Inflammatory and Angiogenic Mediators Are Differentially Ex-Pressed in Patients with Post-COVID-19 Syndrome with Normal and Abnormal Spirometry Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Evaluation of the Recruitment Instrument and Participants Selection
2.3. Evaluation of Pulmonary Function
2.4. Blood Sample Collection and RNA Isolation
2.5. Analysis of Relative Gene Expression
2.6. MMP-9 Determination
2.7. Statistical Analysis
3. Results
3.1. Instrument Evaluation, Participants’ Demographic Characteristics, and Clinical Parameters
3.2. Pulmonary Function and Comorbidities Associated with Pulmonary Alteration
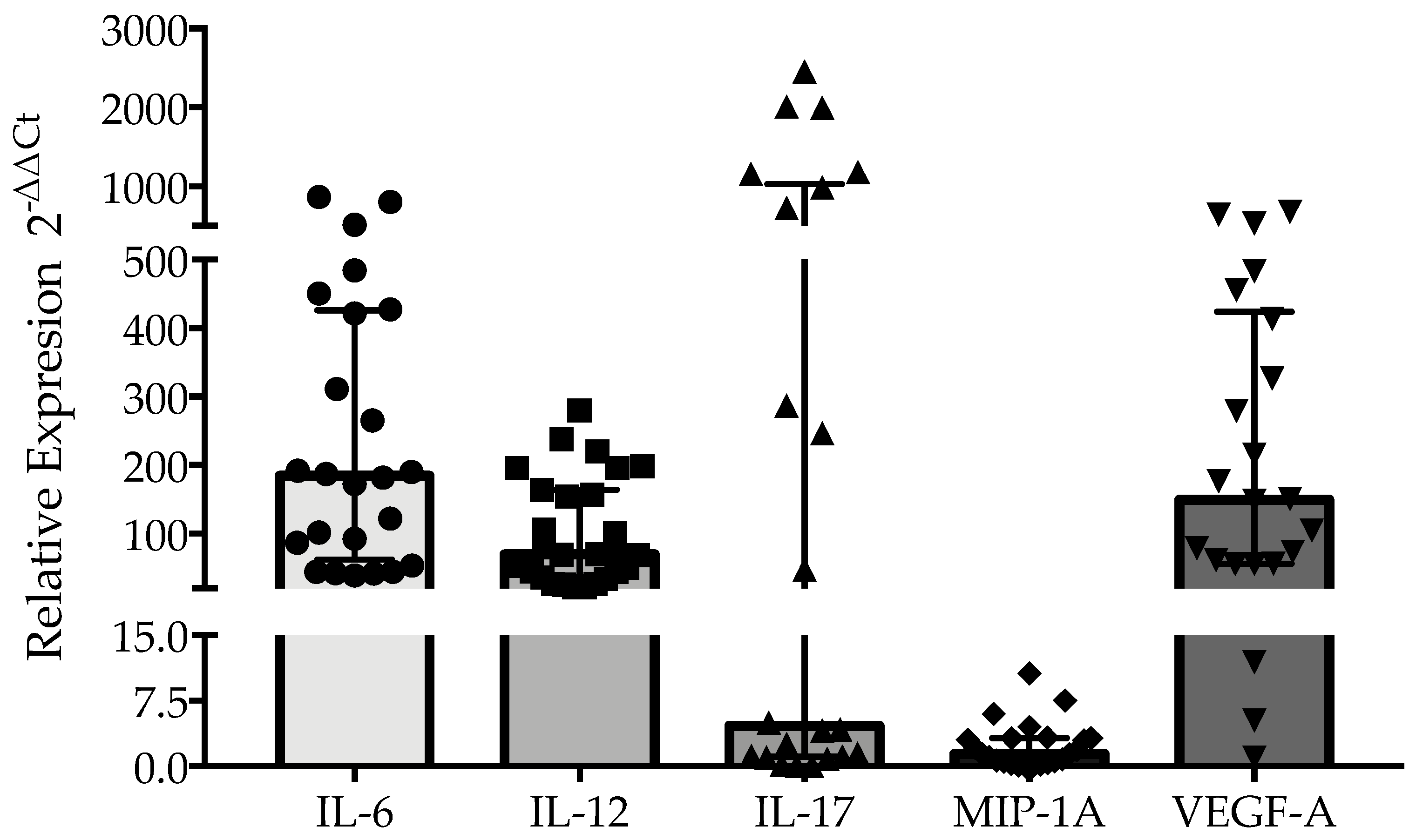
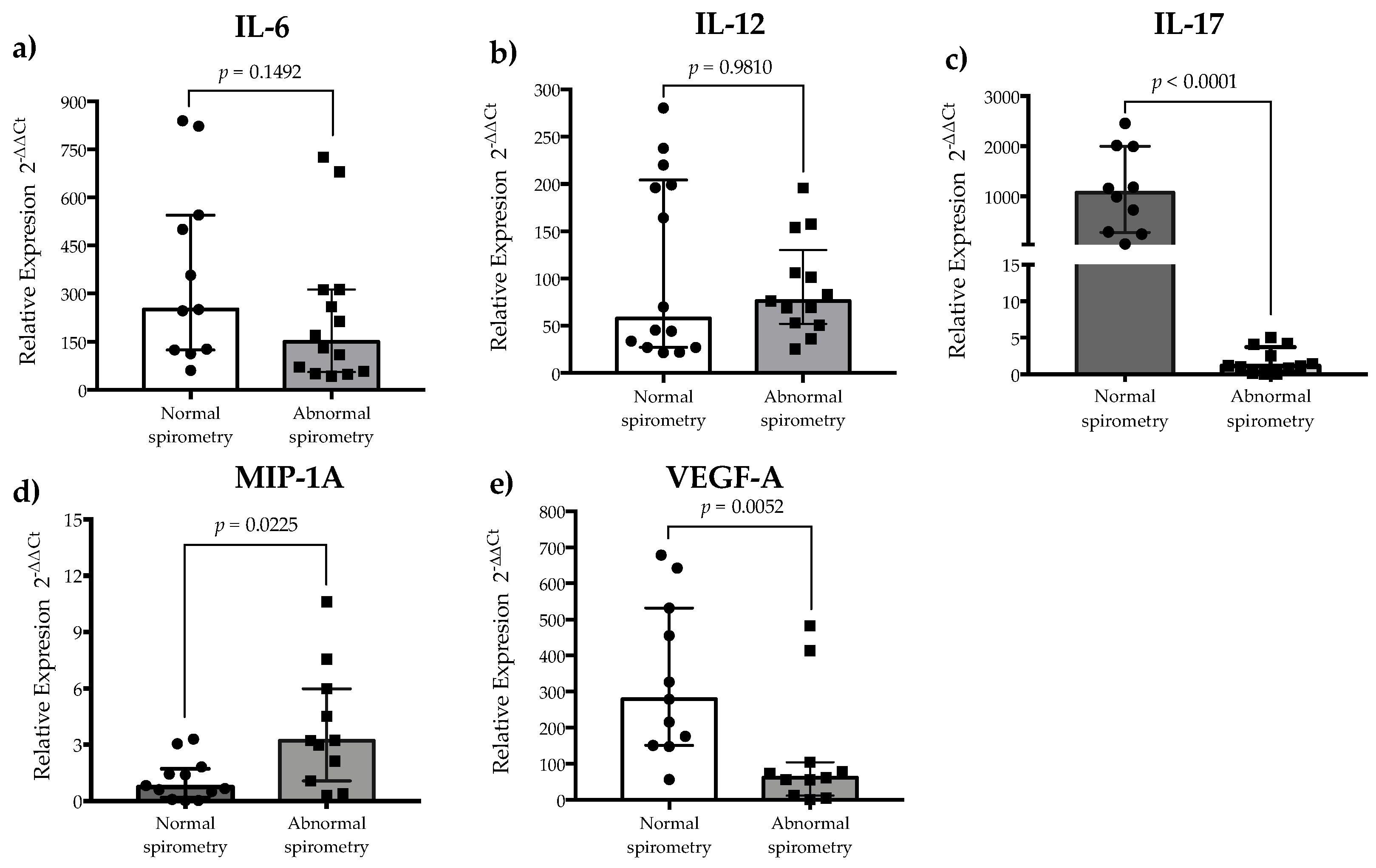
3.3. Inflammatory Cytokine Expression and Plasma Levels of MMP-9 Are Associated with Pulmonary Alteration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CI 95% | Confidence interval of 95% |
| FEV1 | Forced expiratory volume in the first second |
| FVC | Forced vital capacity |
| ICU | Intensive care unit |
| MIP | Macrophage inflammatory protein |
| MMP-9 | Matrix metalloproteinase-9 |
| OR | Odds ratio |
| PBMCs | Peripheral blood mononuclear cells |
| ROS | Reactive oxygen species |
| SARS-CoV-2 | Severe acute respiratory syndrome-coronavirus-2 |
| VEGF | Vascular endothelial growth factor |
References
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Wigén, J.; Löfdahl, A.; Bjermer, L.; Elowsson Rendin, L.; Westergren-Thorsson, G. Converging Pathways in Pulmonary Fibrosis and COVID-19—The Fibrotic Link to Disease Severity. Respir. Med. X 2020, 2, 100023. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of Adults with Noncritical COVID-19 Two Months after Symptom Onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.-O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary Function and Radiological Features 4 Months after COVID-19: First Results from the National Prospective Observational Swiss COVID-19 Lung Study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Fong, S.-W.; Young, B.E.; Chan, Y.-H.; Lee, B.; Amrun, S.N.; Chee, R.S.-L.; Yeo, N.K.-W.; Tambyah, P.; Pada, S.; et al. Persistent Symptoms and Association With Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019 Patients. Open Forum Infect. Dis. 2021, 8, ofab156. [Google Scholar] [CrossRef] [PubMed]
- Lerum, T.V.; Aaløkken, T.M.; Brønstad, E.; Aarli, B.; Ikdahl, E.; Lund, K.M.A.; Durheim, M.T.; Rodriguez, J.R.; Meltzer, C.; Tonby, K.; et al. Dyspnoea, Lung Function and CT Findings 3 Months after Hospital Admission for COVID-19. Eur. Respir. J. 2021, 57, 2003448. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yao, Z.; Wu, K.; Zheng, J. Patient Follow-up after Discharge after COVID-19 Pneumonia: Considerations for Infectious Control. J. Med. Virol. 2020, 92, 2412–2419. [Google Scholar] [CrossRef]
- Ríos, C.P.S. Función pulmonar y frecuencia de síndrome post-COVID-19 en pacientes recuperados de neumonía por SARS-CoV-2 mediante enfoque completo de telemedicina. Respirar 2021, 13, 127–138. [Google Scholar]
- González-Santamaría, J.; Arámburo-Gálvez, J.G.; Beltrán-Cárdenas, C.E.; Mora-Melgem, J.A.; Figueroa-Salcido, O.G.; Ramírez-Torres, G.I.; Cárdenas-Torres, F.I.; Carvalho Gomes, I.; Geralda André, T.; Macêdo-Callou, M.A.; et al. Design, Assessment, and Validation of a Questionnaire to Estimate Food-Dependent Exercise-Induced Anaphylaxis Prevalence in Latin American Population. Healthcare 2020, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Arámburo-Gálvez, J.G.; Carvalho Gomes, I.; André, T.G.; Beltrán-Cárdenas, C.E.; Macêdo-Callou, M.A.; Braga Rocha, É.M.; Mye-Takamatu-Watanabe, E.A.; Rahmeier-Fietz, V.; Figueroa-Salcido, O.G.; Cárdenas-Torres, F.I.; et al. Translation, Cultural Adaptation, and Evaluation of a Brazilian Portuguese Questionnaire to Estimate the Self-Reported Prevalence of Gluten-Related Disorders and Adherence to Gluten-Free Diet. Medicina 2019, 55, 593. [Google Scholar] [CrossRef]
- Vásquez-Aguilar, F.; Vergara-Jiménez, M.d.J.; Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Cárdenas-Torres, F.I.; Ontiveros, N.; Martínez-López, E.; Barrón-Cabrera, E. The Role of Diet and Physical Activity in Shaping COVID-19 Severity: Design, Validation, and Application of a Retrospective Questionnaire. Healthcare 2024, 12, 813. [Google Scholar] [CrossRef]
- US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994; National Center for Health Statistics: Hyattsville, MD, USA, 1994.
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Man, M.A.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Ilie, A.C.; Burtic, S.-R.; Fildan, A.P.; Fizedean, C.M.; Jianu, A.M.; Negrean, R.A.; et al. Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 189. [Google Scholar] [CrossRef]
- Mambelli, F.; de Araujo, A.C.V.S.C.; Farias, J.P.; de Andrade, K.Q.; Ferreira, L.C.S.; Minoprio, P.; Leite, L.C.C.; Oliveira, S.C. An Update on Anti-COVID-19 Vaccines and the Challenges to Protect Against New SARS-CoV-2 Variants. Pathogens 2025, 14, 23. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.; Jiang, L.; Dua, K.; Hansbro, P.M.; Liu, G. SARS-CoV-2 Induces Transcriptional Signatures in Human Lung Epithelial Cells That Promote Lung Fibrosis. Respir. Res. 2020, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Orlov, M.; Wander, P.L.; Morrell, E.D.; Mikacenic, C.; Wurfel, M.M. A Case for Targeting Th17 Cells and IL-17A in SARS-CoV-2 Infections. J. Immunol. 2020, 205, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. Elevated Plasma Levels of Selective Cytokines in COVID-19 Patients Reflect Viral Load and Lung Injury. Natl. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef]
- Sabioni, L.; De Lorenzo, A.; Lamas, C.; Muccillo, F.; Castro-Faria-Neto, H.C.; Estato, V.; Tibirica, E. Systemic Microvascular Endothelial Dysfunction and Disease Severity in COVID-19 Patients: Evaluation by Laser Doppler Perfusion Monitoring and Cytokine/Chemokine Analysis. Microvasc. Res. 2021, 134, 104119. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, S.; Gorgun, S. The Protective Role of IL-17 and IL-22 in COVID-19 Infection. Iran. J. Immunol. 2024, 21, 225–233. [Google Scholar] [CrossRef]
- Sadeghi, A.; Tahmasebi, S.; Mahmood, A.; Kuznetsova, M.; Valizadeh, H.; Taghizadieh, A.; Nazemiyeh, M.; Aghebati-Maleki, L.; Jadidi-Niaragh, F.; Abbaspour-Aghdam, S.; et al. Expression of Concern: Th17 and Treg Cells Function in SARS-CoV2 Patients Compared with Healthy Controls. J. Cell. Physiol. 2021, 236, 2829–2839. [Google Scholar] [CrossRef]
- Arslan, N.G.; Aksakal, Ş.; Yılmam, İ.; Görgün, S. VEGF, IL-17 and IgG4 Levels of Patients with Lung Sequelae in Post-COVID-19 Period. Tuberk Toraks 2022, 70, 179–186. [Google Scholar] [CrossRef]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 Alpha)/CCL3: As a Biomarker. Gen. Methods Biomark. Res. Their Appl. 2015, 223–249. [Google Scholar] [CrossRef]
- Ziegenhagen, M.W.; Schrum, S.; Zissel, G.; Zipfel, P.F.; Schlaak, M.; Müller-Quernheim, J. Increased Expression of Proinflammatory Chemokines in Bronchoalveolar Lavage Cells of Patients with Progressing Idiopathic Pulmonary Fibrosis and Sarcoidosis. J. Investig. Med. 1998, 46, 223–231. [Google Scholar]
- Goodman, R.B.; Strieter, R.M.; Martin, D.P.; Steinberg, K.P.; Milberg, J.A.; Maunder, R.J.; Kunkel, S.L.; Walz, A.; Hudson, L.D.; Martin, T.R. Inflammatory Cytokines in Patients with Persistence of the Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 1996, 154, 602–611. [Google Scholar] [CrossRef]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; de Jesus, A.A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An Immune-Based Biomarker Signature Is Associated with Mortality in COVID-19 Patients. JCI Insight 2021, 6, e144455. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Bagheri-Hosseinabadi, Z.; Kaeidi, A.; Rezvani, M.; Taghipour Khaje Sharifi, G.; Abbasifard, M. Evaluation of the Serum Levels of CCL2, CCL3, and IL-29 after First and Second Administrations of the COVID-19 Vaccine (Oxford–AstraZeneca). Immunobiology 2024, 229, 152789. [Google Scholar] [CrossRef]
- Norooznezhad, A.H.; Mansouri, K. Endothelial Cell Dysfunction, Coagulation, and Angiogenesis in Coronavirus Disease 2019 (COVID-19). Microvasc. Res. 2021, 137, 104188. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, B.; Wang, Y.; Tan, S.; Xu, Q.; Wang, Z.; Zhou, K.; Liu, H.; Ren, Z.; Jiang, Z. Ang-1 and VEGF: Central Regulators of Angiogenesis. Mol. Cell. Biochem. 2025, 480, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.I.; Zachary, I. The Vascular Endothelial Growth Factor (VEGF) Family: Angiogenic Factors in Health and Disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Pine, A.B.; Meizlish, M.L.; Goshua, G.; Chang, C.-H.; Zhang, H.; Bishai, J.; Bahel, P.; Patel, A.; Gbyli, R.; Kwan, J.M.; et al. Circulating Markers of Angiogenesis and Endotheliopathy in COVID-19. Pulm. Circ. 2020, 10, 2045894020966547. [Google Scholar] [CrossRef] [PubMed]
- Davey, A.; McAuley, D.F.; O’Kane, C.M. Matrix Metalloproteinases in Acute Lung Injury: Mediators of Injury and Drivers of Repair. Eur. Respir. J. 2011, 38, 959–970. [Google Scholar] [CrossRef]
- D’Avila-Mesquita, C.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellini-Diab, M.J.; Couto, D.M.S.; et al. MMP-2 and MMP-9 Levels in Plasma Are Altered and Associated with Mortality in COVID-19 Patients. Biomed. Pharmacother. 2021, 142, 112067. [Google Scholar] [CrossRef]
- Demir, N.A.; Kirik, S.Y.; Sumer, S.; Ural, O.; Kiratlı, H.E.; Vatansev, H.; Hayatsal, E.P.; Arslan, U.; Cebeci, H.; Demir, L.S. An Evaluation of Matrix Metalloproteinase-9 (Mmp-9) and Tissue Inhibitor Metalloproteinase-1 (Timp-1) Serum Levels and the Mmp-9/Timp-1 Ratio in COVID-19 Patients. Afr. Health Sci. 2023, 23, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Savic, G.; Stevanovic, I.; Mihajlovic, D.; Jurisevic, M.; Gajovic, N.; Jovanovic, I.; Ninkovic, M. MMP-9/BDNF Ratio Predicts More Severe COVID-19 Outcomes. Int. J. Med. Sci. 2022, 19, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Holter, J.C.; Holten, A.R.; Müller, K.E.; Lind, A.; Bekken, G.K.; Dudman, S.; Aukrust, P.; Dyrhol-Riise, A.M.; Heggelund, L. Distinct and Early Increase in Circulating MMP-9 in COVID-19 Patients with Respiratory Failure. J. Infect. 2020, 81, e41–e43. [Google Scholar] [CrossRef] [PubMed]

| Demographic Characteristics | n (%) | |
|---|---|---|
| Sex | Male | 16 (36) |
| Female | 29 (64) | |
| Age (m ± SD) | 46 ± 14.05 | |
| BMI (m ± SD) | 29.37 ± 5.68 | |
| Comorbidities | Obesity | 21 (47) |
| Arterial hypertension | 13 (29) | |
| Diabetes mellitus | 5 (11) | |
| Cardiomyopathic ischemia | 1 (2) | |
| Substance abuse | Alcohol | 21 (47) |
| Tobacco | 8 (18) | |
| Type of vaccine administered | First dose (n = 43) | |
| Pfizer | 26 (58) | |
| Cansino | 9 (20) | |
| Astra Zeneca | 8 (18) | |
| Second dose (n = 38) | ||
| Pfizer | 27 (60) | |
| Astra Zeneca | 11 (24) | |
| Booster (n = 32) | ||
| Astra Zeneca | 19 (42) | |
| Pfizer | 12 (27) | |
| SINOVAC | 1 (2) | |
| Clinical Parameters | n (%) | |
| Hospitalization | 14 (31) | |
| Diagnosis of pneumonia | 15 (33) | |
| Persistent symptoms | Fatigue | 37 (82) |
| Dyspnea | 24 (53) | |
| Dry cough | 21 (47) | |
| Muscle aches | 10 (22) | |
| Sleep disturbances | 9 (20) | |
| Joint pain | 7 (16) | |
| Headache | 7 (16) | |
| Palpitations | 6 (13) | |
| Blurred vision | 5 (11) | |
| Anosmia | 4 (9) | |
| Dizziness | 4 (9) | |
| Variable | Spirometry | OR | CI 95% | p | |
|---|---|---|---|---|---|
| Abnormal (n = 17) n (%) | Normal (n = 28) n (%) | ||||
| Age over 60 years | 5 (29.4) | 3 (10.7) | 3.4 | 0.7–16.9 | 0.22 |
| Female | 14 (82.4) | 15 (53.6) | 0.2 | 0.05–1.05 | 0.06 |
| Obesity | 9 (52.9) | 12 (42.9) | 1.5 | 0.4–5.0 | 0.51+ |
| Pneumonia | 10 (58.8) | 5 (17.9) | 6.5 | 1.6–25.7 | 0.005+ |
| Hospitalization | 9 (52.9) | 5 (17.9) | 5.1 | 1.3–20.1 | 0.014+ |
| Severity (moderate- severe) | 10 (58.8) | 5 (17.9) | 6.5 | 1.6–25.7 | 0.005+ |
| No vaccine before the infection | 7 (41.2) | 7 (25) | 2.1 | 0.5–7.6 | 0.25+ |
| Alcohol | 5 (29.4) | 16 (57.1) | 0.3 | 0.08–1.1 | 0.71+ |
| Tobacco | 4 (23.5) | 4 (14.3) | 1.8 | 0.3–8.6 | 0.45 |
| Arterial hypertension | 8 (47.1) | 5 (17.9) | 4.0 | 1.0–15.8 | 0.04 |
| Diabetes mellitus | 4 (23.5) | 1 (3.6) | 8.3 | 0.8–81.9 | 0.06 |
| Cardiomyopathic ischemia | 1 (5.9) | -- | 2.7 | 1.8–4.0 | 0.37 |
| Persistence of symptoms | |||||
| Fatigue | 14 (82.4) | 23 (82.1) | 1.0 | 0.2–4.9 | 1.0 |
| Anosmia | 1 (5.9) | 3 (10.7) | 0.5 | 0.05–5.4 | 1.0 |
| Dyspnea | 13 (76.5) | 11 (39.3) | 5.0 | 1.29–19.4 | 0.03 |
| Dry cough | 11 (64.7) | 10 (35.7) | 3.3 | 0.9–11.6 | 0.05 |
| Muscle cramps | 5 (29.4) | 5 (17.9) | 1.9 | 0.4–7.9 | 0.46 |
| Joint pain | 2 (11.8) | 5 (17.9) | 0.6 | 0.1–3.5 | 0.69 |
| Sleep disturbances | 7 (41.2) | 2 (7.1) | 9.1 | 1.6–51.4 | 0.01 |
| Headache | 3 (17.6) | 4 (14.3) | 1.2 | 0.25–6.5 | 1.0 |
| Dizziness | 1 (5.9) | 3 (10.7) | 0.5 | 0.05–5.4 | 1.0 |
| Blurred vision | 2 (11.8) | 3 (10.7) | 1.1 | 0.1–7.4 | 1.0 |
| Palpitations | 2 (11.8) | 4 (14.3) | 0.8 | 0.1–4.9 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minjarez-Robles, L.I.; Arámburo-Gálvez, J.G.; Figueroa-Salcido, O.G.; Ornelas-Aguirre, J.M.; Ontiveros, N.; Flores-Mendoza, L.K. Inflammatory and Angiogenic Mediators Are Differentially Ex-Pressed in Patients with Post-COVID-19 Syndrome with Normal and Abnormal Spirometry Results. Healthcare 2025, 13, 1346. https://doi.org/10.3390/healthcare13111346
Minjarez-Robles LI, Arámburo-Gálvez JG, Figueroa-Salcido OG, Ornelas-Aguirre JM, Ontiveros N, Flores-Mendoza LK. Inflammatory and Angiogenic Mediators Are Differentially Ex-Pressed in Patients with Post-COVID-19 Syndrome with Normal and Abnormal Spirometry Results. Healthcare. 2025; 13(11):1346. https://doi.org/10.3390/healthcare13111346
Chicago/Turabian StyleMinjarez-Robles, Laura Ileana, Jesús Gilberto Arámburo-Gálvez, Oscar Gerardo Figueroa-Salcido, José Manuel Ornelas-Aguirre, Noé Ontiveros, and Lilian Karem Flores-Mendoza. 2025. "Inflammatory and Angiogenic Mediators Are Differentially Ex-Pressed in Patients with Post-COVID-19 Syndrome with Normal and Abnormal Spirometry Results" Healthcare 13, no. 11: 1346. https://doi.org/10.3390/healthcare13111346
APA StyleMinjarez-Robles, L. I., Arámburo-Gálvez, J. G., Figueroa-Salcido, O. G., Ornelas-Aguirre, J. M., Ontiveros, N., & Flores-Mendoza, L. K. (2025). Inflammatory and Angiogenic Mediators Are Differentially Ex-Pressed in Patients with Post-COVID-19 Syndrome with Normal and Abnormal Spirometry Results. Healthcare, 13(11), 1346. https://doi.org/10.3390/healthcare13111346







