Outcomes of the Transsphenoidal Approach for ACTH-Secreting Pituitary Tumours and the Role of Postoperative ACTH in Predicting the Late Recurrence of Cushing’s Disease: A Retrospective Analysis of 50 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Aims
2.2. Diagnosis
2.3. Previous Treatment
2.4. Preoperative Diagnosis of Cushing’s Disease
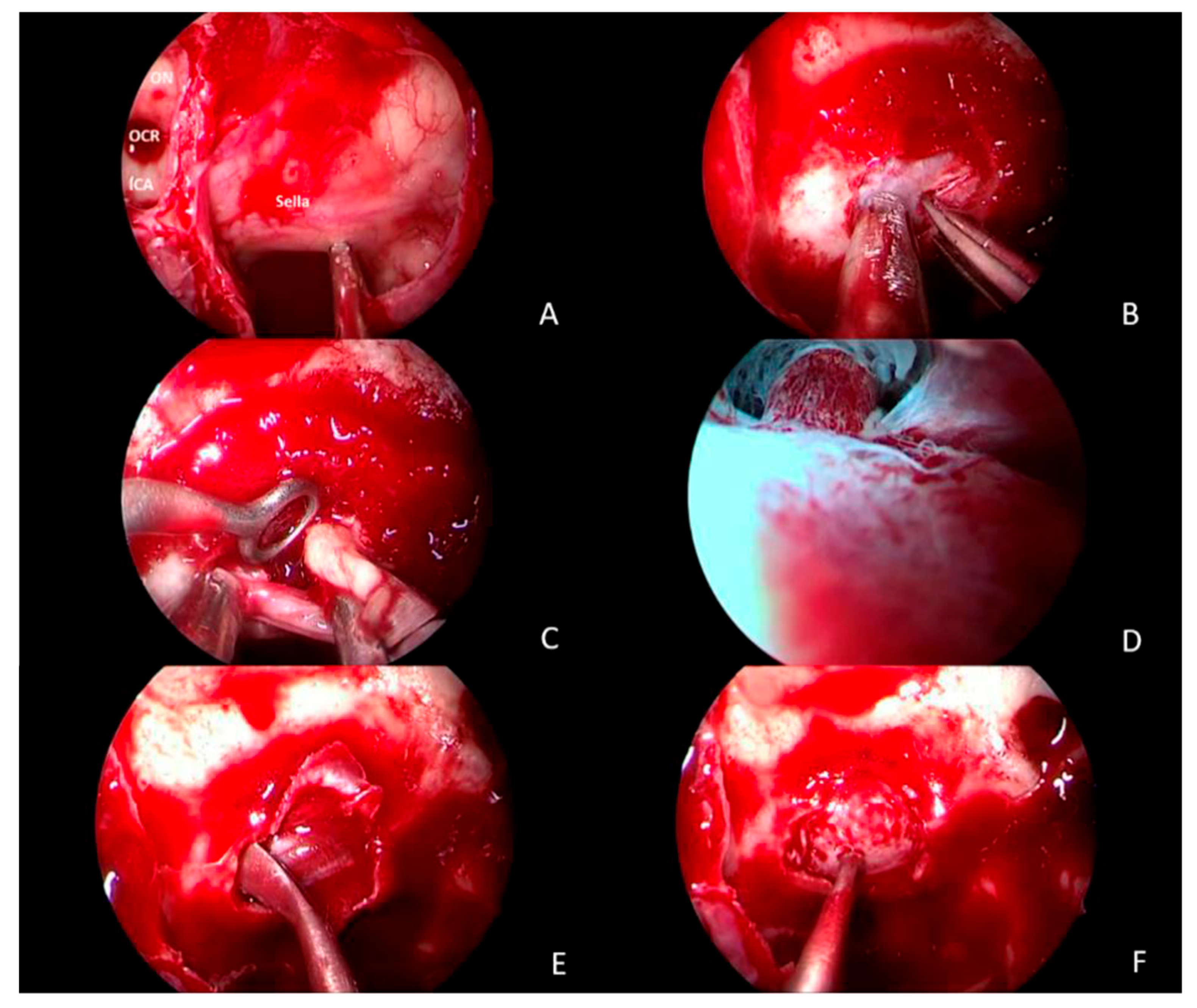
2.5. Surgical Treatment
2.6. Postoperative Management
2.7. Defining Postoperative Remission
2.8. Statistical Analysis
3. Results
3.1. Complications
3.2. Laboratory Analysis
4. Discussion
4.1. Early Management Following Transsphenoidal Surgery for Cushing’s Disease
4.2. The Role of Immediate Serum Cortisol and Urinary Cortisol
4.3. Endocrine Assessment
4.4. Remission
4.5. Factors Influencing Remission
4.6. Long-Term Follow-Up
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avgerinos, P.C.; Chrousos, G.P.; Nieman, L.K.; Oldfield, E.H.; Loriaux, D.L.; Cutler, G.B., Jr. The corticotropin-releasing hormone test in the postoperative evaluation of patients with cushing’s syndrome. J. Clin. Endocrinol. Metab. 1987, 65, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Asuzu, D.; Chatain, G.P.; Hayes, C. Normalized Early Postoperative Cortisol and ACTH Values Predict Nonremission After Surgery for Cushing Disease. J. Clin. Endocrinol. Metab. 2017, 102, 2179–2187. [Google Scholar] [CrossRef]
- Costenaro, F.; Rodrigues, T.C.; Rollin, G.A.; Ferreira, N.P.; Czepielewski, M.A. Evaluation of Cushing’s disease remission after transsphenoidal surgery based on early serum cortisol dynamics. Clin. Endocrinol. 2014, 80, 411–418. [Google Scholar] [CrossRef]
- Hameed, N.; Yedinak, C.G.; Brzana, J. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: A large single center experience. Pituitary 2013, 16, 452–458. [Google Scholar] [CrossRef]
- Roelfsema, F.; Biermasz, N.R.; Pereira, A.M. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: A structured review and meta-analysis. Pituitary 2012, 15, 71–83. [Google Scholar] [CrossRef]
- Ironside, N.; Chatain, G.; Asuzu, D. Earlier post-operative hypocortisolemia may predict durable remission from Cushing’s disease. Eur. J. Endocrinol. 2018, 178, 255–263. [Google Scholar] [CrossRef]
- Pivonello, R.; De Leo, M.; Cozzolino, A.; Colao, A. The Treatment of Cushing’s Disease. Endocr. Rev. 2015, 36, 385–486. [Google Scholar] [CrossRef]
- Renner, U. Recent advances in understanding corticotroph pituitary tumor initiation and progression. F1000Research 2018, 7, 1354. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.N. A change in pituitary magnetic resonance imaging protocol detects ACTH-secreting tumours in patients with previously negative results. Clin. Endocrinol. 2010, 72, 502–506. [Google Scholar] [CrossRef]
- Reincke, M.; Sbiera, S.; Hayakawa, A.; Theodoropoulou, M.; Osswald, A.; Beuschlein, F.; Meitinger, T.; Mizuno-Yamasaki, E.; Kawaguchi, K.; Saeki, Y.; et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 2015, 47, 31–38. [Google Scholar] [CrossRef]
- Jane, J.A.J.; Catalino, M.P.; Laws, E.R.J. Surgical Treatment of Pituitary Adenomas. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278983/ (accessed on 9 March 2022).
- Utz, A.L.; Swearingen, B.; Biller, B.M. Pituitary surgery and postoperative management in Cushing’s disease. Endocrinol Metabol Clin. N. Am. 2005, 34, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K. The diagnosis of Cushing’s syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Nieman, L.K.; Biller, B.M.; Findling, J.W. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 2807–2831. [Google Scholar] [CrossRef]
- Johnston, P.C.; Kennedy, L.; Hamrahian, A.H. Surgical outcomes in patients with Cushing’s disease: The Cleveland clinic experience. Pituitary 2017, 20, 430–440. [Google Scholar] [CrossRef]
- Aranda, G.; Enseñat, J.; Mora, M. Long-term remission and recurrence rate in a cohort of Cushing’s disease: The need for long-term follow-up. Pituitary 2015, 18, 142–149. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.A.; Isidori, A.M. Long-term remission and recurrence rates in Cushing’s disease: Predictive factors in a single-centre study. Eur. J. Endocrinol. 2013, 168, 639–648. [Google Scholar] [CrossRef]
- Lindsay, J.R.; Oldfield, E.H.; Stratakis, C.A.; Nieman, L.K. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J. Clin. Endocrinol. Metab. 2011, 96, 2057–2064. [Google Scholar] [CrossRef]
- Sherlock, M.; Ayuk, J.; Tomlinson, J.W. Mortality in patients with pituitary disease. Endocr. Rev. 2010, 31, 301–342. [Google Scholar] [CrossRef] [PubMed]
- Etxabe, J.; Vazquez, J.A. Morbidity and mortality in Cushing’s disease: An epidemiological approach. Clin. Endocrinol. 1994, 40, 479–484. [Google Scholar] [CrossRef]
- Bou Khalil, R.; Baudry, C.; Guignat, L. Sequential hormonal changes in 21 patients with recurrent Cushing’s disease after successful pituitary surgery. Eur. J. Endocrinol. 2011, 165, 729–737. [Google Scholar] [CrossRef]
- Uvelius, E.; Höglund, P.; Valdemarsson, S.; Siesjö, P. An early post-operative ACTH suppression test can safely predict short- and long-term remission after surgery of Cushing’s disease. Pituitary 2018, 21, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Micko, A.S.; Wöhrer, A.; Wolfsberger, S.; Knosp, E. Invasion of the cavernous sinus space in pituitary adenomas: Endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 2015, 122, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Chatzellis, E.; Alexandraki, K.I.; Androulakis, I.I.; Kaltsas, G. Aggressive pituitary tumors. Neuroendocrinology 2015, 101, 87–104. [Google Scholar] [CrossRef]
- Dwyer, A.J.; Frank, J.A.; Doppman, J.L. Pituitary adenomas in patients with Cushing disease: Initial experience with Gd-DTPA-enhanced MR imaging. Radiology 1987, 163, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Valassi, E.; Biller, B.M.; Swearingen, B. Delayed remission after transsphenoidal surgery in patients with Cushing’s disease. J. Clin. Endocrinol. Metab. 2010, 95, 601–610. [Google Scholar] [CrossRef]
- Invitti, C.; Pecori Giraldi, F.; De Martin, M.; Cavagnini, F. Diagnosis and management of Cushing’s syndrome: Results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J. Clin. Endocrinol. Metab. 1999, 84, 440–448. [Google Scholar] [CrossRef]
- Comtois, R.; Beauregard, H.; Hardy, J.; Robert, F.; Somma, M. High prolactin levels in patients with Cushing’s disease without pathological evidence of pituitary adenoma. Clin. Endocrinol. 1993, 38, 601–607. [Google Scholar] [CrossRef]
- Pouratian, N.; Prevedello, D.M.; Jagannathan, J.; Lopes, M.B.; Vance, M.L.; Laws, E.R.J. Outcomes and management of patients with Cushing’s disease without pathological confirmation of tumor resection after transsphenoidal surgery. J. Clin. Endocrinol. Metab. 2007, 92, 3383–3388. [Google Scholar] [CrossRef]
- Fleseriu, M.; Hamrahian, A.H.; Hoffman, A.R.; Kelly, D.F.; Katznelson, L. Neuroendocrine and Pituitary Scientific Committee. American Association of Clinical Endocrinologists and American College of endocrinology. Disease state clinical review: Diagnosis of recurrence in Cushing disease. Endocr. Pract. 2016, 22, 1436–1448. [Google Scholar] [CrossRef]
- Chee, G.H.; Mathias, D.B.; James, R.A.; Kendall-Taylor, P. Transsphenoidal pituitary surgery in Cushing’s disease: Can we predict outcome? Clin. Endocrinol. 2001, 54, 617–626. [Google Scholar] [CrossRef]
- Flitsch, J.; Knappe, U.J.; Lüdecke, D.K. The use of postoperative ACTH levels as a marker for successful transsphenoidal microsurgery in Cushing’s disease. Zentralbl. Neurochir. 2003, 64, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Fahlbuschm, R.; Buchfelder, M.; Müller, O.A. Transsphenoidal surgery for Cushing’s disease. J. R. Soc. Med. 1986, 79, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, L.; Laws, E.R.; Dodd, R.L. The dynamics of post-operative plasma ACTH values following transsphenoidal surgery for Cushing’s disease. Pituitary 2011, 14, 312–317. [Google Scholar] [CrossRef]
- Locatelli, M.; Vance, M.L.; Laws, E.R. Clinical review: The strategy of immediate reoperation for transsphenoidal surgery for Cushing’s disease. J. Clin. Endocrinol. Metab. 2005, 90, 5478–5482. [Google Scholar] [CrossRef] [PubMed]
- Ram, Z.; Nieman, L.K.; Cutler, G.B.J.; Chrousos, G.P.; Doppman, J.L.; Oldfield, E.H. Early repeat surgery for persistent Cushing’s disease. J. Neurosurg. 1994, 80, 37–45. [Google Scholar] [CrossRef]
- Friedman, R.B.; Oldfield, E.H.; Nieman, L.K. Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg. 1989, 71, 520–527. [Google Scholar] [CrossRef]
- Hassan-Smith, Z.K.; Sherlock, M.; Reulen, R.C. Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. J. Clin. Endocrinol. Metab. 2012, 97, 1194–1201. [Google Scholar] [CrossRef]
- Chen, J.C.; Amar, A.P.; Choi, S.; Singer, P.; Couldwell, W.T.; Weiss, M.H. Transsphenoidal microsurgical treatment of Cushing disease: Postoperative assessment of surgical efficacy by application of an overnight low-dose dexamethasone suppression test. J. Neurosurg. 2003, 98, 967–973. [Google Scholar] [CrossRef]
- Hofmann, B.M.; Hlavac, M.; Martinez, R.; Buchfelder, M.; Müller, O.A.; Fahlbusch, R. Long-term results after microsurgery for Cushing disease: Experience with 426 primary operations over 35 years. J. Neurosurg. 2008, 108, 9–18. [Google Scholar] [CrossRef]
- Saratziotis, A.; Baldovin, M.; Zanotti, C. Prospective Evaluation of Transsphenoidal Pituitary Surgery in Patients with Cushing’s Disease: Delayed Remission and the Role of Postsurgical Cortisol as a Predictive Factor. Healthcare 2024, 12, 1900. [Google Scholar] [CrossRef]
- Maniatis, A.; Cutfield, W.; Dattani, M.; Deal, C.; Collett-Solberg, P.F.; Horikawa, R.; Maghnie, M.; Miller, B.S.; Polak, M.; Sävendahl, L.; et al. Long-Acting Growth Hormone Therapy in Pediatric Growth Hormone Deficiency: A Consensus Statement. J. Clin. Endocrinol. Metab. 2025, 110, e1232–e1240. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, G.; Paraandavaji, E.; Nasab, M.M.M.; Mohammadi, E.; Sadeghi, N.; Tavangar, S.M.; Tehrani, M.R.M.; Dilmaghani, N.A. Clinical and surgical outcomes of pediatric Cushing’s disease following endoscopic transsphenoidal surgery. Child’s Nerv. Syst. 2025, 41, 130. [Google Scholar] [CrossRef] [PubMed]
| Demographic Data | |
|---|---|
| Qualitative Variable | |
| Gender | |
| Female | 34 |
| Male | 16 |
| Previous surgery in another centre | |
| No | 42 |
| Yes | 8 |
| MRI identification of the adenoma | |
| Microadenoma | 36 |
| Macroadenoma | 14 |
| Diagnosis with IPSS | 6 |
| Previous treatment | 9 (7 surgery, 2 radiotherapy) |
| First treatment | 41 |
| Quantitative variable | Mean (S.D.) |
| Age (years) | 37.8 (11.02) |
| Pre-surgery UFC/ULC level (nmol/24 h) | 239.08 (256.15) |
| Pre-surgery cortisol level (nmol/L) | 429.09 (168.04) |
| Pre-surgery ACTH level (ng/mL) | 74.25 (56) |
| BMI (kg/m2) | 35.4 (8.5) |
| Insulin-dependent diabetes | 17.3% |
| Follow-up | 76.5 months |
| Outcome of Surgery | |||
|---|---|---|---|
| Non-Remission | Remission | ||
| N = 50 | (N = 8) | (N = 42) | p-Value |
| Age at surgery (years) | |||
| Median (IQR) | 46.0 (40.0–53.0) | 39.0 (26.0–47.0) | 0.19 |
| Months of follow-up | |||
| Median (IQR) | 58.0 (23.0–106.0 | 76.5 (23.0–122.0) | 0.71 |
| ACTH, preoperative (ng/mL) | |||
| Median (IQR) | 49.0 (38.0–100.0) | 55.0 (44.0–102.0) | 0.60 |
| Morning cortisol, preoperative (nmol/L) | |||
| Median (IQR) | 487.5 (479.2–543.0) | 438.0 (380.0–476.0) | 0.12 |
| UFC, preoperative (nmol/24 h) | |||
| Median (IQR) | 174.0 (128.0–430.9) | 149.0 (109.0–248.0) | 0.56 |
| Cortisol, postoperative (nmol/L) | |||
| Median (IQR) | 449.5 (348.0–522.0) | 57.6 (43.2–100.0) | <0.001 |
| UFC, early postoperative (nmol/24 h) | |||
| Median (IQR) | 187.5 (71.0–319.0) | 40.1 (26.5–99.0) | 0.018 |
| ACTH, postoperative | |||
| Median (IQR) | 42 (29.2–87.4) | 5.1(4.2–13.4) | 0.60 |
| Demographic Data | ||
|---|---|---|
| Qualitative Variable | ||
| Type of complication | Number of patients | Treatment |
| N = 50 | 50 surgeries | |
| Cerebrospinal fluid rhinorrhoea | 3 | Autologous graft with fascia lata |
| Venous thromboembolic disease | 2 | Anticoagulant medicine |
| Fever | 4 | |
| Diabetes insipidus (DI) | 13 | |
| Hypothyroidism | 5 | Medical treatment |
| Anterior panhypopituitarism | 2 | Medical treatment |
| Anterior and posterior panhypopituitarism | 3 | Medical treatment |
| Growth hormone deficiency | 3 | Medical treatment |
| Gender | |||
|---|---|---|---|
| Female | Male | ||
| N = 50 | (N = 34) | (N = 16) | p-Value |
| Age at surgery (years) | |||
| Median (IQR) | 37.6 (33.0–49.0) | 40.0 (29.0–59.0) | 0.68 |
| Months of follow-up | |||
| Median (IQR) | 76.5 (23.0–122.0) | 76.4 (23.0–121.0) | 0.16 |
| ACTH, preoperative (ng/mL) | |||
| Median (IQR) | 49.5 (38.0–71.0) | 109.0 (71.0–135.0) | 0.024 |
| Morning cortisol, preoperative (nmol/L) | |||
| Median (IQR) | 454.0 (389.0–485.0) | 554.0 (459.0–611.0) | 0.22 |
| UFC, preoperative (nmol/24 h) | |||
| Median (IQR) | 139.0 (102.0–240.0) | 218.0 (170.0–434.0) | 0.23 |
| Cortisol, postoperative (nmol/L) | |||
| Median (IQR) | 68.3 (44.0–321.0) | 61.0 (33.0–129.0) | 0.52 |
| UFC, postoperative (nmol/24 h) | |||
| Median (IQR) | 71.0 (31.0–230.0) | 34.0 (26.0–59.0) | 0.11 |
| ACTH, postoperative | |||
| Median (IQR) | 5.2 (4.1–13.4) | 5.1 (4.4–12.7) | 0.60 |
| N = 50 | Microadenoma (N = 36) | Microadenoma (N = 14) | p-Value |
|---|---|---|---|
| Age at surgery (years) | |||
| Median (IQR) | 38.0 (16.0–45.0) | 50.0 (30.0–69.0) | 0.13 |
| Months of follow-up | |||
| Median (IQR) | 76.5 (23.0–122.0) | 60.0 (25.0–121.0) | 0.28 |
| ACTH, preoperative | |||
| Median (IQR) | 48.0 (33.0–76.0) | 85.3 (56.0–119.0) | 0.09 |
| Morning cortisol, preoperative | |||
| Median (IQR) | 469.0 (412.0–569.0) | 454.0 (385.0–490.0) | 0.65 |
| UFC, preoperative | |||
| Median (IQR) | 141.0 (84.0–206.0) | 238.0 (141.0–361.0) | 0.25 |
| Cortisol, preoperative early | |||
| Median (IQR) | 60.0 (43.0–222.0) | 75.5 (57.0–320.0) | 0.75 |
| Cortisol, preoperative late | |||
| Median (IQR) | 28.0 (12.0–271.0) | 97.0 (24.0–372.0) | 0.27 |
| UFC, late | |||
| Median (IQR) | 60.0 (32.0–227.0) | 35.0 (13.0–72.0) | 0.08 |
| ACTH, postoperative | |||
| Median (IQR) | 5.1 (4.3–12.4) | 5.3 (4.6–13.4) | 0.09 |
| Outcome Measures | First Week | 3 Months | 6 Months | 12 Months |
|---|---|---|---|---|
| Serum cortisol (nmol/L) | ||||
| Remission | 95.81 (108.8) | 95.70 (108.81) | 95.22 (108.21) | 91.55 (106.98) |
| Non-remission | 471.16 (134.39) | 470.66 (134.32) | 466.83 (135.28) | 462.83 (132.08) |
| ACTH (pg/mL) | ||||
| Remission | 5.1 (4.2–13.4) | 5.1 (4.1–13.2) | 5.1 (4.1–13.3) | 5.1 (4.2–13.6) |
| Non-remission | 42 (29.2–87.4) | 42 (29.3–87.8) | 42 (29.6–88.1) | 42 (29.7–88.4) |
| UFC (nmol/24 h) | ||||
| Remission | 70.5 (71.66) | 70.05 (70.76) | 70.45 (70.86) | 69.75 (71.22) |
| Non-remission | 186.66 (121.68) | 184.83 (120.63) | 185.33 (121.25) | 185.33 (122.32) |
| ID | Age | Sex | ACTH Pre-Surgery (ng/mL) | ACTH Post-Surgery (pg/mL) | UFC Pre-Surgery (nmol/24 h) | Serum Cortisol Pre-Surgery (nmol/L) | Previous Surgery | Size of Adenoma on MRI | UFC POD1 (nmol/24 h) | Serum Cortisol POD1 (nmol/L) | Serum Cortisol, 4-Month Follow-Up (nmol/L) | UFC Pre-Surgery (nmol/24 h) | Last Follow-Up Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | 65.30 | 4.9 | 138 | 390 | No | Micro | 94.3 | 134.39 | 97.32 | 36.0 | No |
| 2 | 42 | F | 56.29 | 6.2 | 141 | 368 | No | Micro | 110.9 | 160.45 | 110.23 | 87.74 | YES |
| 3 | 45 | M | 68.20 | 4.7 | 120 | 340 | No | Micro | 90.6 | 126.29 | 95.2 | 85.64 | No |
| 4 | 56 | F | 61.29 | 5.0 | 136 | 385 | No | Micro | 120.4 | 136.40 | 85.67 | 62.56 | No |
| 5 | 58 | M | 60.6 | 4.8 | 138 | 368 | No | Micro | 95.8 | 128.9 | 95.8 | 81.3 | No |
| 6 | 44 | F | 71.4 | 4.6 | 129 | 358 | No | Macro | 99.4 | 130.2 | 120.4 | 78.9 | No |
| 7 | 38 | F | 68.4 | 4.7 | 134 | 356 | No | Micro | 110.4 | 126.9 | 109.3 | 85.4 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saratziotis, A.; Baldovin, M.; Zanotti, C.; Munari, S.; Denaro, L.; Hajiioannou, J.; Emanuelli, E. Outcomes of the Transsphenoidal Approach for ACTH-Secreting Pituitary Tumours and the Role of Postoperative ACTH in Predicting the Late Recurrence of Cushing’s Disease: A Retrospective Analysis of 50 Cases. Healthcare 2025, 13, 1395. https://doi.org/10.3390/healthcare13121395
Saratziotis A, Baldovin M, Zanotti C, Munari S, Denaro L, Hajiioannou J, Emanuelli E. Outcomes of the Transsphenoidal Approach for ACTH-Secreting Pituitary Tumours and the Role of Postoperative ACTH in Predicting the Late Recurrence of Cushing’s Disease: A Retrospective Analysis of 50 Cases. Healthcare. 2025; 13(12):1395. https://doi.org/10.3390/healthcare13121395
Chicago/Turabian StyleSaratziotis, Athanasios, Maria Baldovin, Claudia Zanotti, Sara Munari, Luca Denaro, Jiannis Hajiioannou, and Enzo Emanuelli. 2025. "Outcomes of the Transsphenoidal Approach for ACTH-Secreting Pituitary Tumours and the Role of Postoperative ACTH in Predicting the Late Recurrence of Cushing’s Disease: A Retrospective Analysis of 50 Cases" Healthcare 13, no. 12: 1395. https://doi.org/10.3390/healthcare13121395
APA StyleSaratziotis, A., Baldovin, M., Zanotti, C., Munari, S., Denaro, L., Hajiioannou, J., & Emanuelli, E. (2025). Outcomes of the Transsphenoidal Approach for ACTH-Secreting Pituitary Tumours and the Role of Postoperative ACTH in Predicting the Late Recurrence of Cushing’s Disease: A Retrospective Analysis of 50 Cases. Healthcare, 13(12), 1395. https://doi.org/10.3390/healthcare13121395






