Spatiotemporal Dynamics in the Burden of Lip and Oral Cavity Cancer and Attributable Risk Factors in Asia (1990–2021)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Resources
2.2. Statistical Analysis
3. Results
3.1. Burden of LOC in Asia
3.2. Trends in the ASDR for LOC Attributable to Alcohol Use
3.3. Trends in the ASDR for LOC Attributable to Tobacco Chewing
3.4. Trends in the ASDR for LOC Attributable to Smoking
3.5. Temporal Trends in the ASDR for LOC in Different Age Groups
4. Discussion
4.1. Age-Specific Burden Dynamics: Demographic Shifts and Prevention Priorities
4.2. Gender Disparities in LOC Burden
4.3. Risk Factor Heterogeneity: Tobacco, Alcohol, and Smokeless Tobacco Use
4.4. Socioeconomic Disparities in LOC Burden: Regional Inequities Across SDI Strata
4.5. Diagnostic Delays in LOC Burden
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LOC | lip and oral cancers |
| SDI | sociodemographic Index |
| GBD | global burden of disease |
| DALYs | disability-adjusted life years |
| ASDR | age-standardized DALY rate |
| EAPC | estimated annual percentage change |
References
- Noravesh, F.; Behbahan, S.E.B.; Saeediankia, A.; Bahadorimonfared, A.; Looha, M.A.; Mohammadi, G. Burden, trends, projections, and spatial patterns of lip and oral cavity cancer in Iran: A time-series analysis from 1990 to 2040. BMC Public Health 2025, 25, 1282. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wen, H.; Bai, J.; Yu, C. The mortality of oral cancer attributable to tobacco in China, the US, and India. J. Cancer Res. Clin. Oncol. 2023, 149, 16741–16752. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Dou, W.; Liu, W.; Li, J.; Han, X.; Li, S.; Wu, X.; Wang, F.; Xu, X.; Li, J. Global, regional, and national burden of oral cancer and its attributable risk factors from 1990 to 2019. Cancer Med. 2023, 12, 13811–13820. [Google Scholar] [CrossRef]
- Nagao, T.; Warnakulasuriya, S. Screening for oral cancer: Future prospects, research and policy development for Asia. Oral Oncol. 2020, 105, 104632. [Google Scholar] [CrossRef]
- Malkani, N.; Kazmi, S.; Rashid, M.U. Epidemiological Assessment of Oral Cancer Burden in Pakistan. Cancer Investig. 2021, 39, 842–853. [Google Scholar] [CrossRef]
- Laaksonen, M.; Xie, L.; Shang, Z. Burden of oral cancer in Asia from 1990 to 2019: Estimates from the Global Burden of Disease 2019 study. PLoS ONE 2022, 17, e0265950. [Google Scholar] [CrossRef]
- Reitsma, M.B.; Fullman, N.; Ng, M.; Salama, J.S.; Abajobir, A.; Abate, K.H.; Abbafati, C.; Abera, S.F.; Abraham, B.; Abyu, G.Y.; et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Rao, S.V.K.; Mejia, G.; Roberts-Thomson, K.; Logan, R. Epidemiology of Oral Cancer in Asia in the Past Decade- An Update (2000–2012). Asian Pac. J. Cancer Prev. 2013, 14, 5567–5577. [Google Scholar] [CrossRef]
- Lee, Y.C.A.; Li, S.; Chen, Y.; Li, Q.; Chen, C.J.; Hsu, W.L.; Lou, P.J.; Zhu, C.; Pan, J.; Shen, H.; et al. Tobacco smoking, alcohol drinking, betel quid chewing, and the risk of head and neck cancer in an East Asian population. Head Neck 2018, 41, 92–102. [Google Scholar] [CrossRef]
- Ren, Z.H.; Hu, C.Y.; He, H.R.; Li, Y.J.; Lyu, J. Global and regional burdens of oral cancer from 1990 to 2017: Results from the global burden of disease study. Cancer Commun. 2020, 40, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, F.; Zhao, G.; Zhang, X.; Zhang, X.; Li, T.; Hu, C.; Zhu, W.; Li, D. Trends in the global burden of oral cancer joint with attributable risk factors: Results from the global burden of disease study 2019. Oral Oncol. 2022, 134, 106189. [Google Scholar] [CrossRef]
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Jiang, W.; Wang, C.; Li, X.; Zhou, W. Global burden of pancreatic cancer attributable to metabolic risks from 1990 to 2019, with projections of mortality to 2030. BMC Public Health 2024, 24, 456. [Google Scholar] [CrossRef]
- Go, D.-S.; Kim, Y.-E.; Yoon, S.-J. Subnational Burden of Disease According to the Sociodemographic Index in South Korea. Int. J. Environ. Res. Public Health 2020, 17, 5788. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.S.; Luan, H.H.; Jiang, J.F.; Wu, L.; Li, C.; Leng, W.D.; Zeng, X.T. The spatial and temporal trends of severe periodontitis burden in Asia, 1990–2019: A population-based epidemiological study. J. Periodontol. 2022, 93, 1615–1625. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, K.; Fang, S. Trend analysis of stroke subtypes mortality attributable to high body-mass index in China from 1990 to 2019. BMC Public Health 2024, 24, 2155. [Google Scholar] [CrossRef]
- Lan, Q.-W.; Chen, H.-K.; Huang, Z.-M.; Bao, T.-Y.; Liang, C.-J.; Yi, R.-T.; Huang, Y.-Y.; He, Y.-X.; Huang, X.-Q.; Gu, B.; et al. Global, regional, and national time trends in incidence for tuberculosis, 1990−2019: An age-period-cohort analysis for the Global Burden of Disease 2019 study. Heart Lung 2024, 65, 19–30. [Google Scholar] [CrossRef]
- Grover, S. Aging population in Asia: Are we preparing ourselves enough? Asian J. Psychiatry 2015, 13, 1–2. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y.; Li, H.; Zhang, N.; He, R.; Zhang, R.; Mao, Y.; Zhu, B. Lip and Oral Cavity Cancer Burden and Related Risk Factors in China: Estimates and Forecasts from 1990 to 2049. Healthcare 2022, 10, 1611. [Google Scholar] [CrossRef]
- Batchelor, P. The changing epidemiology of oral diseases in the elderly, their growing importance for care and how they can be managed. Age Ageing 2015, 44, 1064–1070. [Google Scholar] [CrossRef][Green Version]
- Sharma, M.; Onozaki, I.; Nunn, P. TB in older people in Asia: Why it is important. Int. J. Tuberc. Lung Dis. 2021, 25, 521–524. [Google Scholar] [CrossRef]
- Tran, Q.; Maddineni, S.; Arnaud, E.H.; Divi, V.; Megwalu, U.C.; Topf, M.C.; Sunwoo, J.B. Oral cavity cancer in young, non-smoking, and non-drinking patients: A contemporary review. Crit. Rev. Oncol./Hematol. 2023, 190, 104112. [Google Scholar] [CrossRef] [PubMed]
- Lory, P.; Perche, L.; Blanc, J.; Fouquier, B.; Giroux, A.; Thomassin, A.; Devaux, M.; Renaudin, A.; Di Martino, C.; Quipourt, V.; et al. Adherence to oral anti-cancer therapies in older patients is similar to that of younger patients. J. Oncol. Pharm. Pract. 2022, 29, 1154–1162. [Google Scholar] [CrossRef]
- Rai, P.; Goh, C.E.; Seah, F.; Islam, I.; Chia-Wei, W.W.; McLoughlin, P.M.; Loh, J.S.P. Oral Cancer Awareness of Tertiary Education Students and General Public in Singapore. Int. Dent. J. 2023, 73, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; Belloni, P.; De Berardinis, R.; Gandini, S.; Chu, F.; Zorzi, S.; Fumagalli, C.; Santoro, L.; Chiocca, S.; Ansarin, M. A systematic review and meta-analysis of the prognostic role of age in oral tongue cancer. Cancer Med. 2021, 10, 2566–2578. [Google Scholar] [CrossRef]
- Dokuru, D.R.; Horwitz, T.B.; Freis, S.M.; Stallings, M.C.; Ehringer, M.A. South Asia: The Missing Diverse in Diversity. Behav. Genet. 2023, 54, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Amato, A.; Romano, A.; Dipalma, G.; Xhajanka, E.; Baroni, A.; Serpico, R.; Inchingolo, F.; Contaldo, M. Analysis of Risk Factors of Oral Cancer and Periodontitis from a Sex- and Gender-Related Perspective: Gender Dentistry. Appl. Sci. 2022, 12, 9135. [Google Scholar] [CrossRef]
- Sengupta, N.; Sarode, S.C.; Sarode, G.S.; Anand, R. Sex bias phenomenon in oral cancer: An insight. Future Oncol. 2023, 19, 1381–1384. [Google Scholar] [CrossRef]
- Al-Jaber, A.; Al-Nasser, L.; El-Metwally, A. Epidemiology of oral cancer in Arab countries. Saudi Med. J. 2016, 37, 249–255. [Google Scholar] [CrossRef]
- Burja Vladić, M.; Matošić, A.; Špiljak, B.; Šimunović, L.; Puljić, A.; Kilić, D.; Mimica, S.; Jelkić, M.; Andabak Rogulj, A.; Brailo, V.; et al. Preventive Examinations for Early Detection of Oral Cancer Conducted among Alcohol Addicts. Arch. Psychiatry Res. 2024, 60, 205–218. [Google Scholar] [CrossRef]
- Danpanichkul, P.; Pang, Y.; Díaz, L.A.; White, T.M.; Sirimangklanurak, S.; Auttapracha, T.; Suparan, K.; Syn, N.; Jatupornpakdee, P.; Saowapa, S.; et al. Alcohol-Attributable Cancer: Update from the Global Burden of Disease 2021 Study. Aliment. Pharmacol. Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.A.; Nyirjesy, S.C.; Balsiger, R.; Talbot, N.; VanKoevering, K.K.; Haring, C.T.; Old, M.O.; Kang, S.Y.; Seim, N.B. Modifiable risk factors for oral cavity cancer in non-smokers: A systematic review and meta-analysis. Oral Oncol. 2023, 137, 106300. [Google Scholar] [CrossRef]
- Mehrotra, R.; Yadav, A.; Sinha, D.N.; Parascandola, M.; John, R.M.; Ayo-Yusuf, O.; Nargis, N.; Hatsukami, D.K.; Warnakulasuriya, S.; Straif, K.; et al. Smokeless tobacco control in 180 countries across the globe: Call to action for full implementation of WHO FCTC measures. Lancet Oncol. 2019, 20, e208–e217. [Google Scholar] [CrossRef] [PubMed]
- Raimondeau, P.; Manzi, S.; Brucato, N.; Kinipi, C.; Leavesley, M.; Ricaut, F.-X.; Besnard, G. Genome skims analysis of betel palms (Areca spp., Arecaceae) and development of a profiling method to assess their plastome diversity. Gene 2021, 800, 145845. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, S.; Christianson, L.; Rehman, S.; Ekwunife, O.; Samkange-Zeeb, F. Smokeless Tobacco and Oral Potentially Malignant Disorders in South Asia: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2018, 20, 12–21. [Google Scholar] [CrossRef]
- Prinja, S.; Purohit, N.; Kaur, N.; Rajapaksa, L.; Sarker, M.; Zaidi, R.; Bennett, S.; Rao, K.D. The state of primary health care in south Asia. Lancet Glob. Health 2024, 12, e1693–e1705. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kanaan, M.; Siddiqi, K.; Sinha, D.N.; Mehrotra, R. Oral Cancer Risk Assessment for Different Types of Smokeless Tobacco Products Sold Worldwide: A Review of Reviews and Meta-analyses. Cancer Prev. Res. 2022, 15, 733–746. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Causes of oral cancer—An appraisal of controversies. Br. Dent. J. 2009, 207, 471–475. [Google Scholar] [CrossRef]
- Huang, J.; Lucero-Prisno, D.E.; Zhang, L.; Xu, W.; Wong, S.H.; Ng, S.C.; Wong, M.C.S. Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 271–287. [Google Scholar] [CrossRef]
- Al-Zalabani, A. Cancer incidence attributable to tobacco smoking in GCC countries in 2018. Tob. Induc. Dis. 2020, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef]
- Hernández-Morales, A.; González-López, B.S.; Scougall-Vilchis, R.J.; Bermeo-Escalona, J.R.; Velázquez-Enríquez, U.; Islas-Zarazúa, R.; Márquez-Rodríguez, S.; Sosa-Velasco, T.A.; Medina-Solís, C.E.; Maupomé, G. Lip and Oral Cavity Cancer Incidence and Mortality Rates Associated with Smoking and Chewing Tobacco Use and the Human Development Index in 172 Countries Worldwide: An Ecological Study 2019–2020. Healthcare 2023, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Meira, I.A.; Soares, M.S.; Bonan, P.R.; Mélo, C.B.; Piagge, C.S. Delay in diagnosis of oral cancer: A systematic review. Med. Oral Patol. Oral Y Cir. Bucal 2021, 26, e815–e824. [Google Scholar] [CrossRef]
- Lauritzen, B.B.; Jensen, J.S.; Grønhøj, C.; Wessel, I.; von Buchwald, C. Impact of delay in diagnosis and treatment-initiation on disease stage and survival in oral cavity cancer: A systematic review. Acta Oncol. 2021, 60, 1083–1090. [Google Scholar] [CrossRef]
- Kruk, M.E.; Gage, A.D.; Joseph, N.T.; Danaei, G.; García-Saisó, S.; Salomon, J.A. Mortality due to low-quality health systems in the universal health coverage era: A systematic analysis of amenable deaths in 137 countries. Lancet 2018, 392, 2203–2212. [Google Scholar] [CrossRef]
- Borse, V.; Konwar, A.N.; Buragohain, P. Oral cancer diagnosis and perspectives in India. Sens. Int. 2020, 1, 100046. [Google Scholar] [CrossRef] [PubMed]
- D’souza, S.; Addepalli, V. Preventive measures in oral cancer: An overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef]
- Harris, J.A.; Ritchie, C.A.; Hanna, G.J.; McCain, J.P.; Ji, Y.D. The Inequitable Global Burden of Lip and Oral Cancers: Widening Disparities Across Countries. J. Oral Maxillofac. Surg. 2021, 79, 1364–1372. [Google Scholar] [CrossRef]
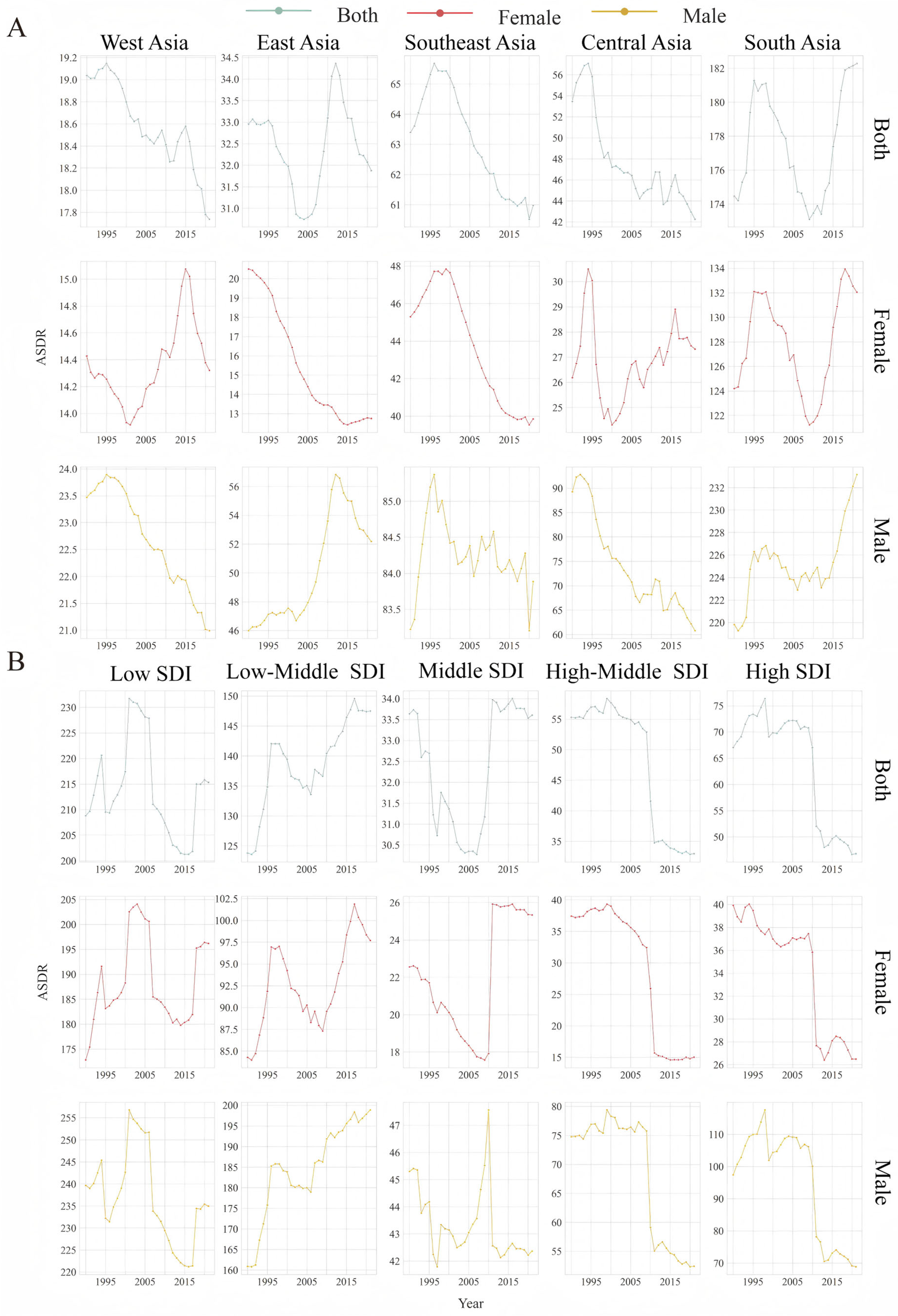
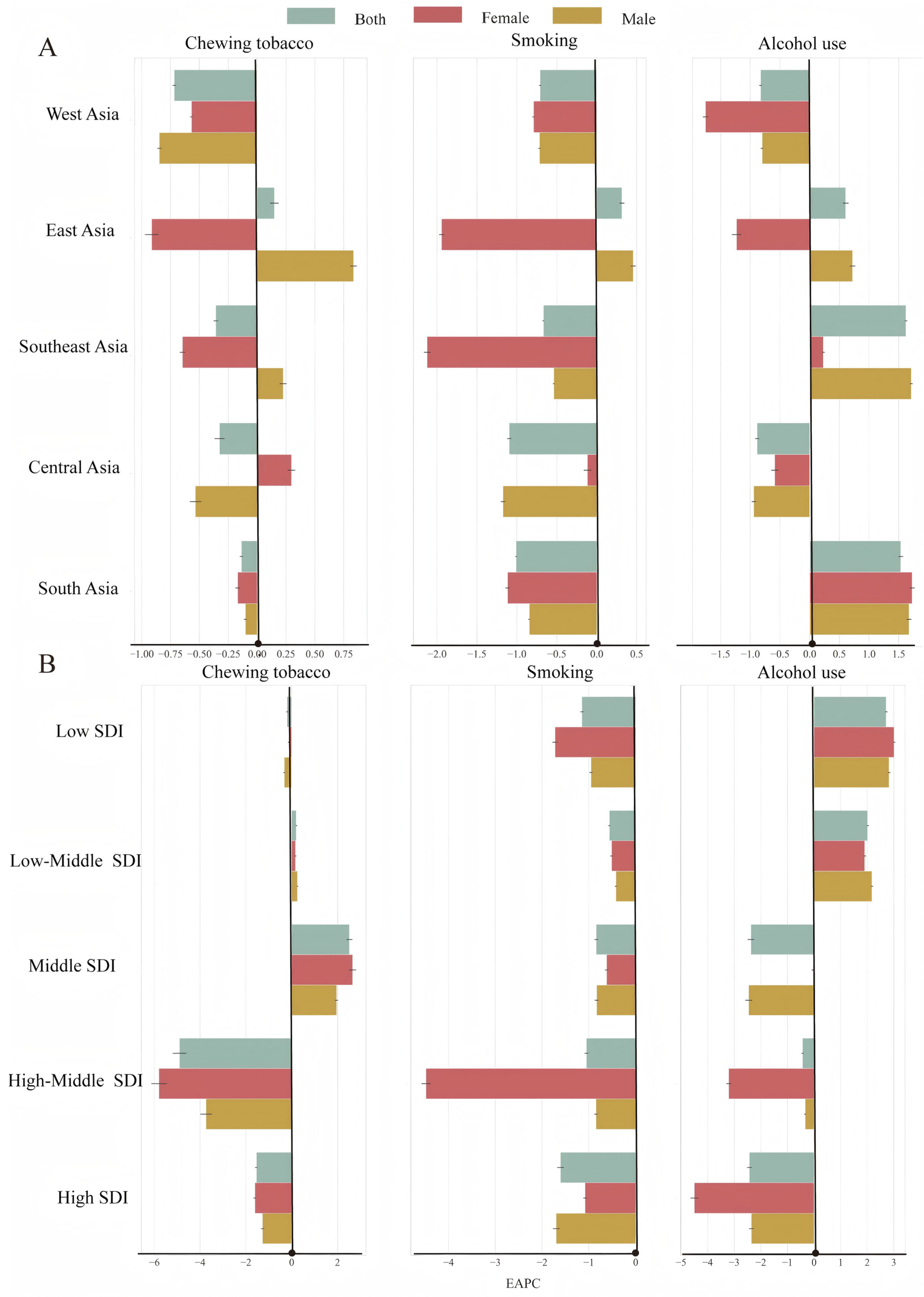
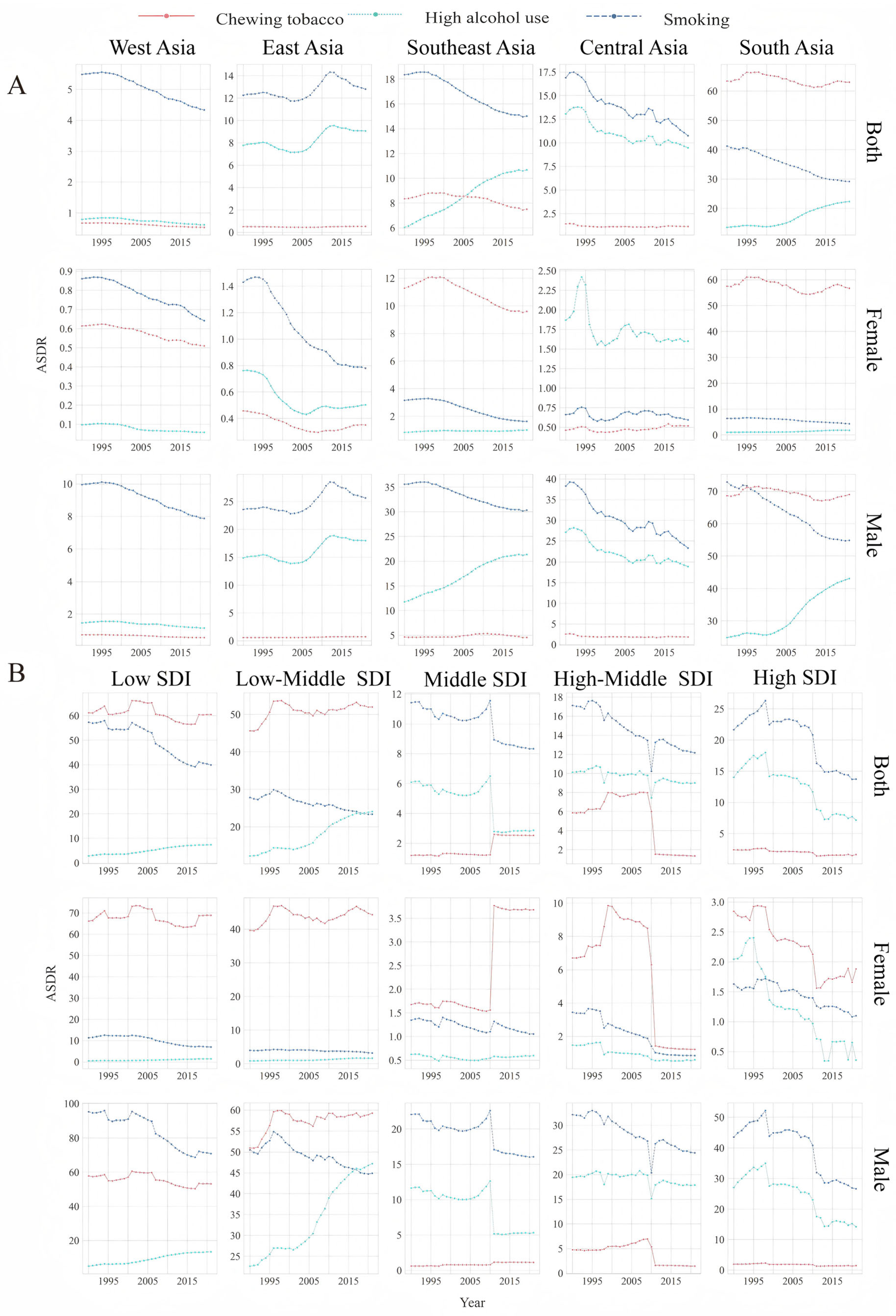
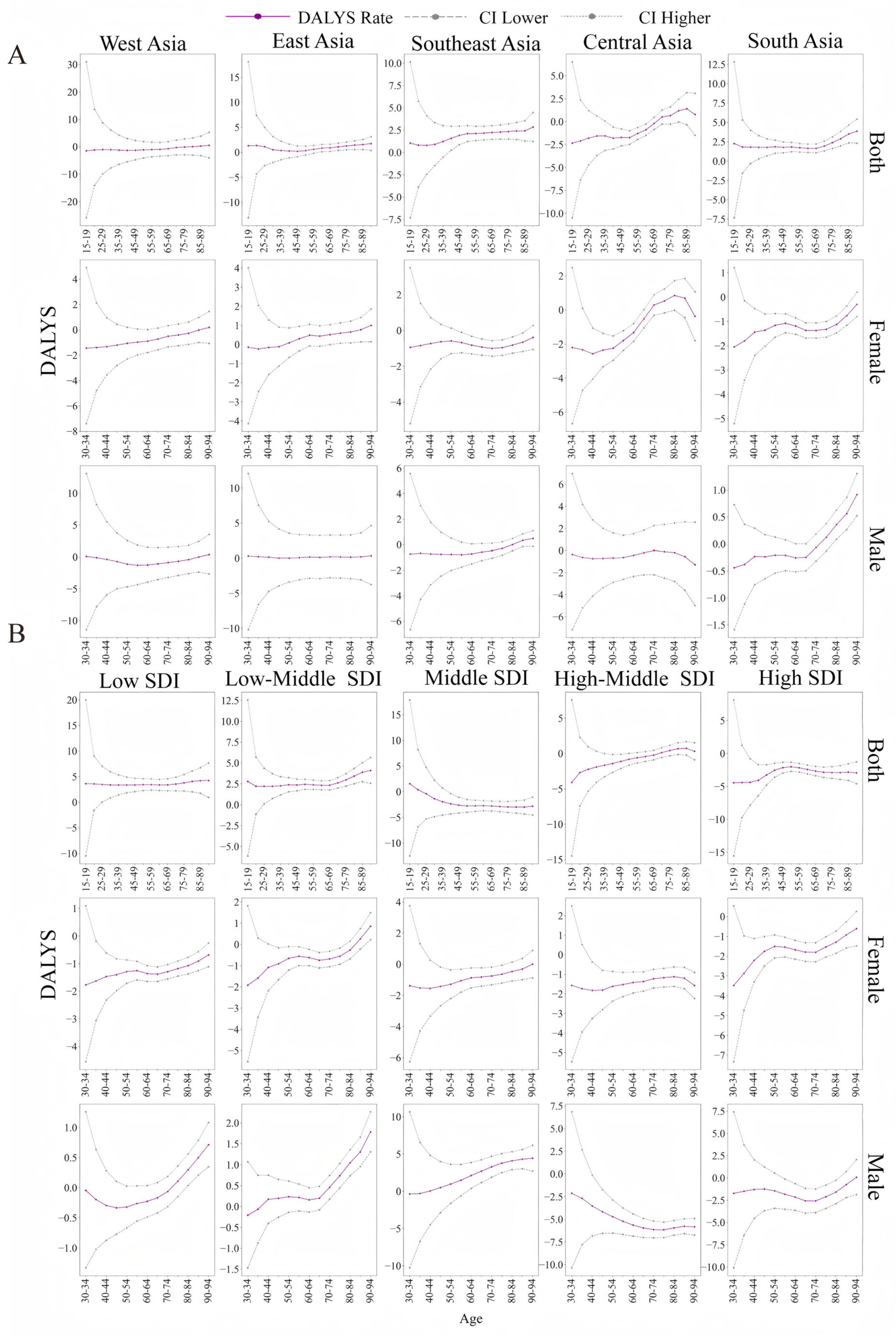
| Location Risk | 1990 | 2021 | 1990–2021 |
|---|---|---|---|
| ASDR (95% UI) | ASDR (95% UI) | EAPC (95% CI) | |
| Global | 69.26 (72.95 to −65.92) | 67.7141 (73.17 to 61.32) | −0.13 (−0.12 to −0.14) |
| Asia | 77.47 (71.14 to 83.80) | 82.23 (73.37 to 90.38) | 0.09 (0.08 to 0.10) |
| Gender | |||
| Male | 103.08 (92.31 to 114.86) | 110.85 (92.68 to 124.52) | 0.17 (0.16 to 0.17) |
| Female | 52.14 (46.21 to 57.61) | 54.55 (48.39 to 61.07) | −0.04 (−0.02 to −0.05) |
| Region | |||
| East Asia | 32.96 (28.12 to 37.65) | 31.88 (25.83 to 38.83) | 0.01 (−0.01 to 0.03) |
| West Asia | 19.04 (16.68 to 21.71) | 17.74 (15.64 to 20.22) | −0.17 (−0.16 to −0.17) |
| Cental Asia | 53.46 (49.82 to 57.83) | 42.26 (36.89 to 48.31) | −0.65 (−0.63 to −0.68) |
| South Asia | 174.46 (156.86 to 192.99) | 182.29 (157.06 to 203.68) | 0.02 (0.01 to 0.03) |
| Southeast Asia | 63.40 (55.13 to 71.04) | 60.97 (69.50 to 52.94) | −0.20 (−0.19 to −0.21) |
| SDI level | |||
| Low SDI | 208.84 (257.69 to 162.58) | 215.36 (279.07 to 162.27) | −0.10 (−0.08 to −0.13) |
| Low-middle SDI | 123.81 (108.94 to 138.72) | 147.51 (125.01 to 167.65) | 0.37 (0.35 to 0.39) |
| Middle SDI | 33.64 (27.57 to 39.53) | 33.61 (41.60 to 25.96) | 0.13 (0.10 to 0.15) |
| High-middle SDI | 55.32 (45.38 to 65.74) | 33.00 (25.87 to 41.54) | −1.76 (−1.33 to −1.21) |
| High SDI | 67.07 (57.49 to 78.27) | 46.81 (39.27 to 55.72) | −1.27 (−1.21 to −1.33) |
| Risk factor | |||
| Alcohol | 9.40 (6.67 to 11.92) | 13.26 (9.48 to 17.19) | 1.033 (1.005 to 1.06) |
| Chewing tobacco | 19.70 (14.79 to 25.15) | 20.29 (15.39 to 24.96) | −0.04 (−0.03 to −0.05) |
| Smoking | 20.94 (15.05 to 26.97) | 17.03 (11.61 to 22.48) | −0.57 (−0.56 to −0.58) |
| East Asia | West East | Central Asia | Southeast Asia | South Asia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | C | S | A | C | S | A | C | S | A | C | S | A | C | S | |
| Net Drift | 0.8 | 0.2 | 0.3 | −0.8 | −0.7 | −0.8 * | −0.9 * | −0.5 | −1.1 * | 1.9 * | −0.5 | 0.8 * | 2.0 * | −0.1 | −1.3 * |
| Local Drift age group | |||||||||||||||
| 15–20 | 1.3 | −1.5 | −2.4 | 1.0 | 2.3 | ||||||||||
| 20–25 | 1.4 | −1.2 | −2.0 | 0.8 | 1.8 | ||||||||||
| 25–30 | 1.1 | −1.0 | −1.8 | 0.8 | 1.8 | ||||||||||
| 30–35 | 0.5 | 0.3 | −0.1 | −1.1 | 0.1 | −1.4 | −1.6 | −0.4 | −2.2 | 0.9 | −0.7 | −1.0 | 1.8 | −0.4 | −2.1 |
| 35–40 | 0.4 | 0.2 | −0.2 | −1.2 | −0.1 | −1.4 | −1.6 | −0.6 | −2.3 | 1.2 | −0.7 | −0.8 | 1.8 | −0.4 | −1.8 |
| 40–45 | 0.3 | 0.2 | −0.2 | −1.3 | −0.4 | −1.3 | −1.8 | −0.7 | −2.6 | 1.6 | −0.7 | −0.7 | 1.9 | −0.2 | −1.4 |
| 45–50 | 0.2 | 0.02 | −0.1 | −1.3 | −0.7 | −1.2 | −1.7 | −0.7 | −2.7 | 1.9 | −0.8 | −0.6 | 1.8 | −0.2 | −1.4 |
| 50–55 | 0.3 | 0.02 | 0.1 | −1.1 | −1.1 | −1.1 | −1.7 | −0.7 | −2.2 | 2.1 | −0.8 | −0.6 | 1.8 | −0.2 | −1.2 |
| 55–60 | 0.6 | 0.1 | 0.3 | −1.0 | −1.3 | −1.0 | −1.3 | −0.6 | −1.8 | 2.1 | −0.8 | −0.7 | 1.7 | −0.2 | −1.1 |
| 60–65 | 0.8 | 0.2 | 0.5 | −1.0 | −1.3 | −0.9 | −0.9 | −0.4 | −1.3 | 2.2 | −0.7 | −0.8 | 1.7 | −0.3 | −1.2 |
| 65–70 | 0.9 | 0.1 | 0.4 | −0.7 | −1.1 | −0.7 | −0.2 | −0.2 | −0.5 | 2.2 | −0.6 | −0.9 | 1.7 | −0.3 | −1.4 |
| 70–75 | 1.1 | 0.2 | 0.5 | −0.3 | −0.9 | −0.5 | 0.5 | 0.0 | 0.3 | 2.3 | −0.5 | −1.0 | 1.9 | −0.1 | −1.4 |
| 75–80 | 1.3 | 0.2 | 0.6 | −0.1 | −0.7 | −0.4 | 0.7 | −0.1 | 0.5 | 2.3 | −0.3 | −1.0 | 2.4 | 0.1 | −1.3 |
| 80–85 | 1.4 | 0.2 | 0.7 | 0.04 | −0.4 | −0.3 | 1.2 | −0.2 | 0.9 | 2.4 | 0.0 | −0.8 | 2.9 | 0.4 | −1.1 |
| 85–90 | 1.6 | 0.2 | 0.8 | 0.3 | −0.0 | −0.0 | 1.4 | −0.6 | 0.7 | 2.4 | 0.3 | −0.7 | 3.5 | 0.6 | −0.8 |
| 90–95 | 1.7 | 0.4 | 1.0 | 0.5 | 0.4 | 0.2 | 0.8 | −1.3 | −0.4 | 2.8 | 0.5 | −0.4 | 3.9 | 0.9 | −0.3 |
| High SDI | High-Middle SDI | Middle SDI | Low-Middle SDI | Low SDI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | C | S | A | C | S | A | C | S | A | C | S | A | C | S | |
| Net Drift | −2.9 * | −1.7 * | −1.7 * | −0.9 * | −5.1 * | −1.5 * | −2.3 | 2.1 * | −0.9 * | 2.6 * | 0.4 * | −0.7 * | 3.5 * | −0.1 | −1.3 * |
| Local Drift age group | |||||||||||||||
| 15–20 | −4.5 | −4.1 | 1.5 | 2.8 | 3.6 | ||||||||||
| 20–25 | −4.4 | −2.7 | 0.4 | 2.2 | 3.6 | ||||||||||
| 25–30 | −4.4 | −2.2 | −0.4 | 2.2 | 3.5 | ||||||||||
| 30–35 | −4.1 | −1.7 | −3.5 | −1.9 | −2.1 | −1.6 | −1.4 | −0.4 | −1.4 | 2.2 | −0.2 | −1.9 | 3.4 | −0.0 | −1.8 |
| 35–40 | −3.3 | −1.5 | −2.9 | −1.7 | −2.7 | −1.7 | −2.0 | −0.3 | −1.5 | 2.3 | −0.1 | −1.6 | 3.4 | −0.2 | −1.6 |
| 40–45 | −2.6 | −1.3 | −2.2 | −1.4 | −3.5 | −1.8 | −2.4 | 0.1 | −1.6 | 2.4 | 0.2 | −1.1 | 3.4 | −0.3 | −1.5 |
| 45–50 | −2.2 | −1.2 | −1.8 | −1.1 | −4.2 | −1.8 | −2.6 | 0.5 | −1.4 | 2.4 | 0.2 | −0.9 | 3.4 | −0.3 | −1.4 |
| 50–55 | −2.0 | −1.5 | −1.5 | −0.8 | −4.7 | −1.6 | −2.8 | 1.0 | −1.3 | 2.5 | 0.2 | −0.7 | 3.4 | −0.3 | −1.3 |
| 55–60 | −2.2 | −1.8 | −1.6 | −0.6 | −5.2 | −1.5 | −2.8 | 1.5 | −1.1 | 2.4 | 0.2 | −0.6 | 3.5 | −0.3 | −1.3 |
| 60–65 | −2.4 | −2.2 | −1.7 | −0.4 | −5.6 | −1.4 | −2.7 | 2.1 | −0.9 | 2.3 | 0.2 | −0.6 | 3.4 | −0.2 | −1.4 |
| 65–70 | −2.7 | −2.6 | −1.8 | −0.2 | −6.0 | −1.4 | −2.8 | 2.7 | −0.8 | 2.3 | 0.2 | −0.8 | 3.4 | −0.2 | −1.4 |
| 70–75 | −2.9 | −2.6 | −1.8 | 0.1 | −6.1 | −1.2 | −2.9 | 3.3 | −0.8 | 2.6 | 0.5 | −0.7 | 3.6 | −0.1 | −1.3 |
| 75–80 | −2.9 | −2.1 | −1.5 | 0.4 | −6.1 | −1.2 | −3.0 | 3.8 | −0.6 | 3.0 | 0.7 | −0.6 | 3.8 | 0.1 | −1.2 |
| 80–85 | −2.9 | −1.6 | −1.3 | 0.7 | −5.9 | −1.1 | −3.0 | 4.1 | −0.5 | 3.4 | 1.1 | −0.3 | 4.1 | 0.3 | −1.1 |
| 85–90 | −2.8 | −0.7 | −0.9 | 0.7 | −5.8 | −1.2 | −3.0 | 4.3 | −0.3 | 3.9 | 1.3 | 0.3 | 4.2 | 0.5 | −0.9 |
| 90–95 | −3.0 | 0.1 | −0.6 | 0.3 | −5.8 | −1.6 | −2.8 | 4.4 | −0.0 | 4.1 | 1.8 | 0.9 | 4.3 | 0.7 | −0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Lu, X.; Ma, R.; Zeng, X. Spatiotemporal Dynamics in the Burden of Lip and Oral Cavity Cancer and Attributable Risk Factors in Asia (1990–2021). Healthcare 2025, 13, 1377. https://doi.org/10.3390/healthcare13121377
Lin D, Lu X, Ma R, Zeng X. Spatiotemporal Dynamics in the Burden of Lip and Oral Cavity Cancer and Attributable Risk Factors in Asia (1990–2021). Healthcare. 2025; 13(12):1377. https://doi.org/10.3390/healthcare13121377
Chicago/Turabian StyleLin, Dan, Xinping Lu, Ri Ma, and Xiaojuan Zeng. 2025. "Spatiotemporal Dynamics in the Burden of Lip and Oral Cavity Cancer and Attributable Risk Factors in Asia (1990–2021)" Healthcare 13, no. 12: 1377. https://doi.org/10.3390/healthcare13121377
APA StyleLin, D., Lu, X., Ma, R., & Zeng, X. (2025). Spatiotemporal Dynamics in the Burden of Lip and Oral Cavity Cancer and Attributable Risk Factors in Asia (1990–2021). Healthcare, 13(12), 1377. https://doi.org/10.3390/healthcare13121377








