The Role of Fixed-Dose Desmopressin in Hemostatic Outcomes of Native and Transplant Kidney Biopsies in a Tertiary Referral Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Management and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hogan, J.J.; Mocanu, M.; Berns, J.S. The native kidney biopsy: Update and evidence for best practice. Clin. J. Am. Soc. Nephrol. 2016, 11, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Schnuelle, P. Renal Biopsy for Diagnosis in Kidney Disease: Indication, Technique, and Safety. J. Clin. Med. 2023, 12, 6424. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.; Brun, C. Aspiration biopsy of the kidney. Am. J. Med. 1951, 11, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Corapi, K.M.; Chen, J.L.T.; Balk, E.M.; Gordon, C.E. Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am. J. Kidney Dis. 2012, 60, 62–73. [Google Scholar] [CrossRef]
- Manno, C.; Strippoli, G.F.M.; Arnesano, L.; Bonifati, C.; Campobasso, N.; Gesualdo, L.; Schena, F.P. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int. 2004, 66, 1570–1577. [Google Scholar] [CrossRef]
- Palsson, R.; Short, S.A.P.; Kibbelaar, Z.A.; Amodu, A.; Stillman, I.E.; Rennke, H.G.; McMahon, G.M.; Waikar, S.S. Bleeding complications after percutaneous native kidney biopsy: Results from the Boston kidney biopsy cohort. Kidney Int. Rep. 2020, 5, 511–518. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Halimi, J.M.; de Fréminville, J.B.; Gatault, P.; Gueguen, J.; Goin, N.; Longuet, H.; Barbet, C.; Bisson, A.; Sautenet, B.; et al. A Universal Bleeding Risk Score in Native and Allograft Kidney Biopsies: A French Nationwide Cohort Study. J. Clin. Med. 2023, 12, 3527. [Google Scholar] [CrossRef]
- Fontana, C.; Diebold, M.; Amico, P.; Hirt-Minkowski, P.; Wehmeier, C.; Hopfer, H.; Menter, T.; Schaub, S.; Steiger, J.; Dickenmann, M. Bleeding risk after native and transplant kidney biopsy—A single-centre observational study. Swiss Med. Wkly. 2025, 155, 4409. [Google Scholar] [CrossRef]
- Thachil, J. Abnormal coagulation tests before kidney biopsies—What next? Clin. Kidney J. 2013, 6, 50–54. [Google Scholar] [CrossRef][Green Version]
- Lim, C.C.; Tan, H.Z.; Tan, C.S.; Healy, H.; Choo, J.; Franca Gois, P.H. Desmopressin acetate to prevent bleeding in percutaneous kidney biopsy: A systematic review. Intern. Med. J. 2021, 51, 571–579. [Google Scholar] [CrossRef]
- Franchini, M. The use of desmopressin as a hemostatic agent: A concise review. Am. J. Hematol. 2007, 82, 731–735. [Google Scholar] [CrossRef]
- Peters, B.; Nasic, S.; Jensen, G.; Stegmayr, B. Renal transplant biopsy complications: Assessment of risk factors and potential of desmopressin to decrease risk of hemorrhage. Acta Radiol. 2020, 61, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.A.; Shahoori, A.; Shahbazian, H.; Sabetnia, L.; Aref, A.; Sattari, A.R.; Ghorbani, A. Desmopressin acetate in percutaneous ultrasound-guided native kidney biopsy in patients with reduced kidney function: A double-blind randomized controlled trial. Iran. J. Kidney Dis. 2022, 16, 238–245. [Google Scholar] [PubMed]
- Athavale, A.; Kulkarni, H.; Arslan, C.D.; Hart, P. Desmopressin and bleeding risk after percutaneous kidney biopsy. BMC Nephrol. 2019, 20, 413. [Google Scholar] [CrossRef]
- Chaturvedy, M.; Jhorawat, R.; Chakrabarti, U.; Bajpai, N.K.; Nalwa, A.; Khera, P. #1289 The effect of desmopressin in reducing post-kidney biopsy bleeding complication in patients with reduced renal function: A randomised control trial. Nephrol. Dial. Transplant. 2024, 39, gfae069-0448-1289. [Google Scholar]
- Fralick, M.; Schneeweiss, S.; Wallis, C.J.D.; Jung, E.H.; Kesselheim, A.S.; Basu, S. Desmopressin and the risk of hyponatremia: A population-based cohort study. PLoS Med. 2019, 16, e1002930. [Google Scholar] [CrossRef]
- Achinger, S.G.; Ayus, J.C. Use of desmopressin in hyponatremia: Foe and friend. Kidney Med. 2019, 1, 65–70. [Google Scholar] [CrossRef]
- Jose, L.; Kaul, A.; Bhadauria, D.; Kushwaha, R.; Nandan, R.; Lal, H.; Prasad, N.; Behera, M.R.; Patel, M.R. Desmopressin Acetate Before Percutaneous Ultrasound-Guided Kidney Biopsy in Patients with Renal Failure—Is it Really Beneficial? Indian J. Nephrol. 2022, 32, 430–434. [Google Scholar] [PubMed]
- Wademan, B.H.; Galvin, S.D. Desmopressin for reducing postoperative blood loss and transfusion requirements following cardiac surgery in adults. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 360–370. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, C.H.; Min, J.Y.; Kim, J.S.; Kim, S.B.; Kim, H. Desmopressin improves platelet function in uremic patients taking antiplatelet agents who require emergent invasive procedures. Ann. Hematol. 2015, 94, 1457–1461. [Google Scholar] [CrossRef]
- Vornicu, A.; Obrişcă, B.; Cotruta, B.; Dulămea, A.O.; Caceaune, N.; Ismail, G. Case Report: Hyponatremia Secondary to Desmopressin Administration Prior to Percutaneous Kidney Biopsy: A Case-Based Review. Front. Med. 2021, 8, 696904. [Google Scholar] [CrossRef] [PubMed]
- Leissinger, C.; Carcao, M.; Gill, J.C.; Journeycake, J.; Singleton, T.; Valentino, L. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014, 20, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Anpalahan, A.; Malacova, E.; Hegerty, K.; Malett, A.; Ranganathan, D.; Healy, H.G.; Gois, P.H.F. Bleeding Complications of Percutaneous Kidney Biopsy: Does Gender Matter? Kidney 2021, 360, 1308–1312. [Google Scholar] [CrossRef]
- Leclerc, S.; Nadeau-Fredette, A.-C.; Elftouh, N.; Lafrance, J.-P.; Pichette, V.; Laurin, L.-P. Use of desmopressin prior to kidney biopsy in patients with high bleeding risk. Kidney Int. Rep. 2020, 5, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.Y.; Lim, C.C.; Thangaraju, S.; Siow, B.; Chin, Y.M.; Hao, Y.; Lee, P.H.; Foo, M.; Tan, C.S.; Kee, T. Bleeding complications and adverse events after desmopressin acetate for percutaneous renal transplant biopsy. Ann. Acad. Med. 2020, 49, 52–64. [Google Scholar] [CrossRef]
- Shidham, G.B.; Siddiqi, N.; A Beres, J.; Logan, B.; Nagaraja, H.; Shidham, S.G.; Piering, W.F. Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology 2005, 10, 305–310. [Google Scholar] [CrossRef]
- Eiro, M.; Katoh, T.; Watanabe, T. Risk factors for bleeding complications in percutaneous renal biopsy. Clin. Exp. Nephrol. 2005, 9, 40–45. [Google Scholar] [CrossRef]
- Manno, C.; Bonifati, C.; Torres, D.D.; Campobasso, N.; Schena, F.P. Desmopressin acetate in percutaneous ultrasound-guided kidney biopsy: A randomized controlled trial. Am. J. Kidney Dis. 2011, 57, 850–855. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Chanchlani, R.; Connolly, B.; Langlois, V. Pre-procedure desmopressin acetate to reduce bleeding in renal failure: Does it really work? Nephron Clin. Pract. 2014, 128, 45–48. [Google Scholar] [CrossRef]
- Lim, C.C.; Siow, B.; Choo, J.C.J.; Chawla, M.; Chin, Y.M.; Kee, T.; Lee, P.H.; Foo, M.; Tan, C.S. Desmopressin for the prevention of bleeding in percutaneous kidney biopsy: Efficacy and hyponatremia. Int. Urol. Nephrol. 2019, 51, 995–1004. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Federici, A.B. Management of inherited von Willebrand disease. Best Pract. Res. Clin. Haematol. 2001, 14, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.; Bansal, S.; Lal, A.; Kohli, H.S.; Rathi, M. Role of Desmopressin Acetate before Percutaneous Ultrasound-Guided Kidney Biopsy in Patients with Kidney Dysfunction. Indian J. Nephrol. 2024, 34, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Franquiz, M.J.; Hines, M.C.; Yeung, S.Y.A. Comparison of two weight-based desmopressin dosing strategies for spontaneous bleeding. Ann. Pharmacother. 2018, 52, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Furqan, F.; Sham, R.; Kouides, P. Efficacy and safety of half-dose desmopressin for bleeding prophylaxis in bleeding disorder patients undergoing predominantly low to moderate risk invasive procedures. Am. J. Hematol. 2020, 95, E285–E287. [Google Scholar] [CrossRef]
- Mohinani, A.; Patel, S.; Tan, V.; Kartika, T.; Olson, S.; DeLoughery, T.G.; Shatzel, J. Desmopressin as a hemostatic and blood sparing agent in bleeding disorders. Eur. J. Haematol. 2023, 110, 470–479. [Google Scholar] [CrossRef]
- Chakrabarti, U.; Jhorawat, R.; Bajpai, N.K.; Nalwa, A.; Das, T.; Khera, P.; Sharma, P.; Chaturvedy, M. Effect of Desmopressin on Post Kidney Biopsy Bleeding Complications in Patients with Reduced Kidney Function: A Randomized Controlled Trial. Kidney360 2025. [Google Scholar] [CrossRef]
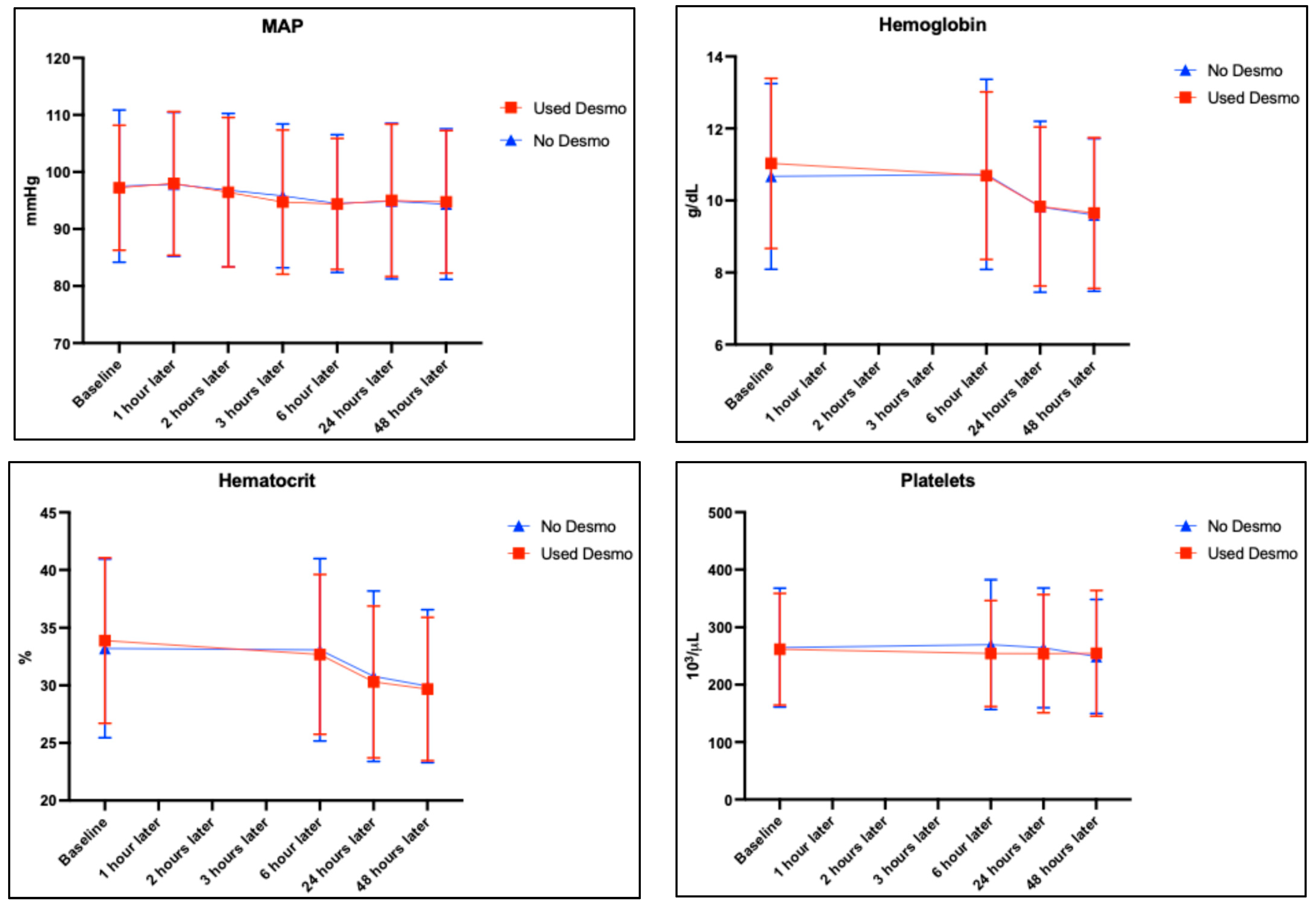
| Variable | Desmopressin Used (n = 210) | No Desmopressin (n = 200) | p-Value † |
|---|---|---|---|
| Gender, n (%) Male Female | 126 (60.0%) 84 (40.0%) | 127 (63.5%) 73 (36.5%) | 0.466 |
| Age (in years) Mean ± (SD) Median (range, IQR) | 42.3 ± (14.7) 40.0 (18–83, 24.8) | 42.9 ± (15.9) 42.0 (18–85, 26.0) | 0.685 |
| Age (in years), n (%) Young adults: 18–34 Middle-aged adults: 35–49 Older adults: 50–64 Elderly: ≥ 65 | 72 (34.3%) 64 (30.5%) 57 (27.1%) 17 (8.1%) | 75 (37.5%) 52 (26.0%) 54 (27.0%) 19 (9.5%) | 0.741 |
| BMI, n (%) <18.5 (underweight) 18.5–24.9 (healthy weight) 25–29.9 (overweight) ≥30 (obesity) | 6 (2.9%) 40 (19.0%) 66 (31.4%) 98 (46.7%) | 6 (3.0%) 51 (25.5%) 57 (28.5%) 86 (53.0%) | 0.470 |
| Comorbidities, n (%) ‡ None Hypertension CKD Diabetes mellitus Carcinomas Liver disease Others ¶ | 30 (14.3%) 114 (54.3%) 159 (75.7%) 70 (33.3%) 8 (3.8%) 8 (3.8%) 51 (24.3%) | 31 (15.5%) 110 (55.0%) 146 (73.0%) 65 (32.5%) 8 (4.0%) 6 (3.0%) 40 (20%) | 0.730 0.885 0.529 0.858 0.921 0.652 - |
| CCI scores, n (%) Mild (1–2) Moderate (3–4) Severe (≥5) | 127 (60.5%) 63 (30.0%) 20 (9.5%) | 131 (65.5%) 56 (28.0%) 13 (6.5%) | 0.424 |
| Medication history, n (%) ‡ None Antiplatelets Anticoagulants Antiplatelets and anticoagulants Steroids | 86 (41.0%) 20 (9.5%) 59 (28.1%) 2 (1.0%) 81 (38.6%) | 77 (38.5%) 24 (12.0%) 61 (30.5%) 5 (2.5%) 88 (44.0%) | 0.612 0.418 0.593 0.227 0.264 |
| Antithrombotic therapy continued through biopsy, n (%) # Antiplatelets Anticoagulants | 9 (45.9%) 38 (64.4%) | 10 (41.7%) 40 (65.6%) | 0.811 0.824 |
| Type of procedure, n (%) Naive kidney biopsy Transplant kidney biopsy | 157 (74.8%) 53 (25.2%) | 117 (58.5%) 83 (41.5%) | <0.001 |
| Preprocedural MAP (in mmHg) Mean ± (SD) Median (range, IQR) | 97.3 ± (11.0) 97.7 (61–122, 15.2) | 97.5 ± (13.4) 97.8 (62–137, 18.5) | 0.821 |
| Preprocedural HTN, n (%) No Yes | 119 (56.7%) 91 (43.3%) | 109 (54.5%) 91 (45.5%) | 0.659 |
| Pre-BUN (mg/dL) Mean ± (SD) Median (range, IQR) | 16.2 ± (10.2) 14.2 (2.0–68, 12.8) | 15.0 ± (9.9) 12.0 (2.5–54, 13.6) | 0.239 |
| Pre-ALT (U/L) Mean ± (SD) Median (range, IQR) | 18.1 ± (10.0) 15.0 (5.0–47, 15.0) | 22.0 ± (17.1) 16.5 (5–125, 15.8) | 0.055 |
| Pre-AST (U/L) Mean ± (SD) Median (range, IQR) | 19.3 ± (9.2) 17 (5.0–54, 8.0) | 21.2 ± (13.6) 17.0 (6.0–93, 9.8) | 0.241 |
| Pre-sodium (U/L) Mean ± (SD) Median (range, IQR) | 135.7 ± (4.1) 137 (121–146, 6.0) | 135.1 ± (4.4) 136 (122–148, 6.0) | 0.121 |
| eGFR, n (%) Normal (≥60 mL/min) Reduced (<60 mL/min) Missing data (n = 1 + 2) | 20 (9.6%) 189 (90.4%) | 23 (11.6%) 175 (88.4%) | 0.502 |
| CKD stages, n (%) § Stage 1/2 Stage 3a Stage 3b Stage 4 Stage 5 Missing data (n = 1 + 2) ¶ | 20 (9.6%) 29 (13.9%) 46 (22.0%) 45 (21.5%) 69 (33.0%) - | 24 (12.1%) 31 (15.7%) 45 (22.7%) 56 (28.3%) 42 (21.2%) - | 0.095 |
| Undergoing dialysis, n (%) No Yes | 143 (68.1%) 67 (31.9%) | 139 (69.5%) 61 (30.5%) | 0.759 |
| AKI stages, n (%) None Stage 1 Stage 2 Stage 3 | 75 (35.7%) 23 (11.0%) 15 (7.1%) 97 (46.2%) | 53 (26.5%) 29 (14.5%) 18 (9.0%) 100 (50.0%) | 0.208 |
| Blood parameters, Ψ n (%) Low hemoglobin Low hematocrit Low platelet count Missing data (n = 2 + 2) ¶ | 139 (66.5%) 155 (74.2%) 13 (6.3%) - | 146 (73.7%) 153 (77.3%) 15 (7.6%) - | 0.112 0.465 0.598 - |
| Minor bleeding, n (%) No Yes | 180 (85.7%) 30 (14.3%) | 177 (88.5%) 23 (11.5%) | 0.401 |
| Major bleeding, n (%) No Yes | 200 (95.2%) 10 (4.8%) | 180 (90.0%) 20 (10.0%) | 0.042 |
| Composite bleeding, Φ n (%) No Yes | 174 (82.9%) 36 (17.1%) | 167 (83.5%) 33 (16.5%) | 0.862 |
| Need for blood transfusion, n (%) No Yes | 200 (95.2%) 10 (4.8%) | 181 (90.5%) 19 (9.5%) | 0.061 |
| Length of hospital stay (in days) Mean ± (SD) Median (range, IQR) | 10.7 ± (12.6) 7 (1–99, 11.0) | 18.4 ± (40.3) 10 (1–371, 15.0) | 0.009 |
| Length of hospital stay (in days) <3 3–10 >10 Missing data (n = 1) ¶ | 45 (21.4%) 92 (43.8%) 73 (34.8%) | 41 (20.6%) 58 (29.1%) 100 (50.3%) | 0.003 |
| Variable | All (n = 210) | High Risk † (n = 187) | Low Risk (n = 23) | p-Value ‡ |
|---|---|---|---|---|
| Gender, n (%) Male Female | 126 (60.0%) 84 (40.0%) | 113 (60.4%) 74 (39.6%) | 13 (56.5%) 10 (43.5%) | 0.718 |
| Age (in years) Mean ± (SD) Median (range, IQR) | 42.3 ± (14.7) 40.0 (18–83, 24.8) | 43.2 ± (14.9) 43.0 (18–83, 23.0) | 34.6 ± (10.3) 36.0 (19–58, 13.5) | 0.008 |
| Type of procedure, n (%) Naive kidney biopsy Transplant kidney biopsy | 157 (74.8%) 53 (25.2%) | 137 (73.3%) 50 (26.7%) | 20 (87.0%) 3 (13.0%) | 0.241 |
| Time of administration, n (%) Pre-procedure Post-procedure Missing data (n = 4) | 197 (95.6%) 9 (4.4%) | 178 (96.7%) 6 (3.3%) | 19 (86.4%) 3 (13.6%) | 0.089 |
| Dose received (in mcg/kg) Mean ± (SD) Median (range, IQR) | 0.26 ± (0.07) 0.25 (0.14–0.53, 0.09) | 0.26 ± (0.07) 0.25 (0.14–0.53, 0.09) | 0.26 ± (0.07) 0.24 (0.16–0.42, 0.12) | 0.573 |
| Dosage groups, n (%) Adequate (0.3–0.4 mcg/kg) Underdosed (<0.3 mcg/kg) Overdosed (>0.4 mcg/kg) | 50 (23.8%) 153 (72.9%) 7 (3.3%) | 44 (23.5%) 137 (73.3%) 6 (3.2%) | 6 (26.1%) 16 (69.6%) 1 (4.3%) | 0.916 |
| Composite bleeding Φ n (%) No Yes | 174 (82.9%) 36 (17.1%) | 156 (83.4%) 31 (16.6%) | 18 (78.3%) 5 (21.7%) | 0.744 |
| Variable | Bleeding Events † | Hypotension | Hyponatremia | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Desmopressin used No Yes | 1.06 (0.62 to 1.82) | 0.826 | 0.83 (0.42 to 1.61) | 0.574 | 0.99 (0.62 to 1.57) | 0.952 |
| Gender Male Female | 0.87 (0.51 to 1.51) | 0.624 | 0.61 (0.32 to 1.19) | 0.147 | 1.32 (0.82 to 2.16) | 0.259 |
| Age group Less than 50 years 50 years and older | 1.44 (0.80 to 2.67) | 0.229 | 0.94 (0.46 to 1.97) | 0.877 | 0.79 (0.48 to 1.31) | 0.362 |
| BMI <25 under and healthy ≥25 over and obese | 0.99 (0.53 to 1.80) | 0.981 | 1.38 (0.64 to 2.81) | 0.388 | 0.96 (0.56 to 1.61) | 0.876 |
| Comorbidity None Yes | 1.10 (0.50 to 2.33) | 0.801 | 1.41 (0.50 to 3.62) | 0.493 | 0.26 (0.08 to 0.70) | 0.015 |
| CCI scores Mild (1–2) Moderate/severe (≥3) | 0.92 (0.50 to 1.73) | 0.800 | 0.74 (0.34 to 1.61) | 0.454 | 0.56 (0.34 to 0.93) | 0.025 |
| Antithrombotic drugs No Yes | 1.09 (0.61 to 1.96) | 0.776 | 0.84 (0.42 to 1.73) | 0.635 | 0.83 (0.51 to 1.37) | 0.468 |
| Type of biopsy Naive kidney Transplant kidney | 1.75 (0.94 to 3.39) | 0.085 | 1.27 (0.60 to 2.85) | 0.551 | 0.57 (0.35 to 0.94) | 0.026 |
| Bleeding events No Yes | — | — | 1.16 (0.47 to 2.55) | 0.731 | 1.39 (0.75 to 2.52) | 0.283 |
| Hypotension events No Yes | 0.86 (0.39 to 2.10) | 0.719 | — | — | 0.76 (0.36 to 1.63) | 0.460 |
| Hyponatremia events No Yes | 0.71 (0.39 to 1.31) | 0.264 | 0.75 (0.36 to 1.60) | 0.440 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bifari, N.; Alatawi, Y.; Abdel-Razaq, W.S.; Shawaqfeh, M.S.; Albekairy, A.M.; Hejaili, F.; Shattat, G.F.; Alkathiri, M.; Alrajhi, Y.A.; Al Sulaiman, K.A.; et al. The Role of Fixed-Dose Desmopressin in Hemostatic Outcomes of Native and Transplant Kidney Biopsies in a Tertiary Referral Hospital. Healthcare 2025, 13, 1553. https://doi.org/10.3390/healthcare13131553
Bifari N, Alatawi Y, Abdel-Razaq WS, Shawaqfeh MS, Albekairy AM, Hejaili F, Shattat GF, Alkathiri M, Alrajhi YA, Al Sulaiman KA, et al. The Role of Fixed-Dose Desmopressin in Hemostatic Outcomes of Native and Transplant Kidney Biopsies in a Tertiary Referral Hospital. Healthcare. 2025; 13(13):1553. https://doi.org/10.3390/healthcare13131553
Chicago/Turabian StyleBifari, Nisrin, Yasser Alatawi, Wesam S. Abdel-Razaq, Mohammad S. Shawaqfeh, Abdulkareem M. Albekairy, Fayez Hejaili, Ghassan F. Shattat, Mohammed Alkathiri, Yousef A. Alrajhi, Khalid A. Al Sulaiman, and et al. 2025. "The Role of Fixed-Dose Desmopressin in Hemostatic Outcomes of Native and Transplant Kidney Biopsies in a Tertiary Referral Hospital" Healthcare 13, no. 13: 1553. https://doi.org/10.3390/healthcare13131553
APA StyleBifari, N., Alatawi, Y., Abdel-Razaq, W. S., Shawaqfeh, M. S., Albekairy, A. M., Hejaili, F., Shattat, G. F., Alkathiri, M., Alrajhi, Y. A., Al Sulaiman, K. A., & Alkatheri, A. M. (2025). The Role of Fixed-Dose Desmopressin in Hemostatic Outcomes of Native and Transplant Kidney Biopsies in a Tertiary Referral Hospital. Healthcare, 13(13), 1553. https://doi.org/10.3390/healthcare13131553






