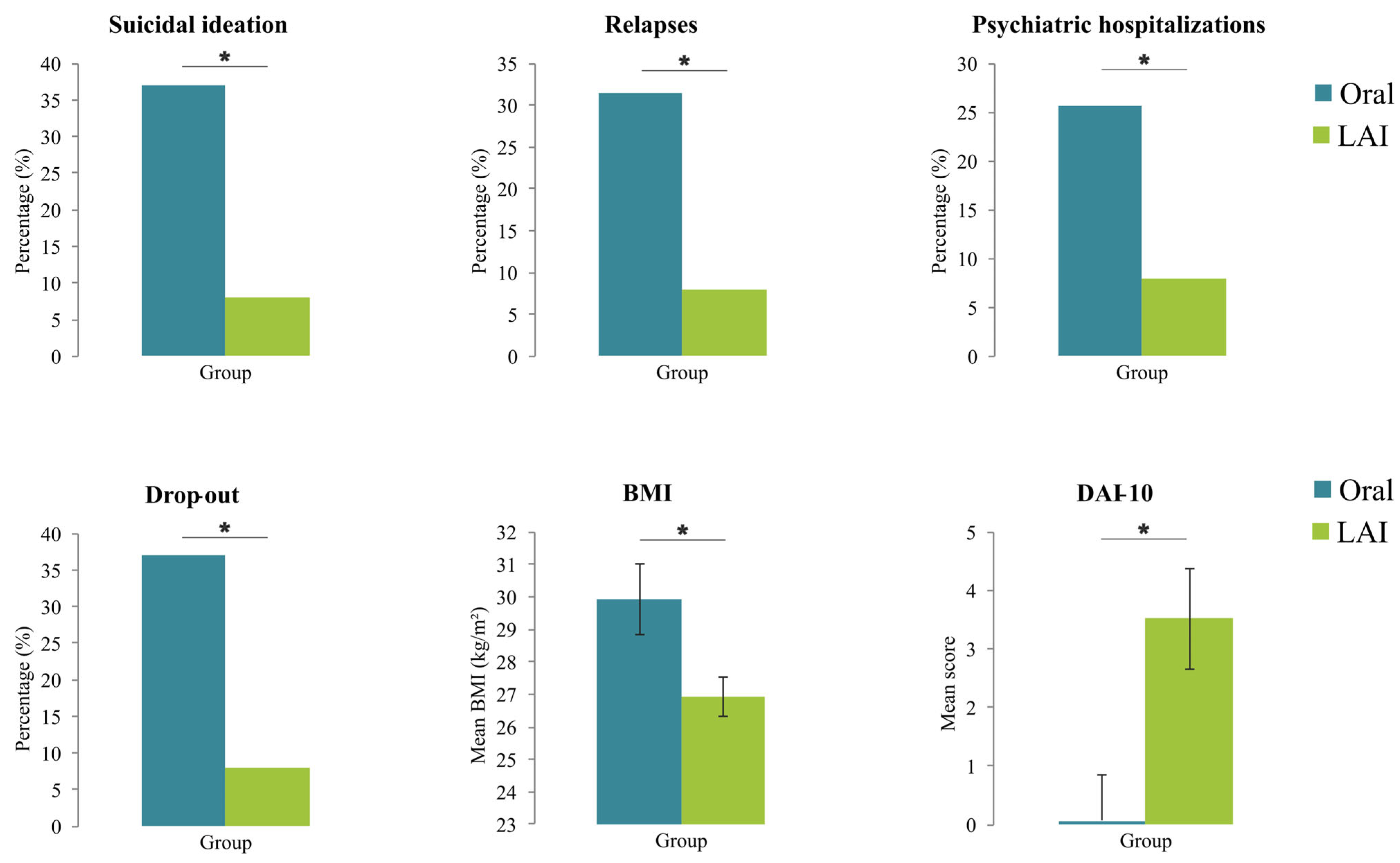
| Variables | Oral Antipsychotic (n = 35) | LAI (n = 25) | χ2 or F | p-Value | Cramér’s V or η2 |
|---|---|---|---|---|---|
| Suicidal ideation, No. (%) | 13 (37.1%) | 2 (8.0%) | 6.61 | 0.010 | 0.29 |
| Relapses, No. (%) | 11 (31.4%) | 2 (8.0%) | 4.72 | 0.030 | 0.24 |
| Psychiatric hospitalizations, No. (%) | 9 (25.7%) | 1 (4.0%) | 4.95 | 0.026 | 0.24 |
| Drop out, No. (%) | 13 (37.1%) | 2 (8.0%) | 6.61 | 0.010 | 0.29 |
| BMI, mean ± SD | 29.93 ± 6.38 | 26.92 ± 3.07 | 5.89 | 0.019 | 0.09 |
| DAI-10, mean ± SD | 0.06 ± 4.72 | 3.52 ± 4.28 | 8.76 | 0.005 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardi, F.; Moccia, L.; Kotzalidis, G.D.; Boggio, G.; Brugnami, A.; Sfratta, G.; Janiri, D.; Sani, G.; Simonetti, A. Clinical Outcomes in Patients with Schizophrenia Treated with Long-Acting Injectable vs. Oral Antipsychotics: A Naturalistic Study. Healthcare 2025, 13, 1709. https://doi.org/10.3390/healthcare13141709
Bardi F, Moccia L, Kotzalidis GD, Boggio G, Brugnami A, Sfratta G, Janiri D, Sani G, Simonetti A. Clinical Outcomes in Patients with Schizophrenia Treated with Long-Acting Injectable vs. Oral Antipsychotics: A Naturalistic Study. Healthcare. 2025; 13(14):1709. https://doi.org/10.3390/healthcare13141709
Chicago/Turabian StyleBardi, Francesca, Lorenzo Moccia, Georgios D. Kotzalidis, Gianluca Boggio, Andrea Brugnami, Greta Sfratta, Delfina Janiri, Gabriele Sani, and Alessio Simonetti. 2025. "Clinical Outcomes in Patients with Schizophrenia Treated with Long-Acting Injectable vs. Oral Antipsychotics: A Naturalistic Study" Healthcare 13, no. 14: 1709. https://doi.org/10.3390/healthcare13141709
APA StyleBardi, F., Moccia, L., Kotzalidis, G. D., Boggio, G., Brugnami, A., Sfratta, G., Janiri, D., Sani, G., & Simonetti, A. (2025). Clinical Outcomes in Patients with Schizophrenia Treated with Long-Acting Injectable vs. Oral Antipsychotics: A Naturalistic Study. Healthcare, 13(14), 1709. https://doi.org/10.3390/healthcare13141709









