New Perspectives on the Efficacy of Catgut Embedment in Acupoint Combined with Rehabilitation Training for Pediatric-Cerebral-Palsy Motor Function Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Aim
3. Methods
3.1. Protocol and Registration
3.2. Inclusion and Exclusion Criteria
3.3. Search Strategies
3.4. Data Extraction and Quality Assessment
3.5. Data Analysis
4. Results
4.1. Study Selection
4.2. Study Characteristics
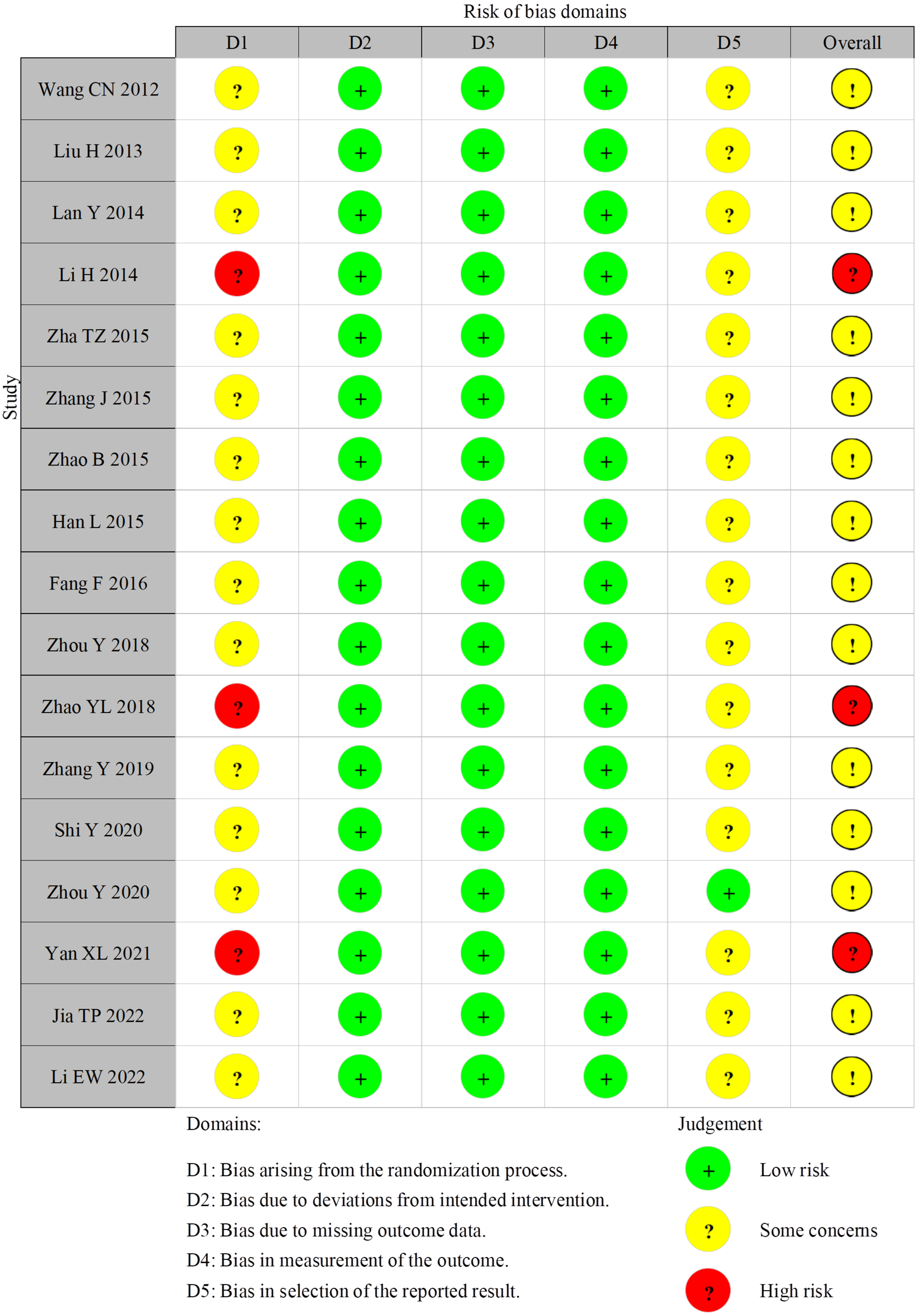
4.3. Risk of Bias of Included Studies
4.4. Primary Outcomes
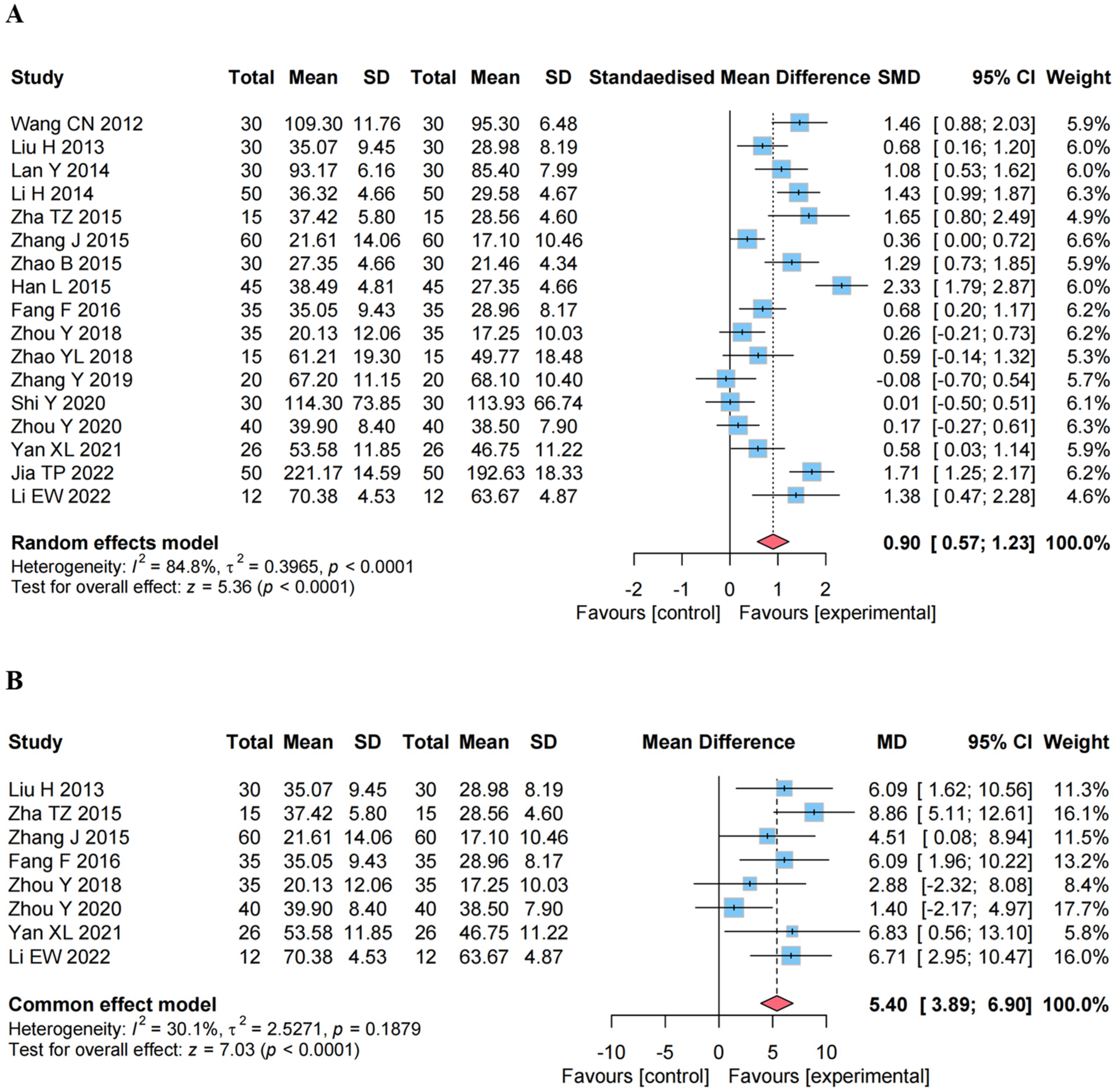
4.4.1. Effect on the GMFM
4.4.2. Effect on the GMFM Percentage
Sensitivity Analysis
Subgroup Analysis
4.5. Secondary Outcomes
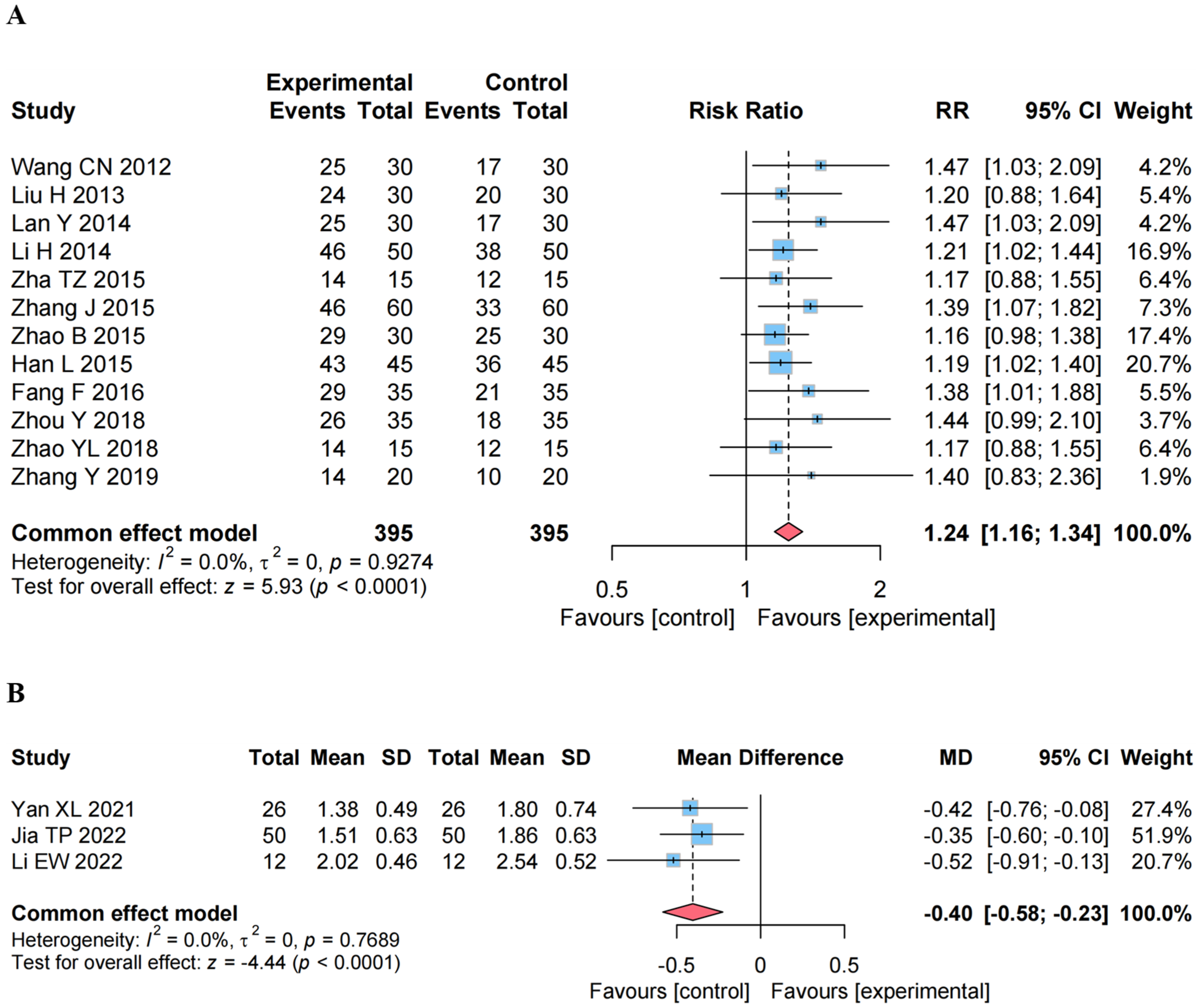
4.5.1. Effect on the ER
4.5.2. Effect on the MAS
4.5.3. Effect on the AEs
4.5.4. Sensitivity Analysis on Secondary Outcomes
4.6. Publication Bias
4.7. Evidence Evaluation
5. Discussion
5.1. Summary of the Findings
5.2. Perceptions of PCPMFDs
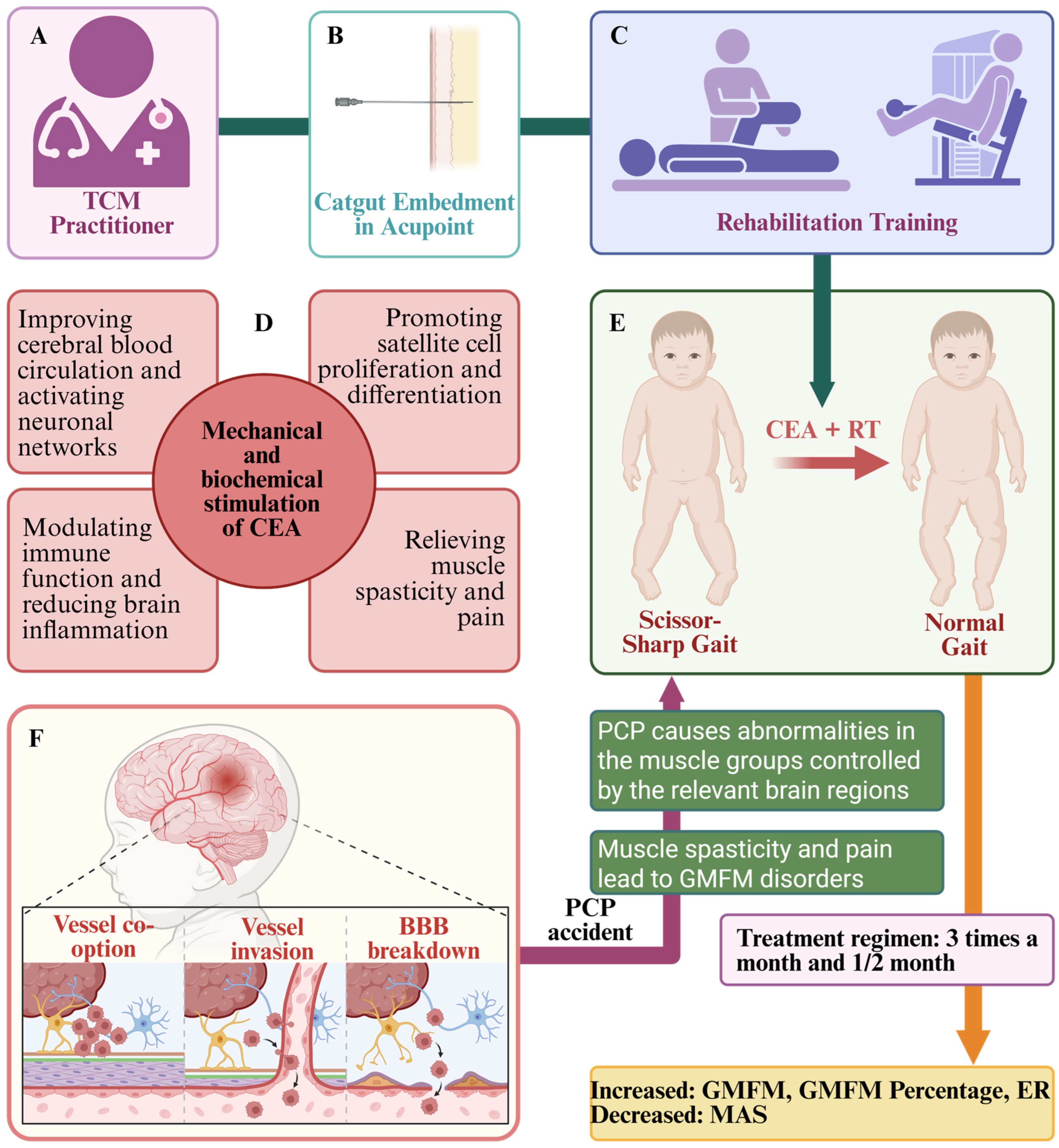
5.3. Possible Mechanisms of CEA to Improve MFDs
5.4. Clinical Relevance
5.5. Limitations and Implications for Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McIntyre, S.; Goldsmith, S.; Webb, A.; Ehlinger, V.; Hollung, S.J.; McConnell, K.; Arnaud, C.; Smithers-Sheedy, H.; Oskoui, M.; Khandaker, G.; et al. Global prevalence of cerebral palsy: A systematic analysis. Dev. Med. Child. Neurol. 2022, 64, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, M.; Sarecka-Hujar, B.; Kopyta, I. Cerebral Palsy: Current Opinions on Definition, Epidemiology, Risk Factors, Classification and Treatment Options. Neuropsychiatr. Dis. Treat. 2020, 16, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Vos, R.C.; Becher, J.G.; Voorman, J.M.; Gorter, J.W.; van Eck, M.; van Meeteren, J.; Smits, D.-W.; Twisk, J.W.; Dallmeijer, A.J.; van Schie, P.; et al. Longitudinal Association Between Gross Motor Capacity and Neuromusculoskeletal Function in Children and Youth With Cerebral Palsy. Arch. Phys. Med. Rehabil. 2016, 97, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Uysal, İ.; Özden, F.; Tümtürk, İ.; İmerci, A. The effectiveness of dual task exercise training on balance, mobility, physical performance, and quality of life in children with cerebral palsy: A single-blind randomized controlled trial. Ir. J. Med. Sci. 2024, 193, 2123–2124. [Google Scholar] [CrossRef]
- Waqas, S.; Ahmad, A.; Goulardins, J.B.; Hassan, Z.; Hanif, A.; Tariq, M. Conjunct Effects of Transcranial Direct Current Stimulation with Mirror Therapy on Motor Control and Muscle Performance in Spastic Quadriplegic Cerebral Palsy Children: A Randomized Clinical Trial. J. Multidiscip. Healthc. 2025, 18, 1195–1216. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.-C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Mc Namara, M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef]
- Saleh, N.; Salaheldin, A.M.; Badawi, M.; El-Bialy, A. Rehabilitative game-based system for enhancing physical and cognitive abilities of neurological disorders. Cogn. Neurodyn. 2025, 19, 48. [Google Scholar] [CrossRef]
- Arumí-Trujillo, C.; Verdejo-Amengual, F.J.; Martínez-Navarro, O.; Vink, J.J.T.; Valenzuela-Pascual, F. The effectiveness of non-invasive brain stimulation in enhancing lower extremity function in children with spastic cerebral palsy: Protocol for a systematic review and meta-analysis. MethodsX 2024, 14, 103141. [Google Scholar] [CrossRef]
- Blaszczyk, I.; Foumani, N.P.; Ljungberg, C.; Wiberg, M. Questionnaire about the adverse events and side effects following botulinum toxin A treatment in patients with cerebral palsy. Toxins 2015, 7, 4645–4654. [Google Scholar] [CrossRef]
- Kaye, A.D.; Cheon, S.Y.; Roque, M.H.; Gibbs, C.; Mott, K.R.; Wandler, A.M.; Munir, S.T.; Lin, J.; Ahmadzadeh, S.; Siddaiah, H.; et al. Efficacy, Indications, and Safety of Intrathecal Baclofen Pump: A Narrative Review. Curr. Pain Headache Rep. 2025, 29, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, Y.; Chen, T.; Mao, Z.-H.; Xu, J.-Y.; Ding, L.; Ruan, W.-C.; Li, H.-F. Body Acupuncture Conjunction with Rehabilitation for Upper Limb Improves Motor Functions in Children with Spastic Hemiplegic Cerebral Palsy: A Pilot Study. J. Evid.-Based Integr. Med. 2025, 30, 2515690X251317438. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Liang, J.Q.; Xu, X.K.; Xu, Y.X.; Chen, G.Z. Safety of Thread Embedding Acupuncture Therapy: A Systematic Review. Chin. J. Integr. Med. 2021, 27, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.H.; Adel-Mehraban, M.S.; Jamali Dastjerdi, M.; Alipour, R. A comprehensive practical review of acupoint embedding as a semi-permanent acupuncture: A mini review. Medicine 2024, 103, e38314. [Google Scholar] [CrossRef]
- Wang, C.N.; Lan, Y.; Wei, X.H.; Lin, N.N. The effect of catgut embedment in acupoint on the walking ability of children with cerebral palsy. Liaoning J. Tradit. Chin. Med. 2012, 39, 2263–2264. [Google Scholar]
- Liu, H.; Xu, L.; Dong, B.Q. Catgut Implantation Treatment of Dyskinetic Cerebral Palsy Randomized Controlled Study. J. Pract. Tradit. Chin. Intern. Medicine 2013, 27, 113–115. [Google Scholar]
- Lan, Y.; Wang, C.N.; Lin, N.N.; Zhang, Z. Clinical Observation on Catgut Embedding Therapy in Treatment of the Foot-drop in 60 Children with Spastic Cerebral Palsy. Med. Innov. Chin. 2014, 11, 106–108. [Google Scholar]
- Li, H. The effect of catgut embedment in acupoint on gross motor function in children with spastic cerebral palsy. Nei MonGol J. Tradit. Chin. Med. 2014, 33, 86–87. [Google Scholar] [CrossRef]
- Zha, T.Z.; Chen, S.Y.; Tang, S.Z. Clinical observation on 15 cases of hypotonic cerebral palsy treated with acupoint catgut implantation. Chin. J. Ethnomed. Ethnopharm 2015, 24, 76. [Google Scholar]
- Zhang, J.; Xu, K.; Ruan, Y. Impacts on motor function in the children of cerebral palsy treated with acupuncture and acupoint embedding therapy. Chin. Acupunct. Moxibustion 2015, 35, 901–904. [Google Scholar]
- Zhao, B. Clinical study on acupoint catgut embedding therapy for the improvement of motor function in children with cerebral palsy. Acupunct. Moxibustion 2015, 4, 4–6. [Google Scholar]
- Han, L.; Zhao, B.; Sun, D.X. Clinical Study of 45 Cases of Child Cerebral Palsy Treated by Embedding Thread in Acupoint. Shandong J. Tradit. Chin. Med. 2015, 34, 598–600. [Google Scholar]
- Fang, F. Clinical Curative Effect Observation on Chinese Medicine Catgut Implantation Treatment for Dyskinetic Cerebral Palsy. Chin. Foreign Med. Treat. 2016, 35, 158–159+168. [Google Scholar]
- Zhou, Y.; Sun, S.; Han, Y.; Liu, H.; Yuan, H.L.; Tian, L.J. Clinical Observation of Acupoint Thread Embedding plus Motor Function Training for Hip Dislocation in Cerebral Palsy. Shanghai J. Acupunct. Moxibustion 2018, 37, 649–652. [Google Scholar]
- Zhao, Y.L.; Jin, B.X.; Zhao, Y.; He, S.F.; Hou, S.L.; Zhou, Y. Effect of Acupoint Catgut Embedding on Standing Balance and Walking Ability in Cerebral Palsy Children with Ataxia. J. Clin. Acupunct. Moxibustion 2018, 34, 38–41. [Google Scholar]
- Zhang, Y.; Shi, Y.; Lei, Y.B.; Tang, Z.J.; Xiong, Z.F.; Lan, J.D. Effect of Thread Embedding at Governor Vessel Points plus Rehabilitation Training on Spastic Cerebral Palsy. Shanghai J. Acupunct. Moxibustion 2019, 38, 870–873. [Google Scholar]
- Shi, Y.; Zhang, Y.; Huang, L.H.; Lei, Y.B.; Tang, Z.J. Study on Effect of Acupoint Catgut Embedding Therapy on Muscle Tension in Children with Spastic Cerebral Palsy Based on Brain-Kidney-Governor Vessel Axis Theory. J. Guangzhou Univ. Tradit. Chin. Med. 2020, 37, 875–880. [Google Scholar]
- Zhou, Y.; Han, Y.; Sun, S.; Fang, L.N.; Niu, X.L.; Yan, M. Clinical observation on effect of acupoint catgut-embedding on functional rehabilitation of children with involuntary exercise caused by cerebral palsy. Chin. J. Integr. Tradit. West. Med. Intensive Crit. Care 2020, 27, 331–333. [Google Scholar]
- Yan, X.L.; Jin, B.X.; Zhou, Y. Clinical Observation on Acupoint Catgut Embedding in the Treatment of Spastic Diplegic Cerebral Palsy in Children. Chin. Med. Mod. Distance Educ. Chin. 2021, 19, 103–105. [Google Scholar]
- Jia, T.P. Clinical Effect of Acupoint Catgut Embedding Combined with Routine Rehabilitation in Children with Cerebral Palsy. Reflexol. Rehabil. Med. 2022, 3, 18–20,24. [Google Scholar]
- Li, E.W.; Zheng, D.P. Analysis of clinical effect of catgut embedding in Jinsuo acupoint on 12 children with spastic cerebral palsy. Chin. Pract. Med. 2022, 17, 129–131. [Google Scholar]
- Jin, B.-X.; Zhao, Y.; Qian, X.-G.; Zhou, Y.; Zhao, Y.-L.; Liu, H.-Z.; Fu, W.-J.; Li, N. Effect of acupoint thread-embedding therapy on the sitting ability of ataxia children with cerebral palsy. Acupunct. Res. 2019, 44, 668–671. [Google Scholar]
- Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.J.H.W. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5. Available online: www.training.cochrane.org/handbook (accessed on 1 January 2025).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.; Chandler, J.; Welch, V.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Salavati, M.; Krijnen, W.; Rameckers, E.; Looijestijn, P.; Maathuis, C.; van der Schans, C.; Steenbergen, B. Reliability of the modified Gross Motor Function Measure-88 (GMFM-88) for children with both Spastic Cerebral Palsy and Cerebral Visual Impairment: A preliminary study. Res. Dev. Disabil. 2015, 45–46, 32–48. [Google Scholar] [CrossRef]
- Koziol, N.A.; Butera, C.D.; Hsu, L.Y.; Pereira, S.A.; Dusing, S.C. Gross Motor Function Measure-66 Item Sets for use with infants and toddlers at high risk for cerebral palsy: Construct validity and responsiveness. Dev. Med. Child. Neurol. 2025. [Google Scholar] [CrossRef]
- Storm, F.A.; Petrarca, M.; Beretta, E.; Strazzer, S.; Piccinini, L.; Maghini, C.; Panzeri, D.; Corbetta, C.; Morganti, R.; Reni, G.; et al. Minimum Clinically Important Difference of Gross Motor Function and Gait Endurance in Children with Motor Impairment: A Comparison of Distribution-Based Approaches. BioMed Res. Int. 2020, 2020, 2794036. [Google Scholar] [CrossRef]
- Lin, L.-L.; Wang, L.-Q.; Yang, J.-W.; Tu, J.-F.; Wang, T.-Q.; Zou, X.; Sun, N.; Liu, C.-Z. Researches status on time-effect of acupuncture. Chin. Acupunct. Moxibustion 2019, 39, 565–570. [Google Scholar]
- Cui, L.Y.; Ding, M.X. Progress of Researches on Central Mechanism of Electroacupuncture Tolerance. Acupunct. Res. 2016, 41, 550–555. [Google Scholar]
- Yuanjie, Y.; Jianyi, X.; Jinyan, X.; Mao, H.; Siyang, Y.; Zhenjin, Y. Acupuncture in the Treatment of Abnormal Muscle Tone in Children with Cerebral Palsy: A Meta-Analysis. Behav. Neurol. 2023, 2023, 4662788. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Xie, D.; Wang, Y. Bioinformatics-based discovery of biomarkers and immunoinflammatory targets in children with cerebral palsy: An observational study. Medicine 2024, 103, e37828. [Google Scholar] [CrossRef] [PubMed]
- Sanches, E.; Ho, D.; van de Looij, Y.; Toulotte, A.A.; Baud, L.; Bouteldja, F.; Barraud, Q.; Araneda, R.; Bleyenheuft, Y.; Brochard, S.; et al. Early intensive rehabilitation reverses locomotor disruption, decrease brain inflammation and induces neuroplasticity following experimental Cerebral Palsy. Brain Behav. Immun. 2024, 121, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.A.; Broda, N.; Gross, W.R.; Menezes, A.F.; Embry, R.B.; Swaroop, V.T.; Chambers, H.G.; Schipma, M.J.; Lieber, R.L.; Domenighetti, A.A. Differential DNA methylation and transcriptional signatures characterize impairment of muscle stem cells in pediatric human muscle contractures after brain injury. FASEB J. 2021, 35, e21928. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, J.; Wu, M.; Li, J.; Song, W.; Zhu, L. The effect of Kinesio Taping on motor function in children with cerebral palsy: A systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 2025, 16, 1527308. [Google Scholar] [CrossRef]
- Qi, Y.-C.; Xiao, X.-J.; Duan, R.-S.; Yue, Y.-H.; Zhang, X.-L.; Li, J.-T.; Li, Y.-Z. Effect of acupuncture on inflammatory cytokines expression of spastic cerebral palsy rats. Asian Pac. J. Trop. Med. 2014, 7, 492–495. [Google Scholar] [CrossRef]
- Yang, D.M.; Wang, X.Z.; Dong, J.Q.; Liu, Z.Z.; Shang, Q. Acupuncture combined with Heixiaoyao powder for children with cerebral palsy and its effect on serum immune indexes and nerve growth related protein. Chin. Acupunct. Moxibustion 2021, 41, 288–292. [Google Scholar]
- Xu, J.B.; Tong, G.L. Efficacy and mechanism of scalp acupuncture for spastic cerebral palsy. Chin. Acupunct. Moxibustion 2023, 43, 163–169. [Google Scholar]
- Zhao, D.-D.; Tang, C.-L.; Huang, S.-Q.; Luo, A.; Zhang, A.-N.; Guo, Q.-H.; Cao, J.; Gao, R.-Q. Effects of Electroacupuncture on Adaptive Hypertrophy of Gastrocnemius and Proliferation and Differentiation of Muscle Satellite Cells in Rats. Acupunct. Res. 2017, 42, 489–495. [Google Scholar]
- Otaif, A.; Alshammari, M.; Gerin, C.G. Can alternative medical methods evoke somatosensory responses and functional improvement? Heliyon 2024, 10, e30010. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Chang, J.; Shi, J.; Zhao, L. Acupuncture combined with language training for aphasia in children with cerebral palsy: A systematic review with meta-analysis and trial sequential analysis. Front. Neurol. 2025, 16, 1502023. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Huang, Y.Y.; Yang, T.H.; Chang, Y.J.; Fu, R.H.; Chen, H.Y. Acupuncture and Acupoints for Managing Pediatric Cerebral Palsy: A Meta-Analysis of Randomized Controlled Trials. Healthcare 2024, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kimoto, M.; Okada, K.; Kawanobe, U.; Sakamoto, H. Impact of lower muscle stiffness on ankle dorsiflexion restriction in children with cerebral palsy evaluated using ultrasound elastography. Clin. Biomech. 2023, 109, 106092. [Google Scholar] [CrossRef] [PubMed]
- Mayorova, L.; Radutnaya, M.; Varyukhina, M.; Vorobyev, A.; Zhdanov, V.; Petrova, M.; Grechko, A. Immediate Effects of Anti-Spastic Epidural Cervical Spinal Cord Stimulation on Functional Connectivity of the Central Motor System in Patients with Stroke- and Traumatic Brain Injury-Induced Spasticity: A Pilot Resting-State Functional Magnetic Resonance Imaging Study. Biomedicines 2023, 11, 2266. [Google Scholar]
- Mailleux, L.; De Beukelaer, N.; Carbone, M.B.; Ortibus, E. Early interventions in infants with unilateral cerebral palsy: A systematic review and narrative synthesis. Res. Dev. Disabil. 2021, 117, 104058. [Google Scholar] [CrossRef]
- Valentin-Gudiol, M.; Bagur-Calafat, C.; Girabent-Farrés, M.; Hadders-Algra, M.; Mattern-Baxter, K.; Angulo-Barroso, R. Treadmill interventions with partial body weight support in children under six years of age at risk of neuromotor delay: A report of a Cochrane systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2013, 49, 67–91. [Google Scholar]
- Liu, Y.; Zhang, X.; Zhang, Z.; Liu, W.; Huang, S.; Liao, H. Effect of sling exercise training on motor function in children with cerebral palsy: A systematic review and meta-analysis. Medicine 2024, 103, e40086. [Google Scholar] [CrossRef]
- Pauluka, E.; Ceolin, L.S.; Fontanela, L.C.; Dos Santos, A.N. Aquatic Compared With Land-Based Exercises on Gross Motor Function of Children/Adolescents With Cerebral Palsy: A Systematic Review With Meta-Analysis. Child. Care Health Dev. 2025, 51, e70023. [Google Scholar] [CrossRef]
- Wang, B.; Huang, H. Effects of various exercise interventions on motor function in cerebral palsy patients: A systematic review and network meta-analysis. Neurol. Sci. 2024, 45, 5915–5927. [Google Scholar] [CrossRef]
- Pedrosa, G.F.; Lima, F.V.; Schoenfeld, B.J.; Lacerda, L.T.; Simões, M.G.; Pereira, M.R.; Diniz, R.C.; Chagas, M.H. Partial range of motion training elicits favorable improvements in muscular adaptations when carried out at long muscle lengths. Eur. J. Sport Sci. 2022, 22, 1250–1260. [Google Scholar] [CrossRef]


| Study ID | Year | Sample Size (Male/Female) | Age of Patients | Outcomes | Treatment Frequency | Treatment Duration | Treatment Points | ||
|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | ||||||
| CEA + RT vs. Acupuncture + RT | |||||||||
| Wang CN | 2012 [15] | 30 (17/13) | 30 (16/14) | 2~6 (3.6 ± 0.5) years | 2~6 (3.6 ± 0.5) years | (1)(3) | Once a week | 3 months | Body Acupoints |
| Liu H | 2013 [16] | 30 (26/4) | 30 (24/6) | 6~24 (16.4 ± 2.5) months | 6~30 (17.3 ± 2.7) months | (1)(2)(3) | Three times a month | 3 months | Body/Scalp Acupoints |
| Lan Y | 2014 [17] | 30 | 30 | (3.55 ± 0.4) years | (3.49 ± 0.5) years | (1)(3) | Once a week | 3 months | Body Acupoints |
| Zhou Y | 2020 [28] | 40 (28/12) | 40 (24/16) | 6~33 (19.4 ± 6.6) months | 8~35 (20.5 ± 7.4) months | (1) | Once a month | 3 months | Body Acupoints |
| CEA + Acupuncture + RT vs. Acupuncture + RT | |||||||||
| Zhang J | 2015 [20] | 60 (40/20) | 60 (36/24) | 6~20 (14 ± 6) months | 7~22 (14 ± 7) months | (1)(2)(3)(5) | Twice a month | 3 months | Body Acupoints |
| Shi Y | 2020 [27] | 30 | 30 | 1~6 years | 1~6 years | (1) | Three times a month | 3 months | Body/Scalp Acupoints |
| CEA + RT vs. RT | |||||||||
| Li H | 2014 [18] | 50 (29/21) | 50 (32/18) | 12~40 (26.6 ± 8.2) months | 12~48 (28.9 ± 6.8) months | (1)(3) | Once a week | 3 months | Body Acupoints |
| Zha TZ | 2015 [19] | 15 (8/7) | 15 (9/6) | (13 ± 2.3) months | (12 ± 2.1) months | (1)(2)(3) | Three times a month | 1 month | Body Acupoints |
| Zhao B | 2015 [21] | 30 (19/11) | 30 (12/18) | 6~34 (13.6 ± 7.2) months | 6~36 (15.9 ± 8.4) months | (1)(3) | Twice a month | 3 months | Body/Scalp Acupoints |
| Han L | 2015 [22] | 45 (29/16) | 45 (32/13) | 6~34 (13.6 ± 7.2) months | 6~36 (15.9 ± 8.4) months | (1)(3) | Twice a month | 3 months | Body/Scalp Acupoints |
| Fang F | 2016 [23] | 35 (21/14) | 35 (20/15) | 6~30 (14.3 ± 5.2) months | 7~30 (14.4 ± 5.5) months | (1)(2)(3) | Three times a month | 3 months | Body/Scalp Acupoints |
| Zhou Y | 2018 [24] | 35 (20/15) | 35 (18/17) | (47.3 ± 7.9) months | (48.2 ± 8.4) months | (1)(2)(3) | Twice a month | 3 months | Body Acupoints |
| Zhao YL | 2018 [25] | 15 (11/4) | 15 (10/5) | 67~106 (81.3 ± 11.9) months | 64~103 (80.2 ± l1.4) months | (1)(2)(3) | Once a week | 2 months | Body/Scalp Acupoints |
| Zhang Y | 2019 [26] | 20 (10/10) | 20 (9/11) | 1~5 (3 ± 1) years | 1~4 (3 ± 1) years | (1)(3) | Three times a month | 3 months | Body/Scalp Acupoints |
| Yan XL | 2021 [29] | 26 (16/10) | 26 (18/8) | 1.42~4.33 (2.17 ± 0.67) years | 1.25~4.42 (2.38 ± 0.87) years | (1)(2)(4) | Once a week | 3 months | Body Acupoints |
| Jia TP | 2022 [30] | 50 (29/21) | 50 (27/23) | 1~7 (2.94 ± 0.75) years | 1~6 (3.09 ± 0.52) years | (1)(4) | Once a week | 3 months | Body/Scalp Acupoints |
| Li EW | 2022 [31] | 12 (8/4) | 12 (6/6) | (4.2 ± 1.2) years | (4.0 ± 1.3) years | (1)(2)(4) | Twice a month | 2 months | Body Acupoints |
| Outcomes | No. of Studies | Certainty Assessment | No. of Patients | Effect | Certainty | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | E | C | Relative (95% CI) | Absolute (95% CI) | ||||
| GMFM | 17 | RCTs | serious a | serious b | not serious | not serious | none | 553 | 553 | - | SMD 0.90 higher (0.57 higher to 1.23 higher) | ⊕⊕◯◯ Low a,b | CRITICAL |
| GMFM Percentage | 8 | RCTs | serious a | not serious | not serious | serious c | none | 253 | 253 | - | MD 5.41 higher (3.52 higher to 7.29 higher) | ⊕⊕◯◯ Low a,c | CRITICAL |
| ER | 3 | RCTs | serious a | not serious | not serious | not serious | none | 335/395 (84.8%) | 259/395 (65.6%) | RR 1.24 (1.16 to 1.34) | 157 more per 1000 (from 105 more to 223 more) | ⊕⊕⊕◯ Moderate a | - |
| MAS | 12 | RCTs | serious a | not serious | not serious | serious c | none | 88 | 88 | - | MD 0.40 lower (0.58 lower to 0.23 lower) | ⊕⊕◯◯ Low a,c | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.-H.; Zhang, X.-Y.; Jiang, H.-Z.; Li, X.-J.; Hao, Y.-F. New Perspectives on the Efficacy of Catgut Embedment in Acupoint Combined with Rehabilitation Training for Pediatric-Cerebral-Palsy Motor Function Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare 2025, 13, 1301. https://doi.org/10.3390/healthcare13111301
Hu Z-H, Zhang X-Y, Jiang H-Z, Li X-J, Hao Y-F. New Perspectives on the Efficacy of Catgut Embedment in Acupoint Combined with Rehabilitation Training for Pediatric-Cerebral-Palsy Motor Function Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare. 2025; 13(11):1301. https://doi.org/10.3390/healthcare13111301
Chicago/Turabian StyleHu, Zhe-Hao, Xin-Yue Zhang, Hong-Zhan Jiang, Xue-Jing Li, and Yu-Fang Hao. 2025. "New Perspectives on the Efficacy of Catgut Embedment in Acupoint Combined with Rehabilitation Training for Pediatric-Cerebral-Palsy Motor Function Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Healthcare 13, no. 11: 1301. https://doi.org/10.3390/healthcare13111301
APA StyleHu, Z.-H., Zhang, X.-Y., Jiang, H.-Z., Li, X.-J., & Hao, Y.-F. (2025). New Perspectives on the Efficacy of Catgut Embedment in Acupoint Combined with Rehabilitation Training for Pediatric-Cerebral-Palsy Motor Function Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare, 13(11), 1301. https://doi.org/10.3390/healthcare13111301





