Comparison of the Frailty Phenotype and the Korean Version of the FRAIL Scale
Abstract
1. Introduction
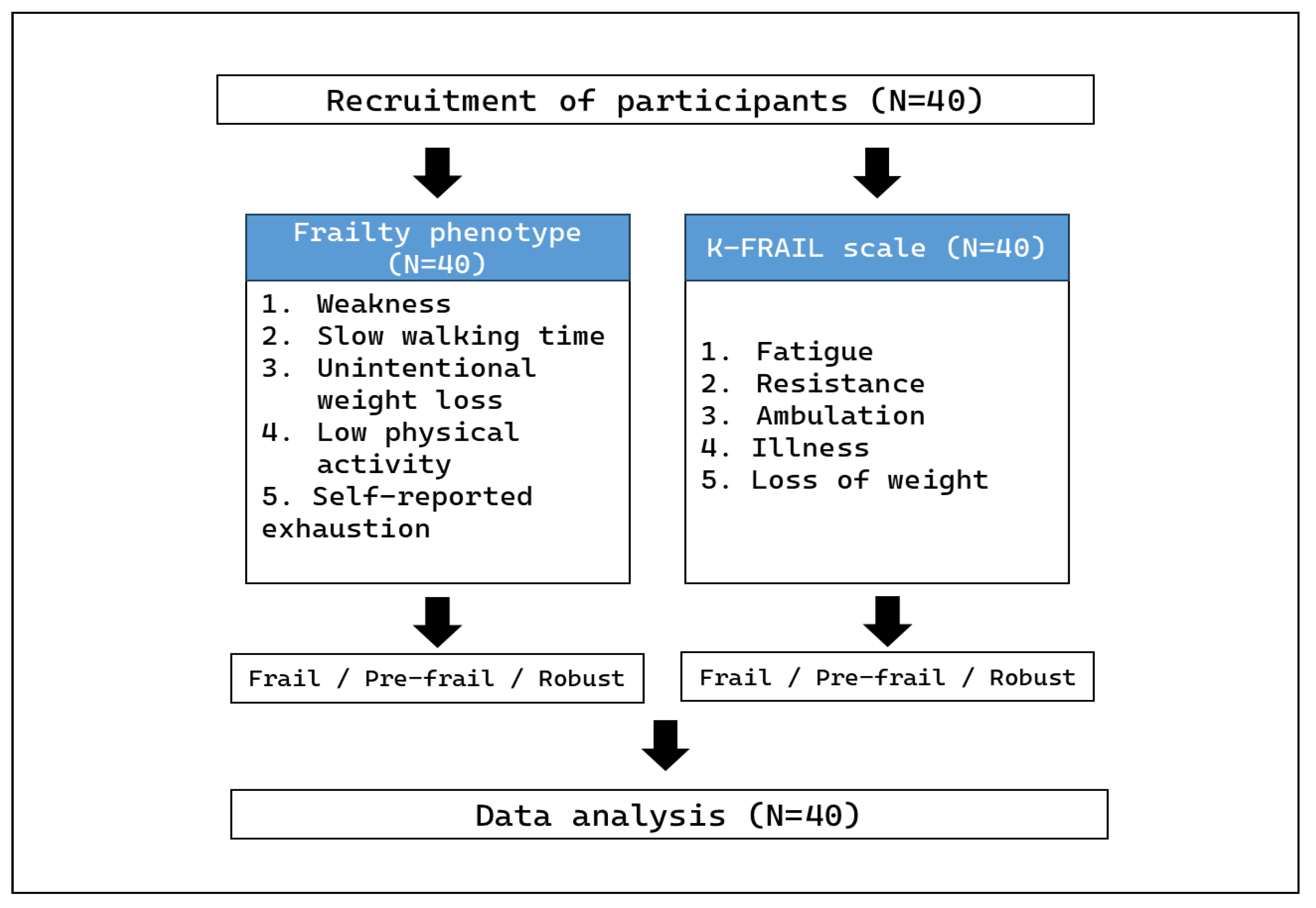
2. Materials and Methods
2.1. Participant Eligibility Criteria
2.2. Frailty Phenotype
2.2.1. Weakness (Measured by Grip Strength)
2.2.2. Slow Walking Speed
2.2.3. Unintentional Weight Loss
2.2.4. Low Physical Activity
2.2.5. Self-Reported Exhaustion
2.3. K-FRAIL Scale
2.3.1. Fatigue
2.3.2. Resistance
2.3.3. Ambulation
2.3.4. Illness
2.3.5. Weight Loss
2.4. Statistical Analysis
3. Results
3.1. The Participant Characteristics
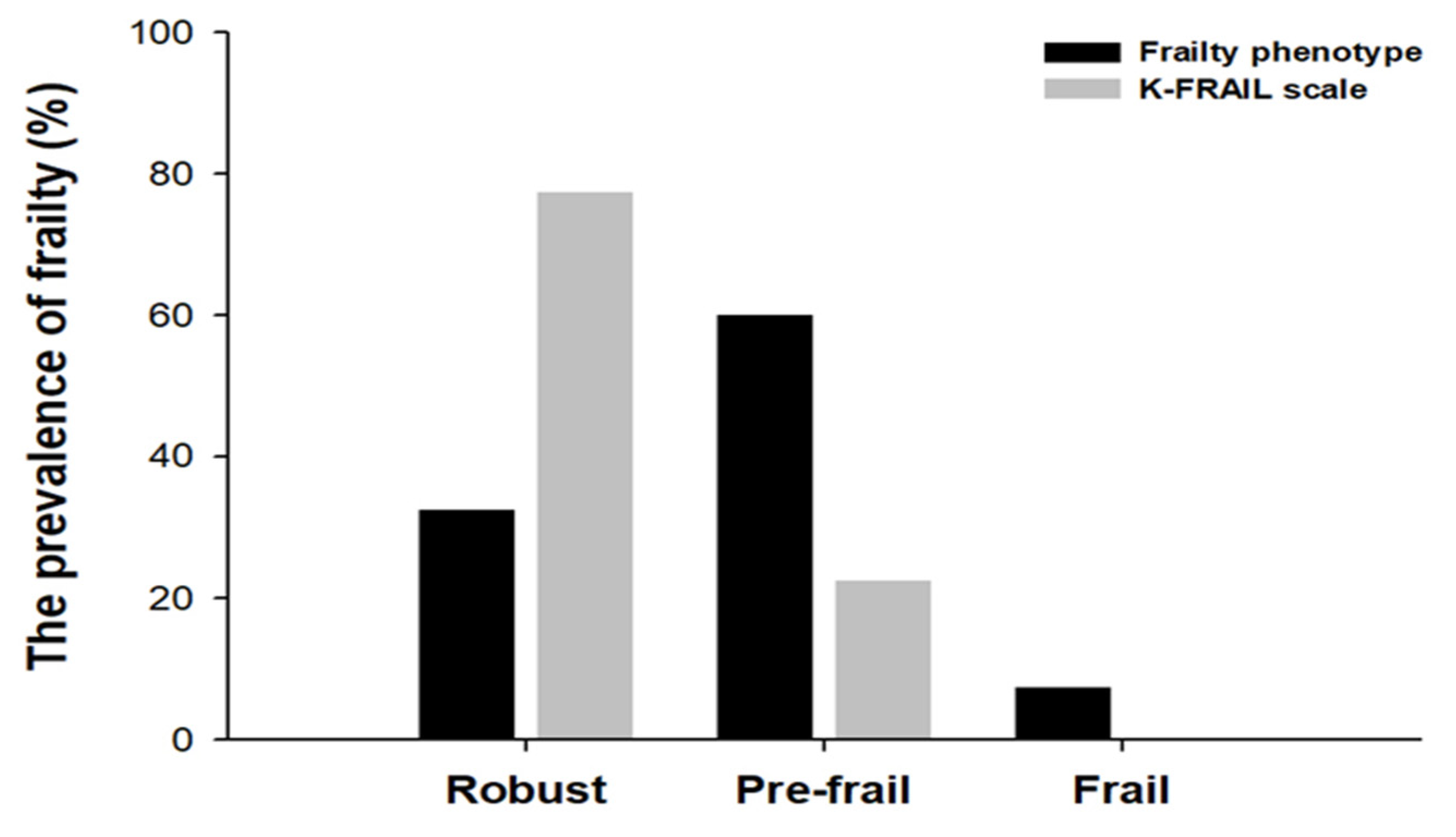
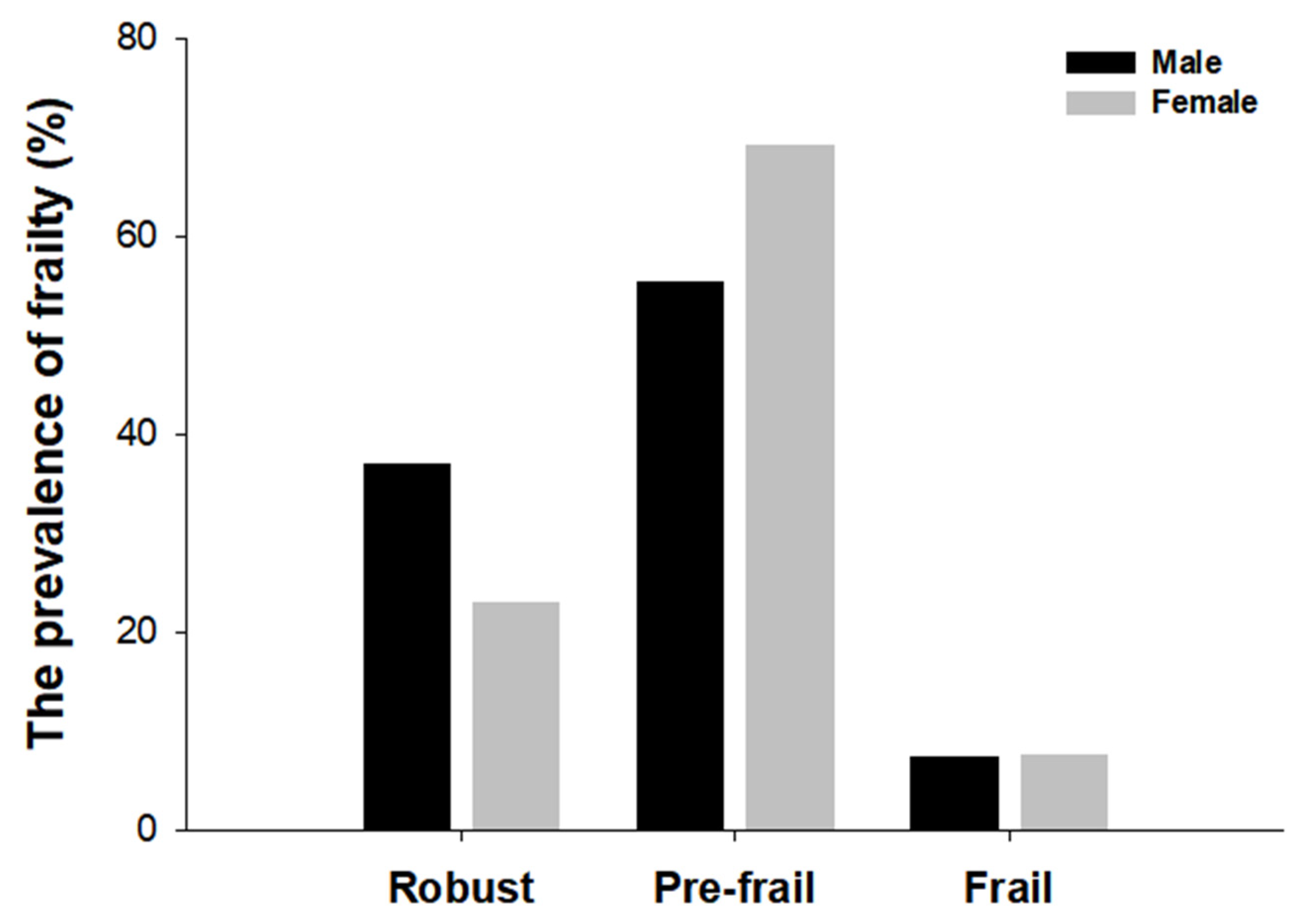
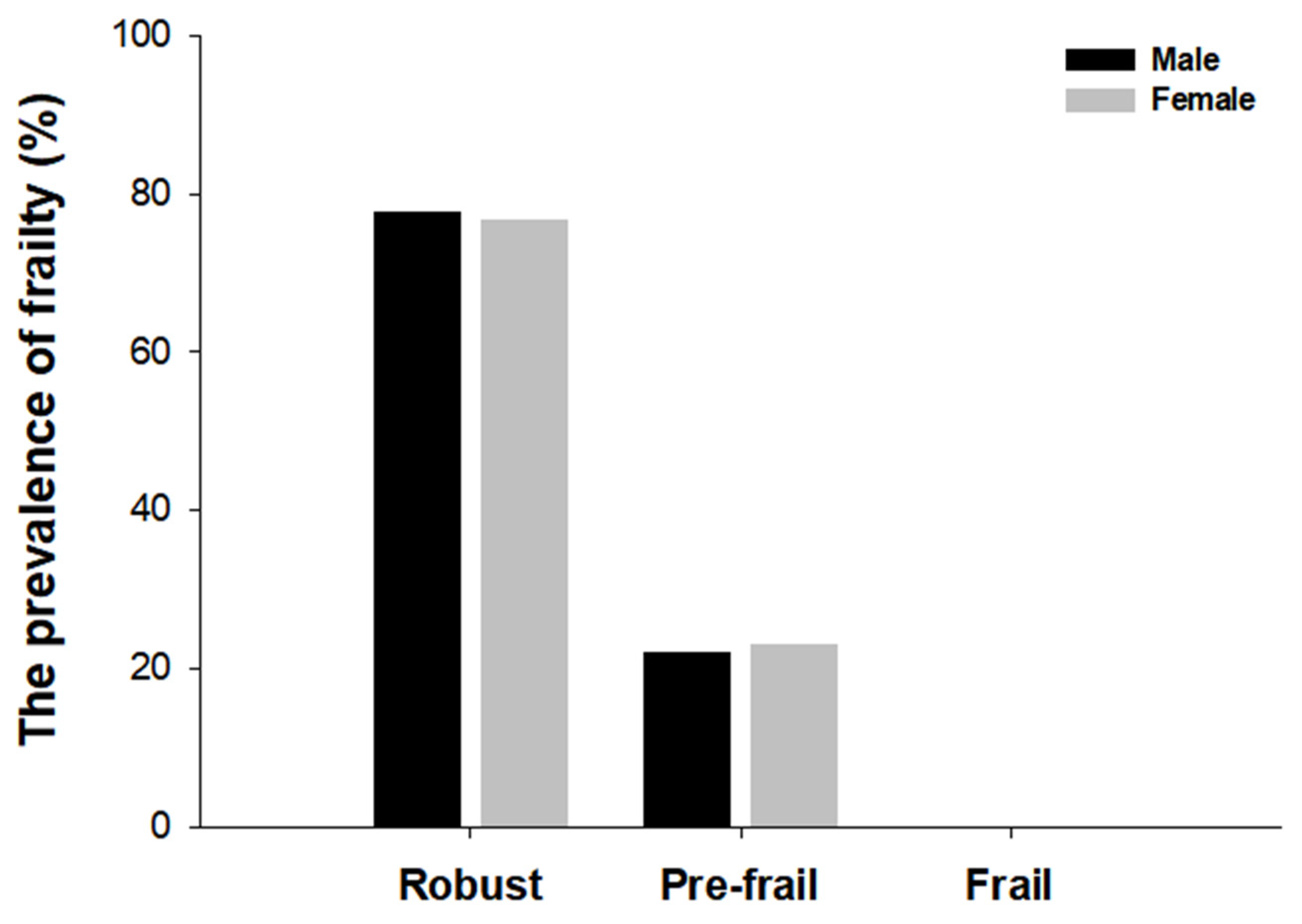
3.2. The Prevalence of Frailty
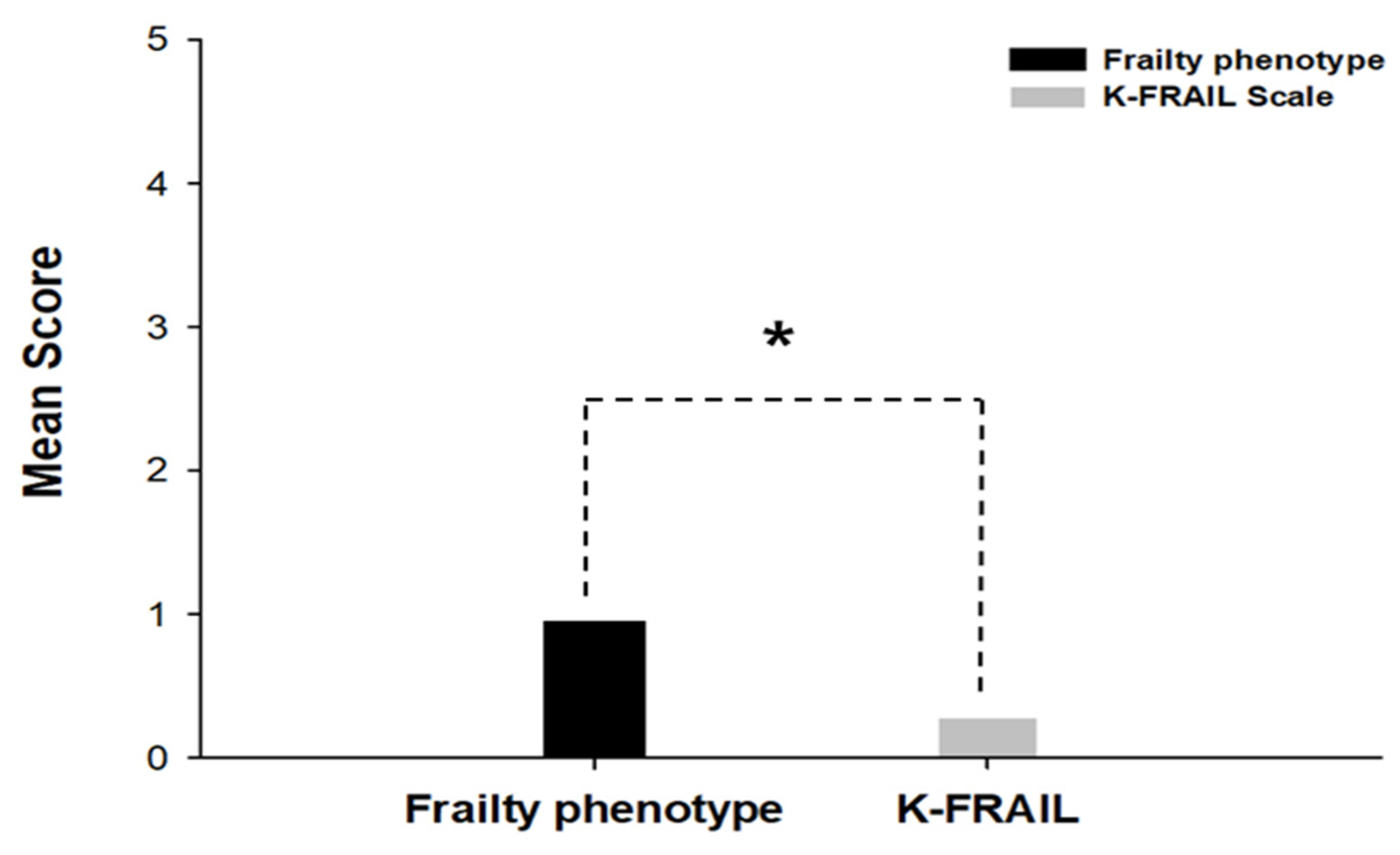
3.3. The Comparison of the Mean Scores
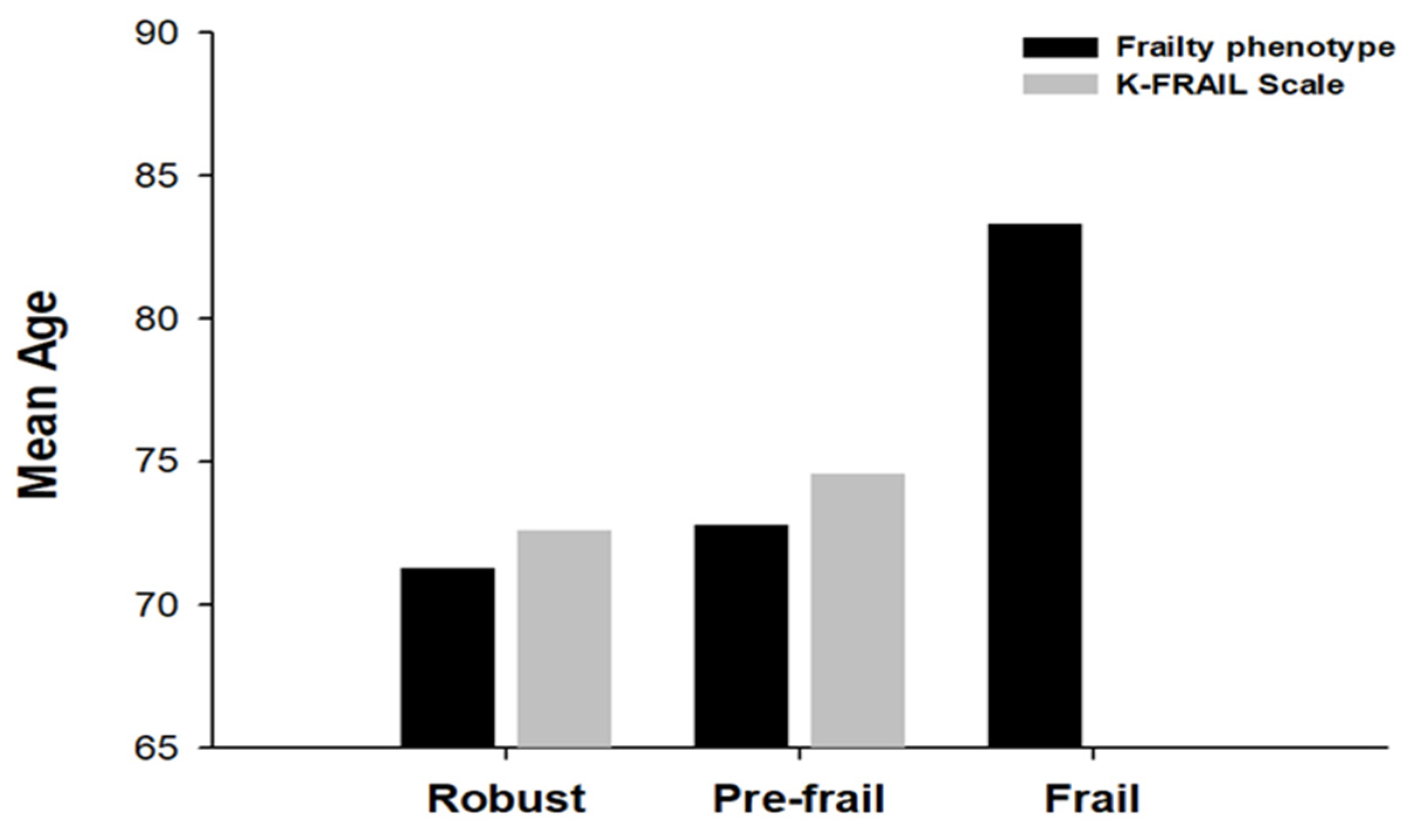
3.4. Mean Age According to Frailty Status
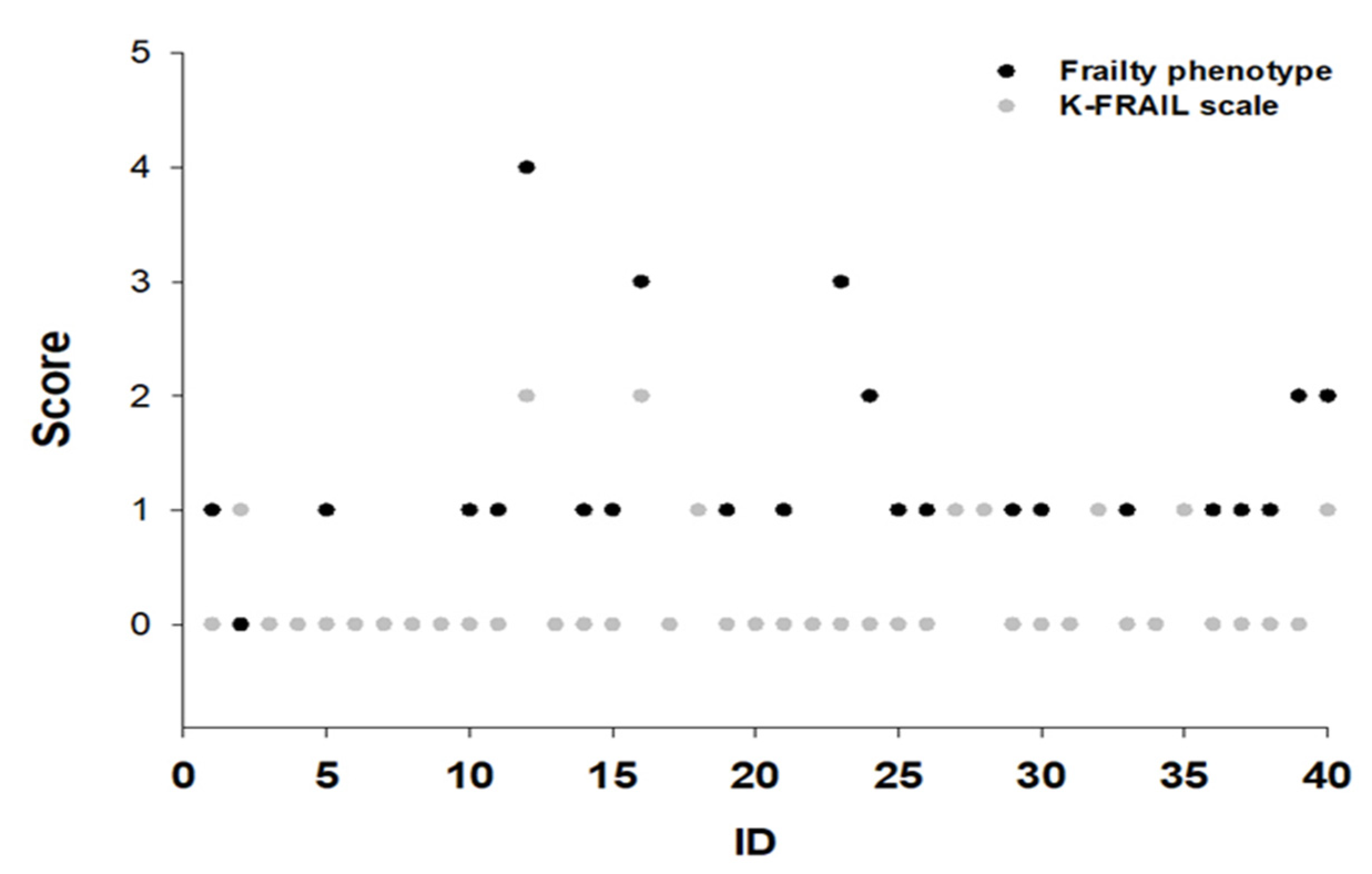
3.5. Individual Frailty Score of the Two Assessment Tools
3.6. Concordance Between the Two Assessment Tools
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar]
- Faller, J.W.; Pereira, D.D.N.; de Souza, S.; Nampo, F.K.; Orlandi, F.d.S.; Matumoto, S. Instruments for the detection of frailty syndrome in older adults: A systematic review. PLoS ONE 2019, 14, e0216166. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Bray, N.W.; Smart, R.R.; Jakobi, J.M.; Jones, G.R. Exercise prescription to reverse frailty. Appl. Physiol. Nutr. Metab. 2016, 41, 1112–1116. [Google Scholar] [CrossRef]
- Varan, H.D.; Deniz, O.; Çöteli, S.; Doğrul, R.T.; Kizilarslanoğlu, M.C.; Göker, B. Validity and reliability of Fried frailty phenotype in Turkish population. Turk. J. Med. Sci. 2022, 52, 524–527. [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. How Might Deficit Accumulation Give Rise to Frailty? J. Frailty Aging 2012, 1, 8–12. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Morley, J.E. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J. Am. Geriatr. Soc. 2012, 60, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Hogan, D.B.; MacKnight, C. Conceptualisation and measurement of frailty in elderly people. Drugs Aging 2000, 17, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Jung, H.-W.; Yoo, H.-J.; Park, S.-Y.; Kim, S.-W.; Choi, J.-Y.; Yoon, S.-J.; Kim, C.-H.; Kim, K.-I. The Korean version of the FRAIL scale: Clinical feasibility and validity of assessing the frailty status of Korean elderly. Korean J. Intern. Med. 2016, 31, 594–600. [Google Scholar] [CrossRef]
- Cesari, M.; Gambassi, G.; van Kan, G.A.; Vellas, B. The frailty phenotype and the frailty index: Different instruments for different purposes. Age Ageing 2014, 43, 10–12. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Han, K. Association Between Decreased Bone Mineral Density, Sarcopenia and Frailty Using Data from Centenarian Healthcare Maintenance. Master’s Thesis, Yonsei University, Seoul, Republic of Korea, 2018. [Google Scholar]
- Chun, M.Y. Validity and reliability of korean version of international physical activity questionnaire short form in the elderly. Korean J. Fam. Med. 2012, 33, 144–151. [Google Scholar] [CrossRef]
- Vaupel, J.W. Biodemography of human ageing. Nature 2010, 464, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 2018, 47, 193–200. [Google Scholar] [CrossRef]
- Walston, J.D.; Bandeen-Roche, K. Frailty: A tale of two concepts. BMC Med. 2015, 13, 185. [Google Scholar] [CrossRef]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.-L.; Walston, J.D.; Kasper, J.D. Frailty in Older Adults: A Nationally Representative Profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; van Hout, H.P.; Heymans, M.W.; van der Horst, H.E.; Frijters, D.H.; van Groenou, M.I.B.; Deeg, D.J.; Huisman, M. Explaining the association between educational level and frailty in older adults: Results from a 13-year longitudinal study in the Netherlands. Ann. Epidemiol. 2014, 24, 538–544.e2. [Google Scholar] [CrossRef]
- Gordon, E.; Peel, N.; Samanta, M.; Theou, O.; Howlett, S.; Hubbard, R. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Custodero, C.; Cella, A.; Demurtas, J.; Zora, S.; Maggi, S.; Barbagallo, M.; Sabbà, C.; Ferrucci, L.; Pilotto, A. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 72, 101498. [Google Scholar] [CrossRef] [PubMed]
- Arosio, B.; Guerini, F.R.; Costa, A.S.; Dicitore, A.; Ferri, E.; Mari, D.; Torresani, E.; Clerici, M.; Cesari, M.; Vitale, G. Vitamin D Receptor Polymorphisms in Sex-Frailty Paradox. Nutrients 2020, 12, 2714. [Google Scholar] [CrossRef]
- Falconer, J.; Hughes, S.L.; Naughton, B.J.; Singer, R.; Chang, R.W.; Sinacore, J.M. Self report and performance-based hand function tests as correlates of dependency in the elderly. J. Am. Geriatr. Soc. 1991, 39, 695–699. [Google Scholar] [CrossRef]
- Beier, F.; Löffler, M.; Nees, F.; Hausner, L.; Frölich, L.; Flor, H. Sensory and motor correlates of frailty: Dissociation between frailty phenotype and frailty index. BMC Geriatr. 2022, 22, 755. [Google Scholar] [CrossRef]
- Nascimento, C.M.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.C.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2019, 132, 42–49. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, epidemiology, and pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar] [CrossRef]
- Alexandre, T.d.S.; Scholes, S.; Santos, J.L.F.; Duarte, Y.A.d.O.; de Oliveira, C. The combination of dynapenia and abdominal obesity as a risk factor for worse trajectories of IADL disability among older adults. Clin. Nutr. 2018, 37 Pt A, 2045–2053. [Google Scholar] [CrossRef]
| Methods | Sex | BMI (kg/m2) | Strength (kg) | Score |
|---|---|---|---|---|
| Weakness (grip strength) | Men | ≤24 | <29 | 1 |
| 24.1~26 | <30 | |||
| 26.1~28 | <30 | |||
| >28 | <32 | |||
| Women | ≤23 | <17 | ||
| 23.1~26 | <17.3 | |||
| 26.1~29 | <18 | |||
| >29 | <21 | |||
| Sex | Height (cm) | Speed (s) | Score | |
| Slow walking time | Men | ≤173 | ≤2.94 | 1 |
| >173 | ≤3.43 | |||
| Women | ≤159 | ≤2.94 | ||
| >159 | ≤3.43 | |||
| Unintentional weight loss | Unintentional weight loss of 4.5 kg or more | Score | ||
| 1 | ||||
| Sex | Kcals/week | Score | ||
| Low physical activity | Men | <383 | 1 | |
| Women | <270 | |||
| Self-reported exhaustion | Questionnaire | days | Score | |
| In last week, how often did you feel tired? | everyday | 1 | ||
| 3~4 days | ||||
| 1 day or none | 0 | |||
| Total score | Frailty | >3 | ||
| Pre-frail | 1~2 | |||
| Non-frail | 0 | |||
| Methods | Questionnaire | Answer | Score |
|---|---|---|---|
| Fatigue | Have you felt fatigued in the past month? | Always | 1 |
| Almost | |||
| Often | 0 | ||
| Sometimes | |||
| Not at all | |||
| Resistance | Is it difficult for you to go up the 10 steps without help? | Yes | 1 |
| No | 0 | ||
| Ambulation | Is it difficult for you to move 300 m without help? | Yes | 1 |
| No | 0 | ||
| Illness | Have you ever been diagnosed with any of the following diseases by a doctor: hypertension, diabetes, cancer, chronic obstructive pulmonary disease, myocardial infarction, cardiac failure, angina pectoris, asthma, arthritis, cerebral infarction, kidney disease? | 5~11 | 1 |
| 0~4 | 0 | ||
| Loss of weight | What is the difference in your current weight compared to your weight in last year? | 5% or more loss | 1 |
| Less than 5% loss | 0 | ||
| Total score | Frailty | >3 | |
| Pre-frail | 1~2 | ||
| Non-frail | 0 | ||
| Factor | N(%) or M ± SD |
|---|---|
| Age | 73.07 ± 5.75 |
| Male | 27 (67.5) |
| Female | 13 (32.5) |
| Systolic blood pressure (SBP) | 146.7 ± 14.86 |
| Diastolic blood pressure (DBP) | 78.1 ± 10.92 |
| Height (cm) | 167.72 ± 8.9 |
| Body weight (kg) | 63.79 ± 10.31 |
| Body mass index | 24.33 ± 3.03 |
| Grip strength (kg) | 33.44 ± 7.72 |
| Walking speed (sec) | 3.32 ± 0.83 |
| Unintentional weight loss (kg) | −0.17 ± 2.21 |
| Physical activity (kcal) | 1969.93 ± 1530.28 |
| Self-reported exhaustion (day) | 0.40 ± 0.74 |
| K-FRAIL | |||||||
|---|---|---|---|---|---|---|---|
| Frailty phenotype | Robust | Pre-Frail | Frail | Total | Kappa | 95% CI | |
| Robust | 12 | 1 | 0 | 13 | 0.161 | 0.009–0.313 | |
| Pre-frail | 18 | 6 | 0 | 24 | |||
| Frail | 1 | 2 | 0 | 3 | |||
| Total | 31 | 9 | 0 | 40 | |||
| Variables | Grip Strength | Slow Walking Speed | Unintentional Weight Loss | Low Physical Activity | Self- Reported Exhaustion | Resistance | Ambulation | Loss of Weight | Fatigue |
|---|---|---|---|---|---|---|---|---|---|
| Grip strength | 1.000 | −0.119 | −0.197 | 0.276 | −0.254 | −0.233 | −0.078 | 0.104 | 0.020 |
| Slow walking speed | 1.000 | 0.201 | −0.282 | 0.094 | 0.426 ** | 0.407 ** | −0.007 | −0.040 | |
| Unintentional weight loss | 1.000 | 0.075 | −0.255 | −0.082 | −0.082 | −0.271 | 0.124 | ||
| Low physical activity | 1.000 | −0.138 | 0.013 | −0.325 * | −0.104 | −0.070 | |||
| Self-reported exhaustion | 1.000 | 0.319 * | 0.328 * | −0.104 | 0.087 | ||||
| Resistance | 1.000 | 0.466 ** | −0.061 | −0.087 | |||||
| Ambulation | 1.000 | −0.046 | −0.065 | ||||||
| Loss of weight | 1.000 | −0.037 | |||||||
| Fatigue | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Cho, I.; Kwak, D. Comparison of the Frailty Phenotype and the Korean Version of the FRAIL Scale. Healthcare 2025, 13, 1352. https://doi.org/10.3390/healthcare13111352
Lee D, Cho I, Kwak D. Comparison of the Frailty Phenotype and the Korean Version of the FRAIL Scale. Healthcare. 2025; 13(11):1352. https://doi.org/10.3390/healthcare13111352
Chicago/Turabian StyleLee, Dongwoo, Inhye Cho, and Dongmin Kwak. 2025. "Comparison of the Frailty Phenotype and the Korean Version of the FRAIL Scale" Healthcare 13, no. 11: 1352. https://doi.org/10.3390/healthcare13111352
APA StyleLee, D., Cho, I., & Kwak, D. (2025). Comparison of the Frailty Phenotype and the Korean Version of the FRAIL Scale. Healthcare, 13(11), 1352. https://doi.org/10.3390/healthcare13111352






