Presence of Drug Interaction Between Penicillin and Hormonal Contraceptives in Women: A Scoping Review
Abstract
1. Introduction
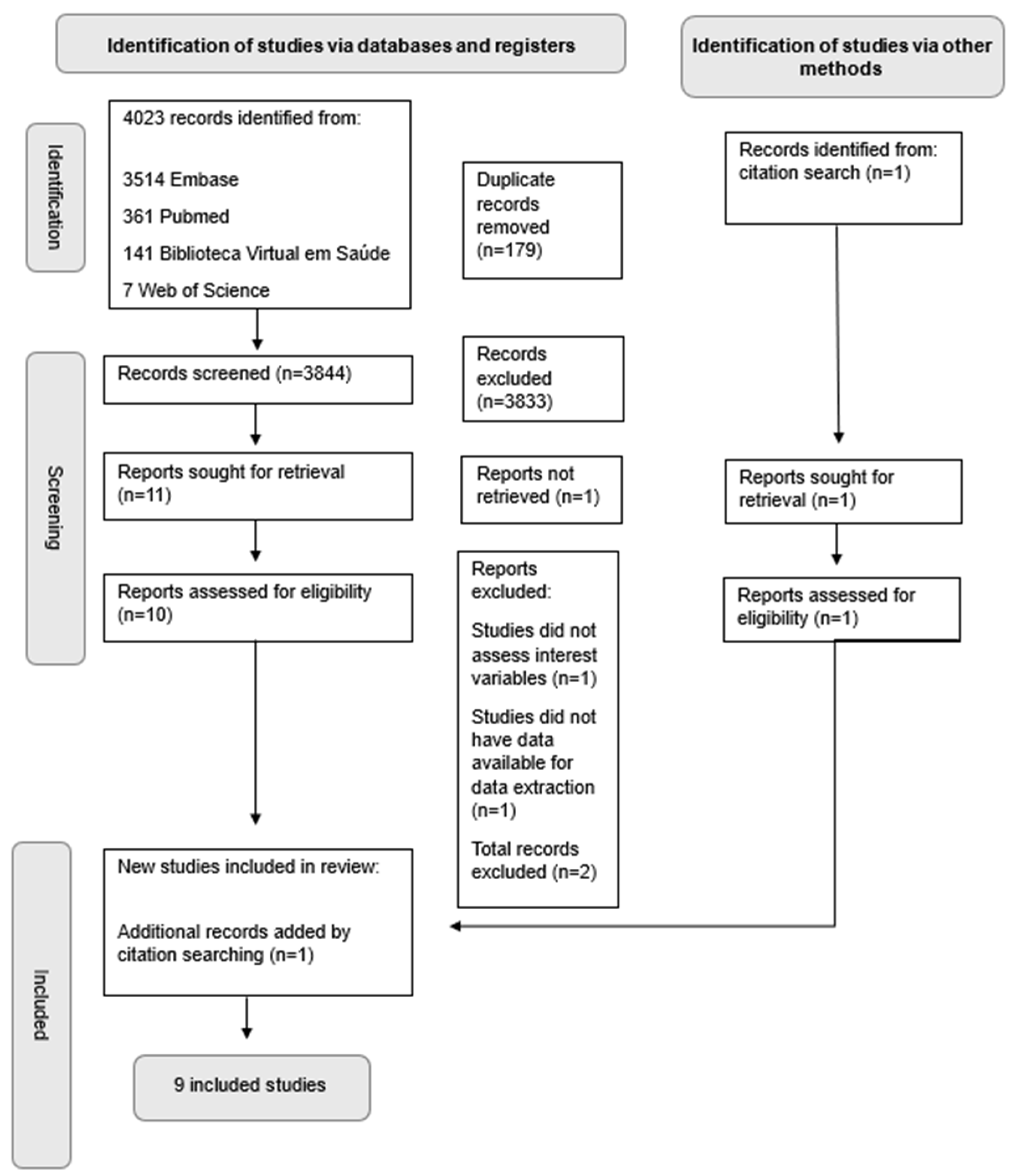
2. Materials and Methods
2.1. Exposure
2.2. Outcome
2.3. Eligibility Criteria
2.4. Information Sources and Search Strategies
2.5. Study Selection
2.6. Data Extraction
2.7. Data Synthesis
3. Results
3.1. Characteristics of Included Studies
3.2. Results of Individual Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| AMS | Antimicrobial Stewardship |
| BDS | Slone Epidemiology Center Birth Defects Study |
| CDC | Control and Prevention |
| COVID-19 | Corona Virus Disease 2019 |
| DIs | Drug Interactions |
| FSRH | UK’s Faculty of Sexual and Reproductive Healthcare |
| MeSH | Medical Subject Headings |
| NSAIDs | Nonsteroidal Anti-inflammatory DrugS |
| NBDPS | National Birth Defects Prevention Study |
| OC | Oral contraceptive |
| OSF | Open Science Framework platform |
| Prisma-ScR | Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews |
| PPIs | Proton Pump Inhibitors |
| UK | United Kingdom |
| USA | United States of America |
References
- Pereira, L.B.; Zanetti, M.O.B.; Rodrigues, J.P.V.; Pereira, L.R.L. Consumo de antibióticos em um hospital de alta complexidade: Padrão de utilização em diferentes enfermarias. Res. Soc. Dev. 2022, 11, e12011225573. [Google Scholar] [CrossRef]
- Quinn, R. Rethinking antibiotic research and development: World War II and the penicillin collaborative. Am. J. Public Health 2013, 103, 426–434. [Google Scholar] [CrossRef]
- Gaynes, R. The Discovery of Penicillin—New Insights After More Than 75 Years of Clinical Use. Emerg. Infect. Dis. 2017, 23, 849–853. [Google Scholar] [CrossRef]
- Capa, N. Alexander Fleming E a Descoberta Da Penicilina. J. Bras. Patol. Med. Lab. 2009, 45, 1. [Google Scholar] [CrossRef]
- Grumach, A.S.; Ferraroni, N.R. O papel da penicilina na medicina moderna. DST—J. Bras. Doenças Sex. Transm. 2006, 18, 7–13. Available online: https://www.bjstd.org/revista/article/view/1176 (accessed on 3 March 2024).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Grau, S.; Echeverria-Esnal, D.; Gómez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P.; et al. Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic. Antibiotics 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Sokolović, D.; Drakul, D.; Vujić-Aleksić, V.; Joksimović, B.; Marić, S.; Nežić, L. Antibiotic consumption and antimicrobial resistance in the SARS-CoV-2 pandemic: A single-center experience. Front. Pharmacol. 2023, 14, 1067973. [Google Scholar] [CrossRef]
- Escola Politécnica de Saúde Venâncio/FIOCRUZ. Available online: https://www.epsjv.fiocruz.br/noticias/reportagem/resistencia-aos-antibioticos-um-problema-que-se-agrava (accessed on 22 May 2025).
- dos Santos, M.L.; Falcão, A.H.P.B.M.; de Jesus, M.M.R.; Oliveira, A.P.S.; Baia, S.J.A.A.; Brito, F.I.S. Increased antibiotic consumption in hospital settings during the Covid-19 pandemic. Braz. J. Health Rev. 2023, 6, 2341–2350. [Google Scholar] [CrossRef]
- Magalhães, V.; Lima, F.C.Y.; Lopes, A.M.M.; Lopes, S.G.; Rousseau, A.M.F.M.E.; Dias, O.C.M.; Menescal, J.I.; Fernandes, C.M.C.L.; Nunes, L.F.F.; Silveira, M.R.; et al. Desafios no combate à resistência antimicrobiana: Abordagem global e local. Braz. J. Implantol. Health Sci. 2025, 7, 248–257. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Antibiotic Use and Stewardship in the United States, 2023 Update: Progress and Opportunities; U.S. Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023.
- Centers for Disease Control and Prevention (CDC). Antibiotic Use and Stewardship in the United States, 2024 Update: Progress and Opportunities; U.S. Centers for Disease Control and Prevention: Atlanta, GA, USA, 2024.
- Zay, Y.K.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2253806. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kuča, K. Antibiotics and Antibiotic Resistance-Flipsides of the Same Coin. Curr. Pharm. Des. 2022, 28, 2312–2329. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.B.; Archanjo, I.P.; Cambraia, M.F.; Miranda, N.R.; Miranda, P.R.; Augusto, P.; Dias, A.M.N.; Mendes, N.B.E.S.; Corrêa, C.R.; Jácome, G.P.O. Profile of patients and antimicrobial resistance of urinary diseases in a tertiary hospital in Juiz de For a—MG. Braz. J. Health Rev. 2023, 6, 14654–14669. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence-Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; He, D.L.; Liao, D.; Khan, M.Z.I.; Huang, C.; He, Y. Strategies and progresses for enhancing targeted antibiotic delivery. Adv. Drug Deliv. Rev. 2022, 189, 114502. [Google Scholar] [CrossRef]
- National Health Surveillance Agency (Anvisa). Resolution—RDC nº 60, of December 17, 2010. Establishes Warning Phrases for Active Ingredients and Excipients in Leaflets and Medication Labeling; Official Gazette of the Union: Brasilia, Brazil, 2010. [Google Scholar]
- Butkiewicz, M.; Restrepo, N.A.; Haines, J.L.; Crawford, D.C. Drug-drug interaction profiles of medication regimens extracted from a de-identified electronic medical records system. AMIA Jt. Summits Transl. Sci. Proc. 2016, 2016, 33–40. [Google Scholar]
- Maseda, D.; Ricciotti, E. NSAID-Gut Microbiota Interactions. Front. Pharmacol. 2020, 7, 1153. [Google Scholar] [CrossRef]
- Moreels, N.; Boven, A.; Gressani, O.; Andersson, F.L.; Vlieghe, E.; Callens, S.; Engstrand, L.; Simin, J.; Brusselaers, N. The combined effect of systemic antibiotics and proton pump inhibitors on Clostridioides difficile infection and recurrence. J. Antimicrob. Chemother. 2024, 79, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Cwiak, C.; Kaunitz, A.M. Hormonal Contraception: Systemic Estrogen and Progestin Preparations. Clin. Obstet. Gynecol. 2021, 64, 721–738. [Google Scholar] [CrossRef]
- Tikkanen, M.J.; Adlercreutz, H.; Pulkkinen, M.O. Effects of antibiotics on oestrogen metabolism. Br. Med. J. 1973, 2, 369. [Google Scholar] [CrossRef]
- Tsabai, C. Potential Drug Interactions in Patients Taking Oral Contraceptive Pills. Am. Fam. Physician 2019, 100, 599–600. [Google Scholar] [PubMed]
- Aronson, J.K.; Ferner, R.E. Analysis of reports of unintended pregnancies associated with the combined use of non-enzyme-inducing antibiotics and hormonal contraceptives. BMJ Evid. Based Med. 2021, 26, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.B.; Haddad, L.B.; Nanda, K.; Curtis, K.M. Drug interactions between non-rifamycin antibiotics and hormonal contraception: A systematic review. Am. J. Obstet. Gynecol. 2018, 218, 88–97.e14. [Google Scholar] [CrossRef]
- Trindade, R.E.D.; Siqueira, B.B.; Paula, T.F.; Felisbino-Mendes, M.S. Contraception use and family planning inequalities among Brazilian women. Cienc. Saude Colet. 2021, 26, 3493–3504. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance: A Threat to Global Health Security; Executive Board 115: New York, NY, USA, 2005. [Google Scholar]
- Lushniak, B.D. Antibiotic resistance: A public health crisis. Public Health Rep. 2014, 129, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Daunt, R.; Curtin, D.; O’Mahony, D. Polypharmacy stewardship: A novel approach to tackle a major public health crisis. Lancet Healthy Longev. 2023, 4, e228–e235. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Brunton, L.L. Goodman & Gilman: As Bases Farmacológicas da Terapêutica, 2nd ed.; McGraw Hill Brasil: Porto Alegre, Brazil, 2012. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Bainton, R. Interaction between antibiotic therapy and contraceptive medication. Oral. Surg. Oral. Med. Oral. Pathol. 1986, 61, 453–455. [Google Scholar] [CrossRef]
- Russo, S.; Tapparelli, E.; D’alessio, L. Contributo allo studio sulla interferenza tra farmaci e contraccettivi orali. Clin. Exp. Obstet. Gynecol. 1979, 6, 70–72. [Google Scholar]
- Silber, T.J. Apparent oral contraceptive failure associated with antibiotic administration. J. Adolesc. Health Care. 1983, 4, 287–289. [Google Scholar] [CrossRef]
- Helms, S.E.; Bredle, D.L.; Zajic, J.; Jarjoura, D.; Brodell, R.T.; Krishnarao, I. Oral contraceptive failure rates and oral antibiotics. J. Am. Acad. Dermatol. 1997, 36, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.T.; Riddoch, G.; Duncombe, P.; Welberry, L.; Chick, P.; Weisberg, E.; Leavesley, G.M.; Baker, H.W. Inadvertent pregnancies in oral contraceptive users. Med. J. Aust. 1989, 150, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Broe, A.; Stage, T.B.; Brøsen, K.; Hallas, J.; Damkier, P. Use of Dicloxacillin and Risk of Pregnancy among Users of Oral Contraceptives. Basic. Clin. Pharmacol. Toxicol. 2018, 123, 288–293. [Google Scholar] [CrossRef]
- Toh, S.; Mitchell, A.A.; Anderka, M.; de Jong-van den Berg, L.T.; Hernández-Díaz, S.; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—A case-crossover study. Contraception 2011, 83, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Back, D.J.; Grimmer, S.F.; Orme, M.L.; Proudlove, C.; Mann, R.D.; Breckenridge, A.M. Evaluation of Committee on Safety of Medicines yellow card reports on oral contraceptive-drug interactions with anticonvulsants and antibiotics. Br. J. Clin. Pharmacol. 1988, 25, 527–532. [Google Scholar] [CrossRef]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef]
- Turcato, T.C.C.; Correa, M.A. Interação medicamentosa pertinente a fármacos antibióticos e agentes anticoncepcionais femininos. Universitas 2017, 1, 1–13. [Google Scholar]
- Trussell, J.; Portman, D. The creeping Pearl: Why has the rate of contraceptive failure increased in clinical trials of combined hormonal contraceptive pills? Contraception 2013, 88, 604–610. [Google Scholar] [CrossRef]
- Archer, J.S.; Archer, D.F. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J. Am. Acad. Dermatol. 2002, 46, 917–923. [Google Scholar] [CrossRef]
- Simmons, K.B.; Haddad, L.B.; Nanda, K.; Curtis, K.M. Drug interactions between rifamycin antibiotics and hormonal contraception: A systematic review. BJOG 2018, 125, 804–811. [Google Scholar] [CrossRef]
- Palomo, L.C.; Simioni, P.U.; Berro, E.C. Interações Medicamentosas Entre Anticoncepcionais Orais E Antibióticos: Uma Breve Revisão. Visão Acad. 2022, 23. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Siemens, S.; Slayter, K.; Mandell, L. Antibiotic and oral contraceptive drug interactions: Is there a need for concern? Can. J. Infect. Dis. 1999, 10, 429–433. [Google Scholar] [CrossRef]
- Roberts, M.S.; Magnusson, B.M.; Burczynski, F.J.; Weiss, M. Enterohepatic circulation: Physiological, pharmacokinetic and clinical implications. Clin. Pharmacokinet. 2002, 41, 751–790. [Google Scholar] [CrossRef] [PubMed]
- Ratain, M.J.; Plunkett, W.K. Principles of Pharmacokinetics. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Prada-Ramallal, G.; Takkouche, B.; Figueiras, A. Bias in pharmacoepidemiologic studies using secondary health care databases: A scoping review. BMC Med. Res. Methodol. 2019, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, P.C.; Bos, J.H.; de Jong van den Berg, L.T. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol. Drug Saf. 2012, 21, 865–871. [Google Scholar] [CrossRef]
- Jacomini, L.C.L.; Silva, N.A. Interações medicamentosas: Uma contribuição para o uso racional de imunossupressores sintéticos e biológicos. Rev. Bras. Reumatol. 2011, 51, 168–174. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Medical Eligibility Criteria for Contraceptive Use, 5th ed.; World Health Organization: New York, NY, USA, 2015. [Google Scholar]
- Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm. Rep. 2016, 65, 1–103. [Google Scholar] [CrossRef]
- Faculty of Sexual and Reproductive Healthcare (FSRH). Drug Interactions with Hormonal Contraception; Clinical Guidance; FSRH: London, UK, 2020. [Google Scholar]
| Authors | Year | Journal | Study Design | Objectives of the Study | Duration of Study | Sample Size | Age (Years) | Country | Source of Information | Unintended Pregnancy Confirmed | Exposure | Hormones Present in Contraceptives | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Russo S, Tapparelli E, D’alessio L. [36] | 1979 | Clin ExpObst Gyn | Case series | Interactions between steroidal oral contraceptives and other drugs, with a special focus on antibiotics | No information | 4 patients with DI | Mean = 24.8 | Italy | No information | Pregnancy test | OC * and oral ampicillin | Norgestrel and Ethinylestradiol |
| 2 | Silber, TJ. [37] | 1983 | J Adolesc Healthcare | Case report | To report a clinical case of possible contraceptive failure associated with the concomitant use of an antibiotic (oxacillin) and an oral contraceptive in an adolescent | Not applicable | 1 patient | 18 | USA | Adolescent clinic | Beta subunit human chorionic gonadotropin in serum test | OC and oxacillin sodium | Norethindrone and estradiol |
| 3 | Bainton, R. [35] | 1986 | Oral Surg Oral Med Oral Pathol | Case report | To report a clinical case of contraceptive failure associated with the concomitant administration of a long-acting penicillin and a low-dose oral contraceptive, and to discuss the possible pharmacological mechanisms of this interaction | Not applicable | 1 patient | 19 | UK | Oral surgery clinic | Pregnancy test | OC and fortified benethamine penicillin injection | Ethinyl estradiol and levonorgestrel |
| 4 | Back et al. [42] | 1988 | Br. J. Clin. Pharmac. | Drug adverse surveillance analysis. | Analysis of adverse reactions to evaluate data on unintended pregnancies in women using OC concomitantly with antiepileptic drugs or antibiotics | 1968–1984 | All yellow cards (with drug adverse reaction) | No information | UK | All yellow cards which indicated a pregnancy in a woman taking an OC plus an antibiotic | Self-report | OC and penicillin | Progestogen, progestogen with estrogens, biphasic, and triphasic preparations—reports indicate that some contained ethinylestradiol and levonorgestrel, though the specific combinations were not specified. |
| 5 | Kovacs et al. [39] | 1989 | Med J Aust | Cross-sectional | To determine the factors associated with unintended pregnancies in OC users and to compare the percentage of OC types used by patients with unintended pregnancies with the national market usage distribution during the same period | 1985–1986 | 72 patients with DI | Range-18–38 years | Australia | The Family Planning Association’s clinics and several centers which undertook terminations of pregnancy | No report | OC and penicillin | Triphasic, 30-µg ethinyloestradiol, 50-µg ethinyloestradiol, norethisterone, and progesterone |
| 6 | Helms et al. [38] | 1997 | J Am Acad Dermatol. | Cross- sectional | To examine the effect of commonly prescribed oral antibiotics (tetracyclines, penicillins, cephalosporins) on the failure rate of OC | No information | 356 patients | 15–19 years of age = 77; 20–24 years of age = 118; 25–29 years of age = 77; 30–34 years of age = 64; 35 and older = 20 | USA | Review of records in three private dermatology offices | Chart record (if reported by the patient to the physician during regular visits) as well as by the survey responses, and confirmed with a follow-up telephone call | OC and oral antibiotics (minocycline, cephalosporin, and penicillin) | No information |
| 7 | Toh et al. [41] | 2011 | Contraception | Case- crossover | To examine the effect of concomitant antibiotic treatment on the risk of breakthrough pregnancy among users of combined OC | 1997–2008 | 1330 patients | <20 = 225; 20–24 = 440; 25–29 = 353; 30–34 = 215; ≥35 = 97; Unknown = 0 | USA | Participants of the Slone Epidemiology Center Birth Defects Study (BDS) and the National Birth Defects Prevention Study (NBDPS) | Trained interviewers using a computer-assisted telephone interview | OC and oral antibiotics | It mentions that they are combined oral contraceptives and that it excluded users of progestin-only OC |
| 8 | Pottegård et al. [40] | 2018 | Basic Clin Pharmacol Toxicol | Case-crossover | To investigate whether the use of dicloxacillin is associated with an increased risk of unintended pregnancy among users of OC | 1997–2015 | 364 women | Median 23 [interquartile range (IQR) 19–29] | Denmark | The researchers sampled women with seemingly unintended pregnancy from two separate data sources: the Abortion Registry (elective abortion) and the Medical Birth Registry (women giving birth)—1997–2015. For both groups we found that they had used oral contraceptives at the time of conception. | No information | OC and dicloxacillin | No information |
| 9 | Aronson JK, Ferner RE. [26] | 2021 | BMJ Evid Based Med | Cross-sectional | To reassess the hypothesis that non-enzyme-inducing antibiotics (such as penicillins, cephalosporins, tetracyclines, etc.) reduce the effectiveness of OC, leading to unintended pregnancies | Not applicable | 74,623 with DI | No information | UK | Reports of suspected adverse drug reactions published by the Medicines and Healthcare products Regulatory Agency (MHRA) for information on suspected adverse drug reactions between 1963 and July 2018. To be included, reports had to mention exposure to a drug of interest linked to a report of an unintended pregnancy | Self-report | OC and oral antibiotics (amoxicillin, ampicillin, cephalexin, ciprofloxacin, erythromycin, metronidazole, nitrofurantoin, oxytetracycline, and trimethoprim) | No information |
| Name of the Medicine | Dose and Duration of Prescribed Penicillin | Number of Women Who Became Pregnant | Author’s Recommendations | |
|---|---|---|---|---|
| 1 | All patients (n = 4) received ampicillin and information on OC * was available for only one woman (Norgestrel 0.25 mg + Etinilestradiol 0.05 mg) | No information | 1 pregnancy from 4 patients | Need to carefully avoid the simultaneous prescription of antibiotics and hormonal contraceptives and inform the patient of this possibility. It can be added that not only the listed drugs, but also the use of any other drug, while taking oral contraceptives, must be carefully screened. It is believed that new studies can highlight new interference |
| 2 | OC: mg norethindrone/0.035 mg estradiol. Antibiotics: oxacillin sodium | 500 mg oxacillin sodium every 6 h for 6 weeks | 1 pregnancy | Advising a different hormonal contraceptive method or an additional contraceptive modality may be indicated in adolescents taking long-term antibiotic medication |
| 3 | OC: ethinyl estradiol 30 mcg and levonorgestrel 150 mcg; Antibiotic: fortified benethamine penicillin injection | Benethamine penicillin 500,000 I.U.; Procaine penicillin 250,000 I.U.; Benzyl penicillin 500,000 I.U | 1 pregnancy | Advising a different hormonal contraceptive method or an additional contraceptive modality may be indicated in adolescents taking long-term antibiotic medication |
| 4 | OC: estrogen, progestogen, biphasic, triphasic, pill. Antibiotics: penicillin | No information | 32 self-reports of DI between penicillin and OC from 63 registers | No recommendation |
| 5 | OC: triphasic, ethinyloestradiol 30 mcg, ethinyloestradiol 50 mcg, norethisterone, progesterone-only. Antibiotics: penicillin | No information | 22 self-reports of DI between penicillin and OC from 72 registers | Additional contraceptive precautions must be taken until at least seven continuous tablets have been taken after an episode which may impair the efficacy of the drug |
| 6 | OC: not reported; antibiotics: minocycline (tetracyclines)—3 patients, cephalosporin (cephalosporins)—2 patients and no penicillin | No information | 5 self-reports of DI between penicillin and OC from 356 registers | The difference in failure rates of OCs when taken concurrently with antibiotics commonly used in dermatology versus OC use alone suggests that these antibiotics do not increase the risk of pregnancy. Physicians and patients need to recognize that the expected OC failure rate, regardless of antibiotic use, is at least 1% per year; it is not yet possible to predict in whom OCs may fail |
| 7 | OC: not reported; antibiotics: ampicillin/amoxicillin and others | No information | There is no difference in the frequency of use of ampicillin/amoxicillin between the case period (4 weeks before conception) and the control period (4–8 weeks before conception) | No association was found between concomitant antibiotic use and the risk of breakthrough pregnancy among OC users. However, due to limited power and potential carryover effects, findings from the study cannot rule out an elevated risk of OC failure among antibiotic users |
| 8 | OC: combination oral contraceptives, progestogen oral contraceptives; antibiotics: dicloxacillin | No information | There is no difference in the use of dicloxacillin at the time of ovulation and control windows | The results implied no association between the use of dicloxacillin and the risk of oral contraceptive failure. However, oral contraceptive failure may have severe social consequences. It is suggested supplementary physical barrier methods be used until 2 weeks after discontinuation of dicloxacillin, at least until further studies confirm the lack of any association |
| 9 | Oral contraceptive and oral antibiotics (amoxicillin, ampicillin, cephalexin, ciprofloxacin, erythromycin, metronidazole, nitrofurantoin, oxytetracycline, and trimethoprim) | No information | 46 unintended pregnancies (62 per 100,000, (95% CI 44 to 79) in those who took antibiotics (amoxicillin, ampicillin, cephalexin, ciprofloxacin, erythromycin, metronidazole, nitrofurantoin, oxytetracycline, and trimethoprim). Antibiotics increased the odds of unintended pregnancy by 6.7-fold | This study provides a signal that antibacterial drugs may reduce the efficacy of hormonal contraceptives. Women taking hormonal contraceptives should be warned that antibiotics may impair their effectiveness. Extra precautions can be taken during a course of antibiotics; an unintended pregnancy is a life-changing event |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis-Oliveira, J.; Cruz, A.J.S.; Guimarães, N.S.; Abreu, M.H.N.G. Presence of Drug Interaction Between Penicillin and Hormonal Contraceptives in Women: A Scoping Review. Healthcare 2025, 13, 1364. https://doi.org/10.3390/healthcare13121364
Reis-Oliveira J, Cruz AJS, Guimarães NS, Abreu MHNG. Presence of Drug Interaction Between Penicillin and Hormonal Contraceptives in Women: A Scoping Review. Healthcare. 2025; 13(12):1364. https://doi.org/10.3390/healthcare13121364
Chicago/Turabian StyleReis-Oliveira, Jennifer, Alex Junio S. Cruz, Nathalia S. Guimarães, and Mauro Henrique N. G. Abreu. 2025. "Presence of Drug Interaction Between Penicillin and Hormonal Contraceptives in Women: A Scoping Review" Healthcare 13, no. 12: 1364. https://doi.org/10.3390/healthcare13121364
APA StyleReis-Oliveira, J., Cruz, A. J. S., Guimarães, N. S., & Abreu, M. H. N. G. (2025). Presence of Drug Interaction Between Penicillin and Hormonal Contraceptives in Women: A Scoping Review. Healthcare, 13(12), 1364. https://doi.org/10.3390/healthcare13121364






