The Efficacy and Safety of a Personalized Protocol Designed to Balance Hemoglobin Levels in Hemodialysis Patients as Led by Nephrology Clinical Nurse Specialists: An Intervention Study
Abstract
1. Introduction
1.1. ESA Therapy
1.2. Nephrology CNS
2. Methods
2.1. Previous Methods Used to Preserve HgB Stability in a Dialysis Unit
2.2. Sample
2.3. Recruitment of Nurses, Qualifications, and Implementation Process
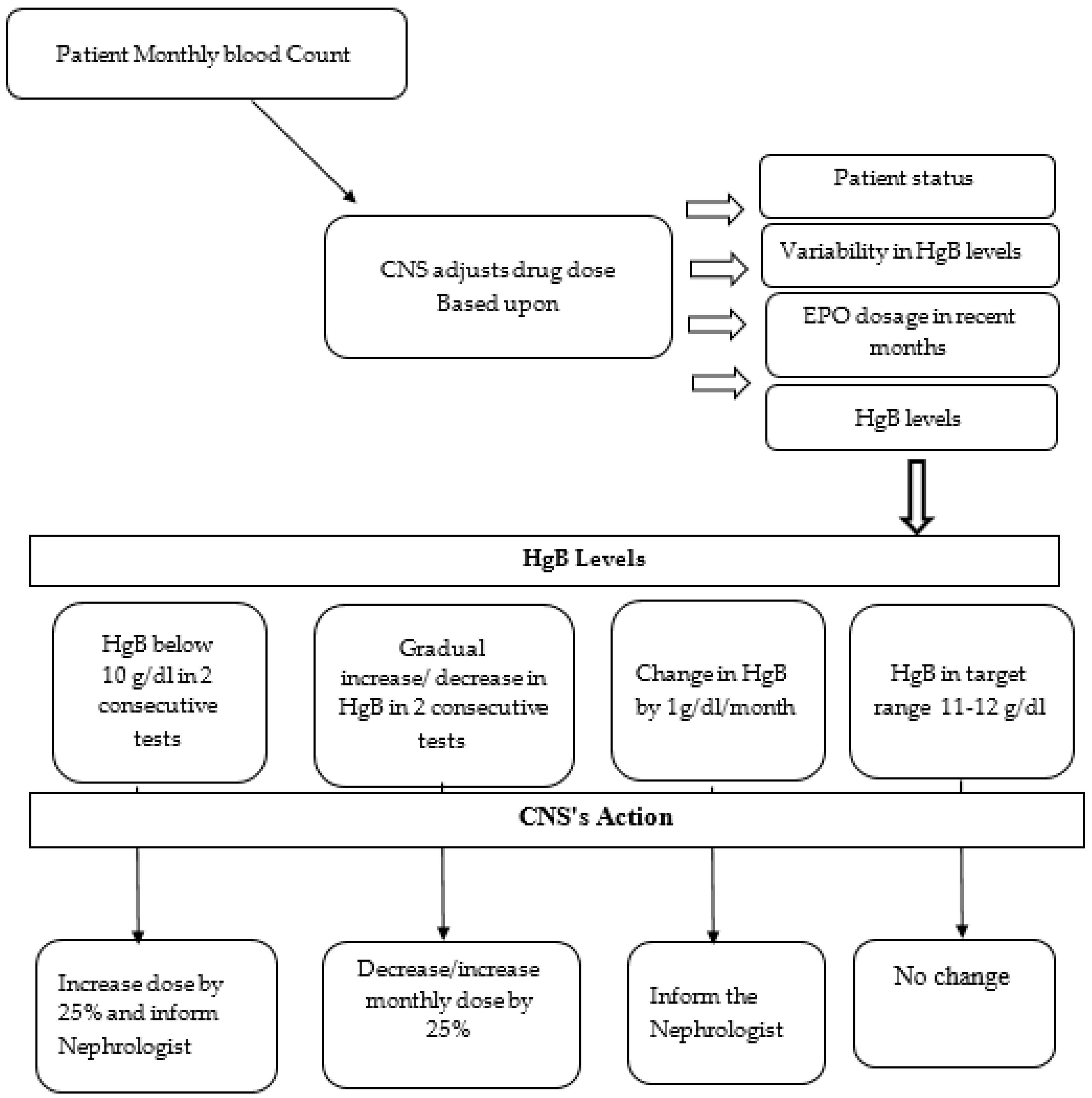
2.4. Protocol Description
2.5. Study Setting
2.6. Study Design
2.7. Ethical Considerations
2.8. Data Collection
2.9. Parameters Measured
2.9.1. Dialysis Quality Indicators
2.9.2. Process Parameters
2.9.3. Hemoglobin Maintenance Parameters
2.9.4. Patient Demographics
2.9.5. Perceived Empowerment of CNS
3. Results
3.1. Dialysis Quality Indicators
3.2. ESA Medical Order Updates and Changes in Dosage
3.3. HgB Level Stability
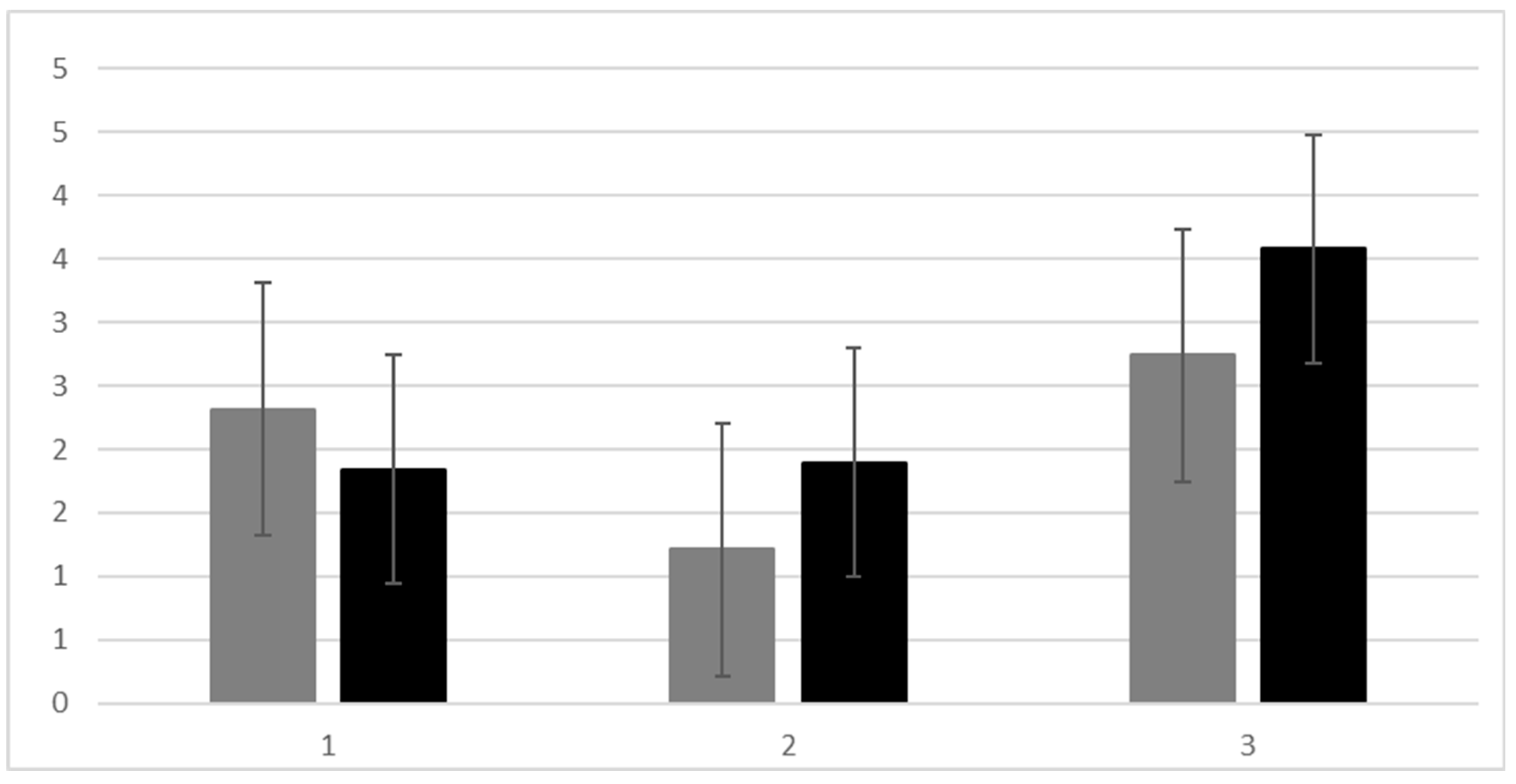
3.4. Perceived Empowerment of CNS
4. Discussion
4.1. Study Limitations
4.2. Future Studies
5. Conclusions
A Closing Note
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gafter-Gvili, A.; Schechter, A.; Rozen-Zvi, B. Iron Deficiency Anemia in Chronic Kidney Disease. Acta Haematol. 2019, 142, 44–50. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Anemia in Chronic Kidney Disease. Available online: https://www.niddk.nih.gov/health-information/kidney-disease/anemia (accessed on 1 May 2025).
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online: https://apps.who.int/iris/handle/10665/85839 (accessed on 1 May 2025).
- Mayo Clinic. Hemoglobin Test. Available online: https://www.mayoclinic.org/tests-procedures/hemoglobin-test/about/pac-20385075 (accessed on 1 May 2025).
- McMurray, J.; Parfrey, P.; Adamson, J.W.; Aljama, P.; Berns, J.S.; Bohlius, J.; Drüeke, T.B.; Finkelstein, F.O.; Fishbane, S.; Ganz, T. Kidney disease: Improving global outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar] [CrossRef]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef]
- Coffey, R.; Ganz, T. Iron homeostasis: An anthropocentric perspective. J. Biol. Chem. 2017, 292, 12727–12734. [Google Scholar] [CrossRef]
- Fishbane, S.; Spinowitz, B. Update on Anemia in ESRD and Earlier Stages of CKD: Core Curriculum 2018. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2018, 71, 423–435. [Google Scholar] [CrossRef]
- Stauffer, M.E.; Fan, T. Prevalence of anemia in chronic kidney disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef] [PubMed]
- Mariani, L.; Stengel, B.; Combe, C.; Massy, Z.A.; Reichel, H.; Fliser, D.; Pecoits-Filho, R.; Lopes, A.A.; Yamagata, K.; Wada, T.; et al. The CKD Outcomes and Practice Patterns Study (CKDopps): Rationale and Methods. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 68, 402–413. [Google Scholar] [CrossRef]
- van Haalen, H.; Jackson, J.; Spinowitz, B.; Milligan, G.; Moon, R. Impact of chronic kidney disease and anemia on health-related quality of life and work productivity: Analysis of multinational real-world data. BMC Nephrol. 2020, 21, 88. [Google Scholar] [CrossRef]
- Wong, M.M.Y.; Tu, C.; Li, Y.; Perlman, R.L.; Pecoits-Filho, R.; Lopes, A.A.; Narita, I.; Reichel, H.; Port, F.K.; Sukul, N.; et al. Anemia and iron deficiency among chronic kidney disease Stages 3-5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: Often unmeasured, variably treated. Clin. Kidney J. 2020, 13, 613–624. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, H.; Chen, Y.; Zhang, Y.; Li, S.; Hu, W.; Yang, R.; Zhang, Z.; Lv, L.; Liu, X. Hemoglobin targets for the anemia in patients with dialysis-dependent chronic kidney disease: A meta-analysis of randomized, controlled trials. Ren. Fail. 2018, 40, 671–679. [Google Scholar] [CrossRef]
- Drew, D.A.; Weiner, D.E.; Sarnak, M.J. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2019, 74, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, J.; Muenz, D.; Zee, J.; Sukul, N.; Speyer, E.; Guedes, M.; Lopes, A.A.; Asahi, K.; van Haalen, H.; James, G.; et al. Associations of Hemoglobin Levels With Health-Related Quality of Life, Physical Activity, and Clinical Outcomes in Persons With Stage 3-5 Nondialysis CKD. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2020, 30, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.; Southcott, E.; MacLaughlin, H.; Wineberg, S. Clinical practice guideline on undernutrition in chronic kidney disease. BMC Nephrol. 2019, 20, 370. [Google Scholar] [CrossRef] [PubMed]
- Wouters, H.; van der Klauw, M.M.; de Witte, T.; Stauder, R.; Swinkels, D.W.; Wolffenbuttel, B.H.R.; Huls, G. Association of anemia with health-related quality of life and survival: A large population-based cohort study. Haematologica 2019, 104, 468–476. [Google Scholar] [CrossRef]
- Gutiérrez, O.M. Treatment of Iron Deficiency Anemia in CKD and End-Stage Kidney Disease. Kidney Int. Rep. 2021, 6, 2261–2269. [Google Scholar] [CrossRef]
- Rogers, J.; Gallaher, E.J.; Dingli, D. Personalized ESA doses for anemia management in hemodialysis patients with end-stage renal disease. Syst. Dyn. Rev. 2018, 34, 121–153. [Google Scholar] [CrossRef]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.-C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef]
- Hain, D.; Bednarski, D.; Cahill, M.; Dix, A.; Foote, B.; Haras, M.S.; Pace, R.; Gutiérrez, O.M. Iron-Deficiency Anemia in CKD: A Narrative Review for the Kidney Care Team. Kidney Med. 2023, 5, 100677. [Google Scholar] [CrossRef]
- Wish, J.B.; Aronoff, G.R.; Bacon, B.R.; Brugnara, C.; Eckardt, K.U.; Ganz, T.; Macdougall, I.C.; Núñez, J.; Perahia, A.J.; Wood, J.C. Positive Iron Balance in Chronic Kidney Disease: How Much is Too Much and How to Tell? Am. J. Nephrol. 2018, 47, 72–83. [Google Scholar] [CrossRef]
- Li, J.L.; Cai, Z.; Zhao, J.; Zhu, X.G.; Li, Q.; Li, Y.S.; Liu, M.C.; Cui, F.Q.; Zhao, W.J.; Niu, W.Q. Association between anemia-related biomarkers and the adequacy of peritoneal dialysis in Chinese patients with chronic kidney disease. Front. Physiol. 2023, 14, 1170537. [Google Scholar] [CrossRef]
- Unger, E.F.; Thompson, A.M.; Blank, M.J.; Temple, R. Erythropoiesis-stimulating agents—Time for a reevaluation. N. Engl. J. Med. 2010, 362, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Vaught, K.; Kerber, S. Evolution of Treatment for Anemia in Chronic Kidney Disease. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2020, 30, e67–e70. [Google Scholar] [CrossRef]
- Gaweda, A.E.; Aronoff, G.R.; Jacobs, A.A.; Rai, S.N.; Brier, M.E. Individualized anemia management reduces hemoglobin variability in hemodialysis patients. J. Am. Soc. Nephrol. JASN 2014, 25, 159–166. [Google Scholar] [CrossRef]
- Bazeley, J.; Wish, J.B. The Evolution of Target Hemoglobin Levels in Anemia of Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Chait, Y.; Germain, M.J.; Hollot, C.V.; Horowitz, J. The Role of Feedback Control Design in Developing Anemia Management Protocols. Ann. Biomed. Eng. 2021, 49, 171–179. [Google Scholar] [CrossRef]
- Ghossein, C.; Serrano, A.; Rammohan, M.; Batlle, D. The role of comprehensive renal clinic in chronic kidney disease stabilization and management: The Northwestern experience. Semin. Nephrol. 2002, 22, 526–532. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, H.S.; Kim, H.Y. Relationships between core factors of knowledge management in hospital nursing organisations and outcomes of nursing performance. J. Clin. Nurs. 2014, 23, 3513–3524. [Google Scholar] [CrossRef]
- Perkins, C.; Kisiel, M. Developing the recognition and response skills of student nurses. Br. J. Nurs. 2013, 22, 715–724. [Google Scholar] [CrossRef]
- Gerrish, K.; McDonnell, A.; Nolan, M.; Guillaume, L.; Kirshbaum, M.; Tod, A. The role of advanced practice nurses in knowledge brokering as a means of promoting evidence-based practice among clinical nurses. J. Adv. Nurs. 2011, 67, 2004–2014. [Google Scholar] [CrossRef]
- Kerr, H.; Donovan, M.; McSorley, O. Evaluation of the role of the clinical Nurse Specialist in cancer care: An integrative literature review. Eur. J. Cancer Care 2021, 30, e13415. [Google Scholar] [CrossRef]
- International Council of Nurses. Guidelines of Advanced Practice Nursing 2020. Available online: https://www.icn.ch/resources/publications-and-reports/guidelines-advanced-practice-nursing-2020 (accessed on 1 May 2025).
- Fulton, J.S.; Mayo, A.M.; Walker, J.A.; Urden, L.D. Core Practice Outcomes for Clinical Nurse Specialists: A Revalidation Study. J. Prof. Nurs. Off. J. Am. Assoc. Coll. Nurs. 2016, 32, 271–282. [Google Scholar] [CrossRef]
- Latter, K.A.; Purser, S.; Chisholm, S.; Robinson, E. Divisional review of the nurse specialist role. Nurs. Stand. 2019, 34, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Tod, A.M.; Redman, J.; McDonnell, A.; Borthwick, D.; White, J. Lung cancer treatment rates and the role of the lung cancer nurse specialist: A qualitative study. BMJ Open 2015, 5, e008587. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Gage, H.; Benton, B.; Lalji, A.; Norton, C.; Andreyev, H.J.N. Gastroenterologist and nurse management of symptoms after pelvic radiotherapy for cancer: An economic evaluation of a clinical randomized controlled trial (the ORBIT study). Clin. Outcomes Res. CEOR 2017, 9, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.; Bos, W.C.; Prins, J.B.; Hoogerbrugge, N.; van Laarhoven, H.W. Breast self-examination education for BRCA mutation carriers by clinical nurse specialists. Clin. Nurse Spec. CNS 2015, 29, E1–E7. [Google Scholar] [CrossRef]
- Macmillan Cancer Support. Cancer Clinical Nurse Specialists. Available online: https://www.macmillan.org.uk/_images/Clinical-Nurse-Specialists_tcm9-283175.pdf (accessed on 1 May 2025).
- Israel Ministry of Health Medical Technologies Information and Research Division; National Center for Disease Control. End-Stage Renal Failure Morbidity in Israel in 2022. Data from the National Registry for Renal Replacement Therapy [Hebrew]. Available online: https://www.gov.il/BlobFolder/reports/dialysis-in-israel-2022/he/files_publications_units_ICDC_dialysisinisrael2022.pdf (accessed on 17 July 2023).
- Israel Ministry of Health. Quality Report of the Israeli Dialysis Department. Available online: https://www.gov.il/BlobFolder/reports/qa-dialysis/he/files_publications_units_quality_assurance_division_QA-Dialysis.pdf (accessed on 17 July 2023).
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 75, S1–S164. [Google Scholar] [CrossRef]
- Oliva-Damaso, N.; Delanaye, P.; Oliva-Damaso, E.; Payan, J.; Glassock, R.J. Risk-based versus GFR threshold criteria for nephrology referral in chronic kidney disease. Clin. Kidney J. 2022, 15, 1996–2005. [Google Scholar] [CrossRef]
- Fishbane, S.; Agoritsas, S.; Bellucci, A.; Halinski, C.; Shah, H.H.; Sakhiya, V.; Balsam, L. Augmented Nurse Care Management in CKD Stages 4 to 5: A Randomized Trial. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2017, 70, 498–505. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Clinical trials in end-stage renal disease-priorities and challenges. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2019, 34, 1084–1089. [Google Scholar] [CrossRef]
- Lægreid, I.K.; Aasarød, K.; Jordhøy, M. End-stage renal disease and recruitment to randomized trials: Why is it so difficult? Results from a survey among Norwegian nephrologists. Scand. J. Urol. Nephrol. 2011, 45, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.-l. Recruitment challenges for end-of-life research. J. Hosp. Palliat. Nurs. 2007, 9, 79–85. [Google Scholar] [CrossRef]
- Ebben, J.P.; Gilbertson, D.T.; Foley, R.N.; Collins, A.J. Hemoglobin level variability: Associations with comorbidity, intercurrent events, and hospitalizations. Clin. J. Am. Soc. Nephrol. CJASN 2006, 1, 1205–1210. [Google Scholar] [CrossRef]
- Matthews, A.; Anne Scott, P.; Gallagher, P.; Corbally, M.A. An exploratory study of the conditions important in facilitating the empowerment of midwives. Midwifery 2006, 22, 181–191. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Bragg-Gresham, J.L.; Fuller, D.S.; Morgenstern, H.; Canaud, B.; Locatelli, F.; Li, Y.; Gillespie, B.; Wolfe, R.A.; Port, F.K.; et al. Facility-level interpatient hemoglobin variability in hemodialysis centers participating in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Associations with mortality, patient characteristics, and facility practices. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 57, 266–275. [Google Scholar] [CrossRef]
- Ream, E.; Wilson-Barnett, J.; Faithfull, S.; Fincham, L.; Khoo, V.; Richardson, A. Working patterns and perceived contribution of prostate cancer clinical nurse specialists: A mixed method investigation. Int. J. Nurs. Stud. 2009, 46, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Cook, O.; McIntyre, M.; Recoche, K.; Lee, S. “Our nurse is the glue for our team”—Multidisciplinary team members’ experiences and perceptions of the gynaecological oncology specialist nurse role. Eur. J. Oncol. Nurs. 2019, 41, 7–15. [Google Scholar] [CrossRef]
- Mayo, A.M.; Ray, M.M.; Chamblee, T.B.; Urden, L.D.; Moody, R. The advanced practice clinical nurse specialist. Nurs. Adm. Q. 2017, 41, 70–76. [Google Scholar] [CrossRef]
- Tao, Z.; Xu, J.; Chen, W.; Yang, Z.; Xu, X.; Liu, L.; Chen, R.; Xie, J.; Liu, M.; Wu, J.; et al. Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J. Med. Virol. 2021, 93, 1478–1488. [Google Scholar] [CrossRef]

| Phase | Phase | Number of Patients’ Dropouts | Updated Number of Participants | % Patients Out of Original Sample | Total Dropouts |
|---|---|---|---|---|---|
| Before | 1 | 1 | 38 | 97.44 | 4 |
| 2 | 0 | 38 | 97.44 | ||
| 3 | 0 | 38 | 97.44 | ||
| 4 | 0 | 38 | 97.44 | ||
| 5 | 2 | 36 | 92.31 | ||
| 6 | 1 | 35 | 89.74 | ||
| Post | 1 | 1 | 34 | 87.18 | 12 |
| 2 | 1 | 33 | 84.62 | ||
| 3 | 2 | 31 | 79.49 | ||
| 4 | 1 | 30 | 76.92 | ||
| 5 | 4 | 26 | 66.67 | ||
| 6 | 3 | 23 | 58.97 |
| N = 39 | |
| Age (Average) | 68.35 (13.8) years |
| Sex | 53% male |
| Marital status | Married 79% |
| Single 5% | |
| Divorced 5% | |
| Widow 11% | |
| Education | |
| Elementary | 25.60 |
| High school | 17.90 |
| Higher/Academic | 30.8% |
| Religion | |
| Jewish | 58.9% |
| Muslim | 38.4% |
| Christian | 2% |
| Place of birth | |
| Israel | 67% |
| North Africa | 10% |
| Russia | 7.60% |
| East Europe | 5% |
| Background disease (more than 1 disease—3) | |
| Diabetes Mellitus | 23 (58.97%) |
| Hypertension | 5 (12.8%) |
| Renal cause of ESRD | 4 (10.25%) |
| Heart failure | 7 (17.94%) |
| Vascular disease | 2 (5.12%) |
| Renal cell carcinoma | 1 (2.56%) |
| Study Period | Month | Patients | Transferrin Saturation 1 (%) | URR 2 (%) | Infectious Disease (Average of Events per Month) | Non-Acute Bleeding (Average of Events per Month) |
|---|---|---|---|---|---|---|
| Nephrologist Balance | 1 | 39 | (11.68) 33.89 | 76.77 | 1.42 events | 0.97 events |
| 2 | 39 | (9.34) 75.79 | ||||
| 3 | 39 | (9.56) 75.25 | ||||
| 4 | 39 | (15.28) 31.04 | (7.01) 75.67 | |||
| 6 | 37 | (7.07) 77.82 | ||||
| 6 | 36 | (7.77) 76.74 | ||||
| CNS Balance | 1 | 34 | (10.24) 31.18 | (8.25) 78.94 | 0.85 events 3 | 0.61 events |
| 2 | 33 | (8.15) 75.86 | ||||
| 3 | 31 | (8.04) 77.85 | ||||
| 4 | 30 | (16.62) 24.75 | (8.84) 77.48 | |||
| 5 | 26 | (8.38) 76.9 | ||||
| 6 | 23 | (7.96) 79 |
| Study Period | Month | Patients | No. Updates of Medical Order (per Month) | Change in ESA Dosage (Number of Patients) |
|---|---|---|---|---|
| Nephrologist Supervision | 1 | 39 | (3.54) 4.74 * | 12 (30.8%) |
| 2 | 39 | (3.84) 4.61 | 12 (33.3%) | |
| 3 | 39 | (3.93) 4.85 * | 15 (38.5%) | |
| 4 | 39 | (3.99) 4.94 | 8 (20.5%) | |
| 5 | 37 | (3.79) 4.69 | 8 (21.6%) | |
| 6 | 36 | (3.66) 4.65 * | 11 (32.4%) | |
| CNS Supervision | 1 | 34 | (3.2) 3.21 * | 9 (23.1%) |
| 2 | 33 | (2.83) 3.42 | 10 (32.3%) | |
| 3 | 31 | (3.08) 3.32 * | 13 (44.8%) | |
| 4 | 30 | (3.55) 3.99 | 8 (29.6%) | |
| 5 | 26 | (3.26) 3.67 | 8 (33.3%) | |
| 6 | 23 | (3.23) 3.64 * | 6 (28.6%) |
| Study Period | Month | Number and (%) of Participants Within Normal Range | Average HgB Levels (SD) | Change in Blood HgB Relative to Previous Period |
|---|---|---|---|---|
| Nephrologist Balance | 1 | 18 (46.15) | 11.29 (1.07) | --- |
| 2 | 18 (46.15) | 11.30 (0.98) | 0.013 | |
| 3 | 18 (46.15) | 11.42 (0.81) | 0.117 | |
| 4 | 16 (41.03) | 11.46 (1.02) | 0.039 | |
| 5 | 17 (45.95) | 11.62 (0.85) | 0.161 | |
| 6 | 12 (33.33) | 11.52 (0.90) | −0.100 | |
| Average HgB–11.4 Average % in normal range-43.13 | ||||
| CNS Balance | 1 | 17 (50.00) | 11.63 (0.84) | 0.117 |
| 2 | 18 (54.55) | 11.44 (0.94) | −0.191 | |
| 3 | 14 (45.16) | 11.37 (0.78) | −0.078 | |
| 4 | 16 (53.33) | 11.27 (1.01) | −0.096 | |
| 5 | 15 (57.69) | 11.35 (0.75) | 0.078 | |
| 6 | 8 (34.78) | 11.50 (0.92) | 0.157 | |
| Average HgB–11.4 Average % in normal range-49.25 | ||||
| Study Period | Consistent/Low Variability-Number of Patients (%) | High Variability-Number of Patients (%) | Difference |
|---|---|---|---|
| Nephrologist supervision (n = 39) | 53.8 (n = 21) | 46.2 (n = 18) | χ2(2) = 1.33 |
| CNS Supervision (n = 24) | 54.1 (n = 13) | 45.8 (n = 11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Israeli, R.; Gabay, G.; Shafran Tikva, S.; Exman, M.; Mor Yosef Levi, I.; Radiano, R.; Alon, R.; Lerman, Y.; Zelker, R. The Efficacy and Safety of a Personalized Protocol Designed to Balance Hemoglobin Levels in Hemodialysis Patients as Led by Nephrology Clinical Nurse Specialists: An Intervention Study. Healthcare 2025, 13, 1317. https://doi.org/10.3390/healthcare13111317
Israeli R, Gabay G, Shafran Tikva S, Exman M, Mor Yosef Levi I, Radiano R, Alon R, Lerman Y, Zelker R. The Efficacy and Safety of a Personalized Protocol Designed to Balance Hemoglobin Levels in Hemodialysis Patients as Led by Nephrology Clinical Nurse Specialists: An Intervention Study. Healthcare. 2025; 13(11):1317. https://doi.org/10.3390/healthcare13111317
Chicago/Turabian StyleIsraeli, Ruth, Gillie Gabay, Sigal Shafran Tikva, Michal Exman, Irit Mor Yosef Levi, Ruth Radiano, Rely Alon, Yulia Lerman, and Revital Zelker. 2025. "The Efficacy and Safety of a Personalized Protocol Designed to Balance Hemoglobin Levels in Hemodialysis Patients as Led by Nephrology Clinical Nurse Specialists: An Intervention Study" Healthcare 13, no. 11: 1317. https://doi.org/10.3390/healthcare13111317
APA StyleIsraeli, R., Gabay, G., Shafran Tikva, S., Exman, M., Mor Yosef Levi, I., Radiano, R., Alon, R., Lerman, Y., & Zelker, R. (2025). The Efficacy and Safety of a Personalized Protocol Designed to Balance Hemoglobin Levels in Hemodialysis Patients as Led by Nephrology Clinical Nurse Specialists: An Intervention Study. Healthcare, 13(11), 1317. https://doi.org/10.3390/healthcare13111317







