The Effectiveness and Harms of PSA-Based Prostate Cancer Screening: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
- Studies on adult males, particularly populations of men aged 50 years and older, evaluating the effectiveness or adverse effects of prostate cancer screening.
- Randomized controlled trials (RCTs), cohort studies, meta-analyses, and systematic reviews.
- Studies reporting mortality outcomes, diagnostic accuracy, or risk–benefit evaluations.
- Non-human or cell-based studies.
- Case reports or studies focusing on treatment rather than screening.
- Non-peer-reviewed articles.
2.4. Study Selection and Data Extraction
2.5. Quality Assessment
2.6. Assessment of Heterogeneity
- (1)
- Differences in study population characteristics (e.g., age range, geographic region);
- (2)
- Variation in screening methods (e.g., PSA alone, PSA + MRI, biomarkers);
- (3)
- Outcome definitions (e.g., prostate-cancer-specific mortality, overall mortality);
- (4)
- Duration of follow-up.
2.7. Assessment of Reporting Bias
- -
- Incomplete outcome reporting or the selective reporting of favorable results;
- -
- Asymmetry in the available evidence (e.g., overrepresentation of large trials or certain geographic regions);
- -
- The lack of trial registry information for some RCTs.
2.8. Data Synthesis Approach
3. Results
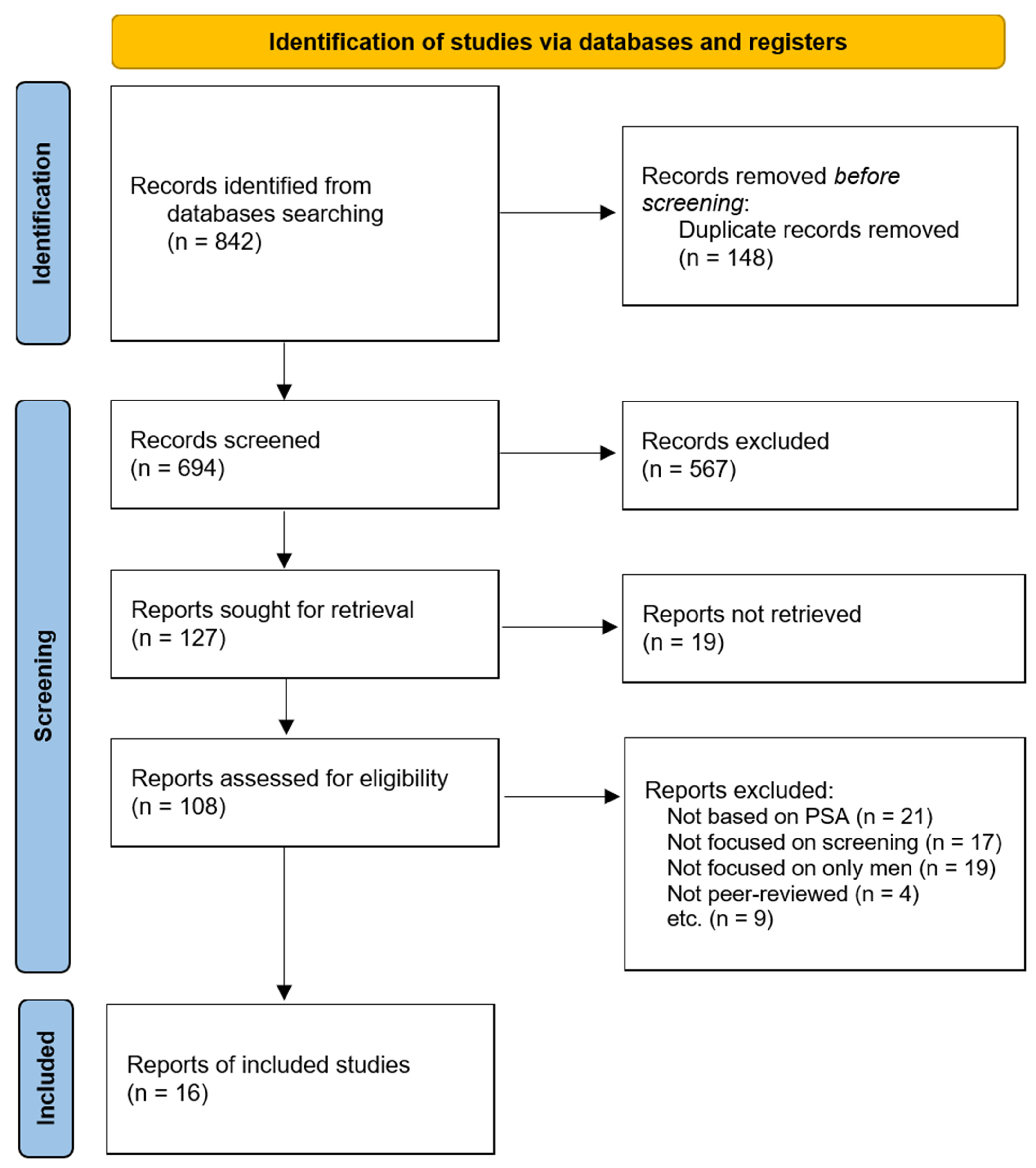
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment Summary
3.4. Effectiveness and Limitations of PSA-Based Screening
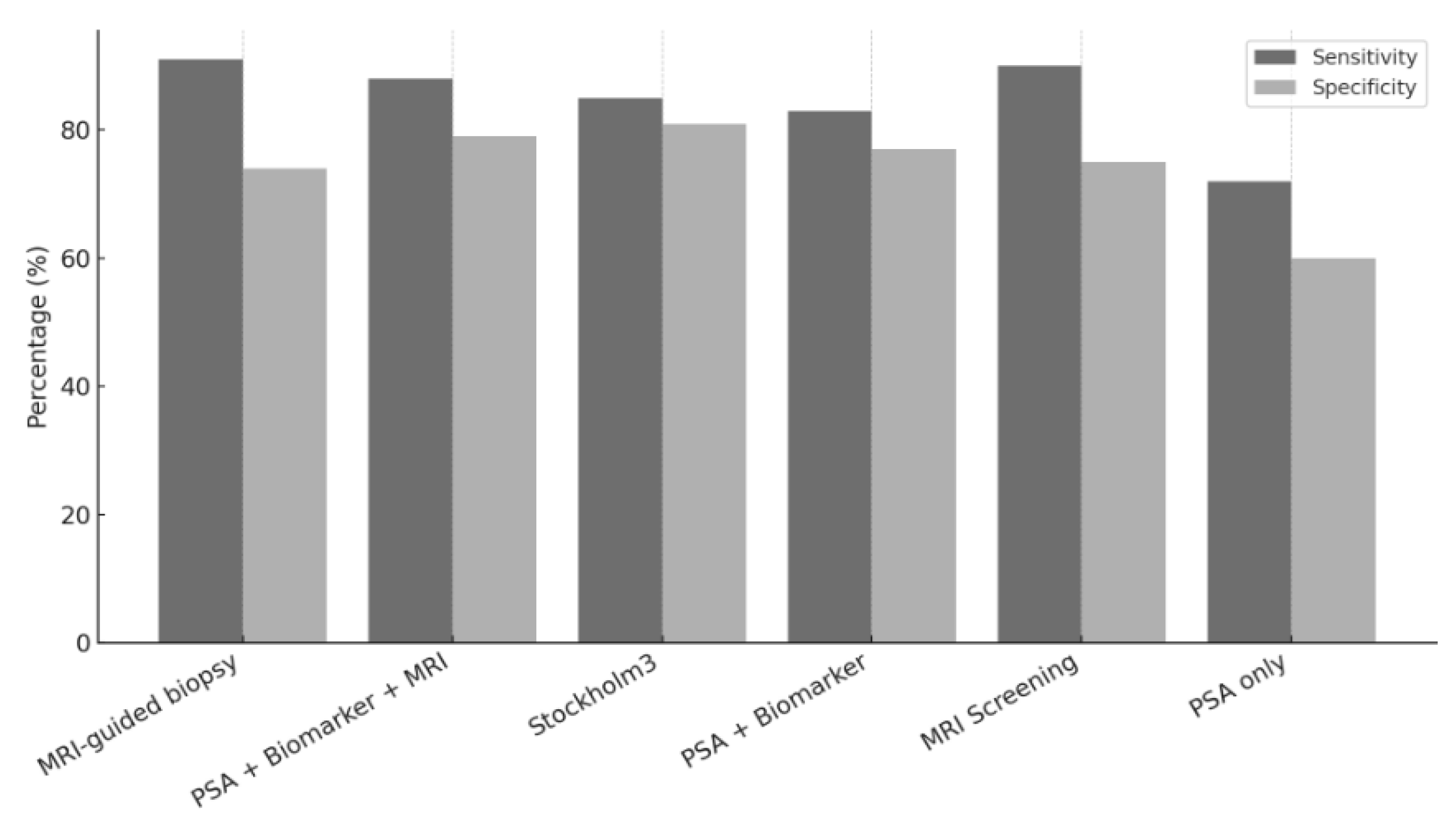
3.5. Diagnostic Accuracy of Screening Strategies
3.6. Screening Recommendations by Age and Risk
3.7. Quality of Evidence: GRADE
4. Discussion
4.1. Benefit–Harm Balance and Overdiagnosis
4.2. Role of Community Health Workers (CHWs) in Equitable Screening
4.3. Advances in Precision Screening
4.4. Policy and Practice Implications
4.5. Strengths and Limitations of This Review
4.6. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Conflicts of Interest
Appendix A. Full Search Strategies for Systematic Review (PRISMA 2020 Compliance)
- Database: PubMed
- Date of final search: 10 January 2025
- (“Prostate Cancer” [MeSH Terms] OR “prostate neoplasms” OR “prostate cancer”) AND (“PSA test” OR “Prostate-Specific Antigen”) AND
- (“Early Detection of Cancer” [MeSH Terms] OR “screening” OR “early diagnosis”) AND (“mortality” OR “death” OR “survival”) AND
- (“Randomized Controlled Trial” [Publication Type] OR “RCT” OR “cohort study” OR “meta- analysis”)
- Limits: English OR Korean language, human studies only
- Database: Embase
- Date of final search: 10 January 2025
- ‘Prostate cancer’/exp OR ‘prostate neoplasm’ OR ‘prostate cancer’ AND
- ‘psa test’ OR ‘prostate specific antigen’ AND
- ‘screening’ OR ‘early diagnosis’/exp AND
- ‘mortality’ OR ‘death’ OR ‘survival’ AND
- ‘randomized controlled trial’/exp OR ‘cohort study’ OR ‘meta-analysis’
- Filters applied: Language (English or Korean), Human studies
- Database: Cochrane Library
- Date of final search: 10 January 2025
- [MeSH descriptor: “Prostatic Neoplasms” explode all trees] AND (“PSA test” OR “Prostate-Specific Antigen”) AND
- [MeSH descriptor: “Early Detection of Cancer”] AND
- (mortality OR death OR survival) AND
- ([MeSH descriptor: “Randomized Controlled Trials as Topic”] OR “RCT” OR “Cohort Study” OR “Meta-Analysis”)
- Limits: English OR Korean, human studies
- Database: KMbase
- Search terms (Korean keywords and English synonyms):
- -
- 전립선암, PSA 검사, 조기진단, 사망률, 생존율, 무작위대조시험, 코호트, 메타분석 등
- -
- Boolean operators: AND/OR 사용, 한국어 논문 포함
- Database: Google Scholar
- Search terms used in Korean and English:
- -
- “Prostate cancer” AND “PSA” AND (“screening” OR “early detection”) AND (“mortality” OR “death”)
- Filters applied: publication year 2014–2024, English/Korean, full text available
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Korea Central Cancer Registry, National Cancer Center. Annual Report of Cancer Statistics in Korea in 2021; Ministry of Health and Welfare: Goyang, Republic of Korea, 2023. [Google Scholar]
- National Cancer Institute. Prostate Cancer Prevention (PDQ). Available online: https://www.cancer.gov/types/prostate/hp/prostate-prevention-pdq (accessed on 12 January 2025).
- Korea Centers for Disease Control and Prevention. Korea National Health and Nutrition Examination Survey (2013–2022); Korea Centers for Disease Control and Prevention: Cheongju, Republic of Korea; Available online: https://www.phwr.org/journal/view.html?doi=10.56786/PHWR.2024.17.7.4 (accessed on 16 January 2025).
- Ha, Y.S.; Kim, K.T.; Nam, W.; Park, H.; Yoo, S.; Lee, C.H.; Chung, H.S.; Choi, W.S.; Kim, J.; Shin, J.; et al. Results of a Nationwide Survey on Initial Diagnosis and Quality of Life in Korean Prostate Cancer Patients. Korean J. Urol. Oncol. 2022, 20, 265–272. [Google Scholar] [CrossRef]
- American Cancer Society. Survival Rates for Prostate Cancer. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 12 January 2025).
- Kim, Y. Prostate Cancer Screening. Korean J. Fam. Pract. 2012, 2, 104–111. [Google Scholar]
- Berkowitz, Z.; Li, J.; Richards, T.B.; Marcus, P.M. Patterns of Prostate-Specific Antigen Test Use in the US, 2005–2015. Am. J. Prev. Med. 2017, 53, 909–913. Available online: https://www.ajpmonline.org/article/S0749-3797(17)30438-5/abstract (accessed on 25 January 2025). [CrossRef]
- Carter, H.B.; Albertsen, P.C.; Barry, M.J.; Etzioni, R.; Freedland, S.J.; Greene, K.L.; Holmberg, L.; Kantoff, P.; Konety, B.R.; Murad, M.H.; et al. Early Detection of Prostate Cancer: AUA Guideline. J. Urol. 2013, 190, 419–426. [Google Scholar] [CrossRef]
- Seetharam Bhat, K.R.; Moschovas, M.C.; Onol, F.F.; Sandri, M.; Rogers, T.; Roof, S.; Rocco, B.; Patel, V.R. Trends in Clinical and Oncological Outcomes of Robot-Assisted Radical Prostatectomy before and after the 2012 US Preventive Services Task Force Recommendation against PSA Screening: A Decade of Experience. BJU Int. 2020, 125, 884–892. [Google Scholar] [CrossRef]
- Pyun, J.H.; Kang, S.H.; Kim, J.Y.; Shin, J.E.; Jeong, I.G.; Kim, J.W.; No, T.I.; Oh, J.J.; Yu, J.H.; Chung, H.S.; et al. Survey Results on the Perception of Prostate-Specific Antigen and Prostate Cancer Screening Among the General Public. Korean J. Urol. Oncol. 2020, 18, 40–46. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J. Urol. 2023, 210, 45–53. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Auvinen, A.; Tammela, T.L.J.; Mirtti, T.; Lilja, H.; Tolonen, T.; Kenttämies, A.; Rinta-Kiikka, I.; Lehtimäki, T.; Natunen, K.; Nevalainen, J.; et al. Prostate Cancer Screening with PSA, Kallikrein Panel, and MRI: The ProScreen Randomized Trial. JAMA 2024, 331, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, T.; Shim, S.R.; Basile, G.; Baboudjian, M.; Koi, T.; Przydacz, M.; Abufaraj, M.; Ploussard, G.; Kasivisvanathan, V.; Rivas, J.G.; et al. Magnetic Resonance Imaging in Prostate Cancer Screening: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Björnebo, L.; Discacciati, A.; Falagario, U.; Vigneswaran, H.T.; Jäderling, F.; Grönberg, H.; Eklund, M.; Nordström, T.; Lantz, A. Biomarker vs MRI-Enhanced Strategies for Prostate Cancer Screening: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e247131. [Google Scholar] [CrossRef]
- Hao, S.; Heintz, E.; Östensson, E.; Discacciati, A.; Jäderling, F.; Grönberg, H.; Eklund, M.; Nordström, T.; Clements, M.S. Cost-Effectiveness of the Stockholm3 Test and Magnetic Resonance Imaging in Prostate Cancer Screening: A Microsimulation Study. Eur. Urol. 2022, 82, 184–192. [Google Scholar] [CrossRef]
- Klotz, L.; Chin, J.; Black, P.C.; Finelli, A.; Anidjar, M.; Bladou, F.; Mercado, A.; Levental, M.; Ghai, S.; Chang, S.D.; et al. Magnetic Resonance Imaging–Targeted Biopsy with Systematic Transrectal Ultrasonography Biopsy for Biopsy-Naive Men at Risk for Prostate Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 534–542. [Google Scholar] [CrossRef] [PubMed]
- American Urological Association. Early Detection of Prostate Cancer: AUA/SUO Guideline (2023). J. Urol. 2023, 209, 275–282. Available online: https://www.auanet.org/guidelines-and-quality/guidelines/early-detection-of-prostate-cancer-guidelines (accessed on 12 January 2025).
- Bratt, O.; Auvinen, A.; Arnsrud Godtman, R.; Hellström, M.; Hugosson, J.; Lilja, H.; Wallström, J.; Roobol, M.J. Screening for prostate cancer: Evidence, ongoing trials, policies and knowledge gaps. BMJ Oncol. 2023, 2, e000039. [Google Scholar] [CrossRef]
- van Harten, M.J.; Roobol, M.J.; van Leeuwen, P.J.; Willemse, P.-P.M.; van den Bergh, R.C.N. Evolution of European prostate cancer screening protocols and summary of ongoing trials. BJU Int. 2024, 134, 31–42. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef]
- Martin, R.M.; Donovan, J.L.; Turner, E.L.; Metcalfe, C.; Young, G.J.; Walsh, E.I.; Lane, J.A.; Noble, S.; Oliver, S.E.; Evans, S.; et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA 2018, 319, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16yr Follow-up of the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Turner, E.L.; Young, G.J.; Metcalfe, C.; Walsh, E.I.; Lane, J.A.; Sterne, J.A.; Noble, S.; Holding, P.; Ben-Shlomo, Y.; et al. Prostate-Specific Antigen Screening and 15-Year Prostate Cancer Mortality: A Secondary Analysis of the CAP Randomized Clinical Trial. JAMA 2024, 331, 1163–1175. [Google Scholar] [CrossRef]
- Vaccarella, S.; Li, M.; Bray, F.; Kvale, R.; Serraino, D.; Lorenzoni, V. Prostate cancer incidence and mortality in Europe and implications for screening activities: Population-based study. BMJ 2024, 386, e077738. [Google Scholar] [CrossRef]
- Mok, Y.; Kimm, H.; Shin, S.Y.; Jee, S.H.; Platz, E.A. Screening Prostate-specific Antigen Concentration and Prostate Cancer Mortality: The Korean Heart Study. Urology 2015, 85, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F.; Crawford, E.D.; Kramer, B.S. Mortality Reduction and Cumulative Excess Incidence (CEI) in the Prostate-Specific Antigen (PSA) Screening Era. JAMA Oncol. 2024, 10, 243–251. [Google Scholar] [CrossRef]
- Rivera, M.P.; Mehta, A.C.; Wahidi, M.M. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e142S–e165S. [Google Scholar] [CrossRef]
- Hooper, G.L.; Allen, R.S.; Payne-Foster, P.; Oliver, J.S. A qualitative study to determine barriers for prostate cancer screening in rural African-American men. Urol. Nurs. 2018, 37, 285–291. [Google Scholar] [CrossRef]
- Wray, R.J.; Nicks, S.E.; Adsul, P.; Elliot, M.; Enard, K.; Jupka, K.; Trainer, A.K.; Hansen, N.; Shahid, M.; Wright-Jones, R.; et al. Promoting informed prostate cancer screening decision-making for African American men in a community-based setting. Cancer Causes Control 2022, 33, 503–514. [Google Scholar] [CrossRef]
- Cho, D.; Gor, B.; Choi, J.; Lee, J.E.; Frost, J.; Roberson, P.; Pettaway, C.A. A Community-Based Prostate Cancer Screening and Education Program for Asian American Men in Medically Underserved Communities. Int. J. Environ. Res. Public Health 2024, 21, 415. [Google Scholar] [CrossRef] [PubMed]
- Attipoe-Dorcoo, S.; Chattopadhyay, S.K.; Verughese, J.; Ekwueme, D.U.; Sabatino, S.A.; Peng, Y. Community Preventive Services Task Force. Engaging Community Health Workers to Increase Cancer Screening: A Community Guide Systematic Economic Review. Am. J. Prev. Med. 2021, 60, e189–e197. [Google Scholar] [CrossRef] [PubMed]
- CHW Resource Hub. Prostate Cancer Screening Program. Available online: https://www.chwresourcehub.org/projects/prostate-cancer-screening-program (accessed on 4 March 2025).
- Dong-gu Public Health Center, Gwangju. Free Prostate Cancer Screening Program Announcement; Dong-gu Public Health Center: Gwangju, Republic of Korea, 2022; Available online: https://www.bsdonggu.go.kr/health/index.donggu?menuCd=DOM_000000404010001000 (accessed on 15 January 2025).
- Eunpyeong-gu Public Health Center, Seoul. Prostate Cancer Health Lecture and Free Screening Notice; Eunpyeong-gu Public Health Center: Seoul, Republic of Korea, 2024; Available online: https://www.ep.go.kr/health/selectBbsNttView.do?key=1667&bbsNo=88&nttNo=62110&searchCtgry=&searchCnd=all&searchKrwd=&pageIndex=209&integrDeptCode= (accessed on 15 January 2025).
- Yangyang-gun Public Health Center, Gangwon. 2023 Prostate Disease Free Screening Announcement; Yangyang-gun Public Health Center: Yangyang, Republic of Korea, 2023; Available online: https://www.yangyang.go.kr/gw/portal/yyc_news_notice?articleSeq=102481&mode=readForm&curPage=1&boardCode=BDAABB01 (accessed on 15 January 2025).
- Goyang-si Public Health Center, Gyeonggi. PSA (Prostate-Specific Antigen) Test Information; Goyang-si Public Health Center: Goyang, Republic of Korea, 2024; Available online: https://www.ggc.go.kr/site/regional/communicate/view/11823?cp=176&listType=list (accessed on 15 January 2025).
| Reference | Year | Setting Country | Study Design | Sample | Key Findings |
|---|---|---|---|---|---|
| Auvinen A et al. [16] | 2024 | Finland | RCT | 12,750 | ProScreen trial: The evaluation of a multimodal screening protocol incorporating PSA, 4Kscore, and MRI demonstrated the improved detection of clinically significant prostate cancer. |
| Fazekas JT et al. [17] | 2024 | Multinational (primarily US) | Meta-analysis | 80,114 | MRI-based screening pathways maintained clinical significance in detecting relevant prostate cancer cases compared to conventional methods. |
| Björnebo et al. [18] | 2024 | Sweden | RCT | 12,743 | Biomarkers vs. MRI for prostate cancer screening: This study compared MRI-based and biomarker-based strategies for prostate cancer screening, highlighting their respective diagnostic efficacies. |
| Hao S et al. [19] | 2022 | Sweden | Simulation model | - | The Stockholm3 risk prediction model was found to be effective in the repeated screening and stratification of prostate cancer risk. |
| Klotz L et al. [20] | 2021 | Canada | RCT | 453 | MRI-targeted biopsy methods demonstrated superior sensitivity compared to traditional TRUS-guided biopsy methods. |
| Wei JT et al. [21] | 2023 | USA | Guideline | - | The AUA/SUO Early Detection Guidelines recommend personalized PSA-based screening strategies tailored to patient risk profiles and shared decision-making. |
| Bratt O et al. [22] | 2023 | Sweden, Europe | Narrative review | - | This review explored the long-term outcomes of prostate cancer screening and proposed future directions for policy refinement. |
| Harten MJ et al. [23] | 2024 | Europe | Narrative review | - | A comprehensive review of prostate cancer screening policies across Europe, focusing on variations in implementation and outcomes. |
| US Preventive Services Task Force [24] | 2018 | USA | Guideline | - | USPSTF PSA Screening Guidelines: PSA screening was shown to provide modest mortality benefits, though it was accompanied by risks of overdiagnosis and overtreatment. |
| Ilic D et al. [25] | 2018 | Europe, US, etc. | Systematic Review | 721,718 | The systematic review indicated both potential benefits in mortality reduction and harms related to overdiagnosis and false positives. |
| Martin RM et al. [26] | 2018 | UK | RCT | 419,582 | PSA Screening and 10-Year Mortality (CAP trial): A single invitation for PSA screening was associated with only a marginal reduction in long-term prostate cancer mortality. |
| Hugosson J et al. [27] | 2019 | Europe (8 countries) | RCT | 182,160 | ERSPC 16-Year Follow-Up Europe: The long-term data suggested a reduction in prostate-cancer-specific mortality following systematic PSA-based screening. |
| Martin RM et al. [28] | 2024 | UK, US, etc. | Meta-analysis | 721,718 | PSA screening reduced prostate-cancer-specific mortality but had no significant impact on overall mortality rates. |
| Vaccarella S et al. [29] | 2024 | Europe (26 countries) | Ecological study (registry data) | - | The study identified notable trends in the overdiagnosis of prostate cancer associated with widespread screening practices. |
| Mok Y et al. [30] | 2015 | South Korea | Cohort | 97,274 | Higher PSA levels at screening were associated with increased prostate cancer mortality in Korean men. |
| Pinsky P et al. [31] | 2024 | US | Cohort (registry data) | 76,693 | PSA screening reduced disease-specific mortality but was also linked to a high rate of overdiagnosis. |
| Screening Tool | Benefits | Risks |
|---|---|---|
| PSA Test | Accessible, cost-effective | Overdiagnosis, possibility of false positive |
| PSA + MRI | High sensitivity, fewer biopsies | High cost, limited MRI access in some regions |
| Stockholm3/Biomarkers | Balanced accuracy, risk-stratified | Requires infrastructure, validation |
| TRUS biopsy | Widely used, standard method | Lower sensitivity, more complications |
| Study [Ref. No.] | Screening Tool | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Klotz L et al. [20] | MRI-Guided Biopsy | 91 | 74 |
| Fazekas JT et al. [17] | MRI Screening | 90 | 75 |
| Auvinen A et al. [16] | PSA + Biomarker + MRI | 88 | 79 |
| Hao S et al. [19] | Stockholm3 | 85 | 81 |
| Björnebo et al. [18] | PSA + Biomarker | 83 | 77 |
| Pinsky P et al. [31] | PSA Alone | 72 | 60 |
| Outcome | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Quality | Ref. No |
|---|---|---|---|---|---|---|---|---|
| Prostate-cancer- specific mortality | RCT/Meta-Analysis | Low | No serious | No serious | No serious | Unlikely | ⬤⬤⬤⬤ High | [27,28] |
| All-cause mortality | RCT | Low | Serious | No serious | Serious | Unlikely | ⬤⬤⬤◯ Moderate | [26] |
| Overdiagnosis rate | Meta-Analysis/Review | Moderate | Serious | No serious | No serious | Likely | ⬤⬤◯◯ Low | [29] |
| Biopsy-related complications | Systematic Review | Low | No serious | Some | Serious | Unclear | ⬤⬤◯◯ Low | [25] |
| Diagnostic accuracy (MRI/biomarkers) | RCT/Observational | Low | No serious | No serious | No serious | Unlikely | ⬤⬤⬤⬤ High | [18,20] |
| Policy and guideline consistency | Guideline/Review | Low | No serious | No serious | No serious | Unlikely | ⬤⬤⬤◯ Moderate | [21,23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, C.-u.; Kang, H. The Effectiveness and Harms of PSA-Based Prostate Cancer Screening: A Systematic Review. Healthcare 2025, 13, 1381. https://doi.org/10.3390/healthcare13121381
Oh C-u, Kang H. The Effectiveness and Harms of PSA-Based Prostate Cancer Screening: A Systematic Review. Healthcare. 2025; 13(12):1381. https://doi.org/10.3390/healthcare13121381
Chicago/Turabian StyleOh, Chung-uk, and Hyekyung Kang. 2025. "The Effectiveness and Harms of PSA-Based Prostate Cancer Screening: A Systematic Review" Healthcare 13, no. 12: 1381. https://doi.org/10.3390/healthcare13121381
APA StyleOh, C.-u., & Kang, H. (2025). The Effectiveness and Harms of PSA-Based Prostate Cancer Screening: A Systematic Review. Healthcare, 13(12), 1381. https://doi.org/10.3390/healthcare13121381






