Effects of Active Spinal Orthosis on Fatty Infiltration in Paraspinal Muscles in Kyphotic Women with Osteoporotic Vertebral Fracture—Sub-Analysis of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
2.3. Study Outcome
2.4. Assessments
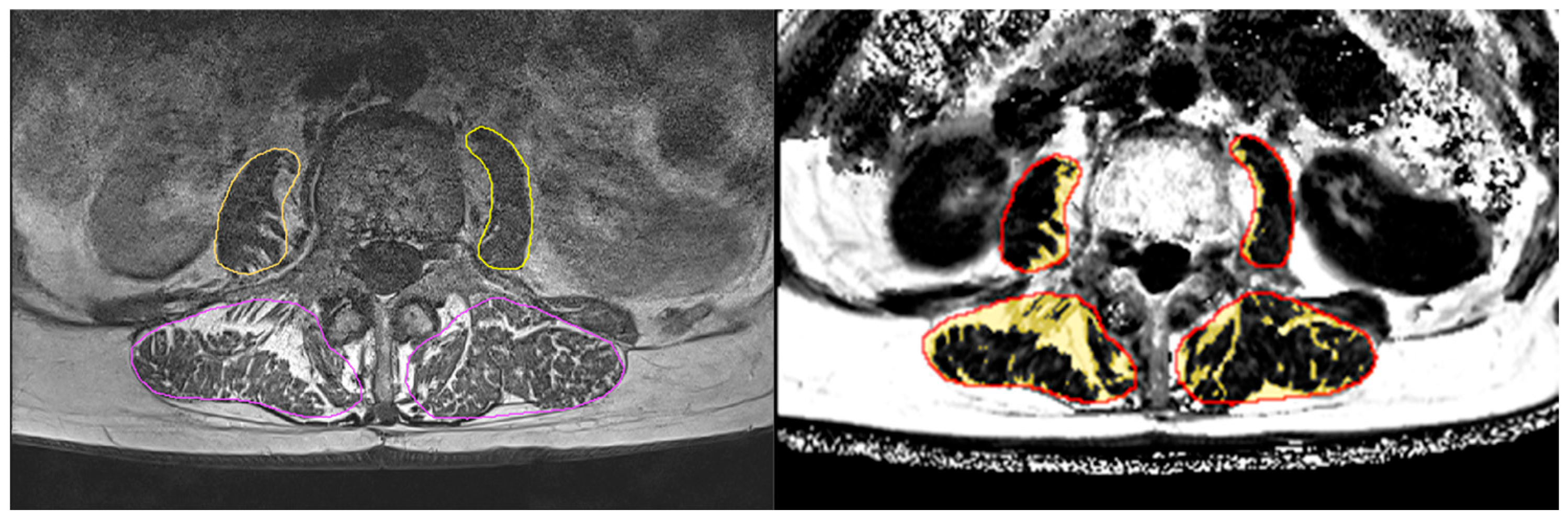
2.4.1. Magnetic Resonance Imaging (MRI)
2.4.2. Maximum Isometric Trunk Strength Assessment
2.4.3. Participant Characteristics and Confounding Factors
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Study Outcomes
Exploratory Outcomes
3.3. Confounding Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCOVA | Analysis of covariance |
| CG | Control group |
| FF | Fat fraction |
| IF | Intra-fascial |
| IMAT | Intermuscular adipose tissue |
| MRI | Magnetic resonance imaging |
| ROI | Region of interest |
| SOG | Spinal orthosis group |
References
- Alin, C.K.; Frisendahl, N.; Kronhed, A.G.; Salminen, H. Experiences of using an activating spinal orthosis in women with osteoporosis and back pain in primary care. Arch. Osteoporos. 2020, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Dionyssiotis, Y.; Trovas, G.; Thoma, S.; Lyritis, G.; Papaioannou, N. Prospective study of spinal orthoses in women. Prosthet. Orthot. Int. 2015, 39, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Hettchen, M.; Willert, S.; von Stengel, S.; Kohl, M.; Kemmler, W. Effects of the “Spinomed active” orthosis on chronic back pain in kyphotic women with osteoporotic vertebral fractures three months and older: A randomized controlled study. Front. Pain. Res. 2022, 3, 1038269. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Begerow, B.; Minne, H.W. Effects of a new spinal orthosis on posture, trunk strength, and quality of life in women with postmenopausal osteoporosis: A randomized trial. Am. J. Phys. Med. Rehabil. 2004, 83, 177–186. [Google Scholar] [CrossRef]
- Pfeifer, M.; Kohlwey, L.; Begerow, B.; Minne, H.W. Effects of two newly developed spinal orthoses on trunk muscle strength, posture, and quality-of-life in women with postmenopausal osteoporosis: A randomized trial. Am. J. Phys. Med. Rehabil. 2011, 90, 805–815. [Google Scholar] [CrossRef]
- Keshavarzi, F.; Arazpour, M. Effect of spinal orthoses on osteoporotic elderly patients kyphosis, back muscles strength, balance and osteoporotic vertebral fractures: (A systematic review and meta-analysis). J. Rehabil. Assist. Technol. Eng. 2024, 11, 20556683241268605. [Google Scholar] [CrossRef]
- Kircher, K.; Chaudry, O.; Nagel, A.M.; Ghasemikaram, M.; Uder, M.; Jakob, F.; Kohl, M.; Kemmler, W.; Engelke, K. Effects of high-intensity training on fatty infiltration in paraspinal muscles in elderly males with osteosarcopenia—The randomized controlled FrOST study. BMC Geriatr. 2024, 24, 141. [Google Scholar] [CrossRef]
- Wesselink, E.O.; Pool, J.J.M.; Mollema, J.; Weber, K.A., 2nd; Elliott, J.M.; Coppieters, M.W.; Pool-Goudzwaard, A.L. Is fatty infiltration in paraspinal muscles reversible with exercise in people with low back pain? A systematic review. Eur. Spine J. 2023, 32, 787–796. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Ma, J. Dixon techniques for water and fat imaging. J. Magn. Reson. Imaging 2008, 28, 543–558. [Google Scholar] [CrossRef]
- Chaudry, O.; Friedberger, A.; Grimm, A.; Uder, M.; Nagel, A.M.; Kemmler, W.; Engelke, K. Segmentation of the fascia lata and reproducible quantification of intermuscular adipose tissue (IMAT) of the thigh. Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; Lauber, D.; Weineck, J.; Hensen, J.; Kalender, W.; Engelke, K. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: Results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Arch. Intern. Med. 2004, 164, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associate: Hillsdale, NJ, USA, 1988; pp. 8–16. [Google Scholar]
- Gießing, J. HIT-Hochintensitätstraining; Novagenics-Verlag: Arnsberg, Germany, 2008. [Google Scholar]
- Kemmler, W.; Weineck, M.; Kohl, M.; von Stengel, S.; Giessing, J.; Fröhlich, M.; Schoene, D. High Intensity Resistance Exercise Training to Improve Body Composition and Strength in Older Men with Osteosarcopenia. Results of the Randomized Controlled Franconian Osteopenia and Sarcopenia Trial (FrOST). Front. Sports Act. Living 2020, 2, 4. [Google Scholar] [CrossRef]
- Kemmler, W.; Kohl, M.; Jakob, F.; Engelke, K.; von Stengel, S. Effects of High Intensity Dynamic Resistance Exercise and Whey Protein Supplements on Osteosarcopenia in Older Men with Low Bone and Muscle Mass. Final Results of the Randomized Controlled FrOST Study. Nutrients 2020, 12, 2341. [Google Scholar] [CrossRef]
- Ghasemikaram, M.; Chaudry, O.; Nagel, A.M.; Uder, M.; Jakob, F.; Kemmler, W.; Kohl, M.; Engelke, K. Effects of 16 months of high intensity resistance training on thigh muscle fat infiltration in elderly men with osteosarcopenia. Geroscience 2021, 3, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.B.; Padwal, J.; Johnson, S.; Englund, E.K.; Ward, S.R.; Shahidi, B. The effect of high-intensity resistance exercise on lumbar musculature in patients with low back pain: A preliminary study. BMC Musculoskelet. Disord. 2019, 20, 290. [Google Scholar] [CrossRef]
- Welch, N.; Moran, K.; Antony, J.; Richter, C.; Marshall, B.; Coyle, J.; Falvey, E.; Franklyn-Miller, A. The effects of a free-weight-based resistance training intervention on pain, squat biomechanics and MRI-defined lumbar fat infiltration and functional cross-sectional area in those with chronic low back. BMJ Open Sport. Exerc. Med. 2015, 1, e000050. [Google Scholar] [CrossRef]
- Hummel, J.; Engelke, K.; Freitag-Wolf, S.; Yilmas, E.; Bartenschlager, S.; Sigurdsson, S.; Gudnason, V.; Glüer, C.C.; Chaudry, O. Trabecular texture and paraspinal muscle characteristics for prediction of first vertebral fracture: A QCT analysis from the AGES cohort. Front. Endocrinol. 2025, 16, 1566424. [Google Scholar] [CrossRef]
- Stohldreier, Y.; Leonhardt, Y.; Ketschau, J.; Gassert, F.T.; Makowski, M.R.; Kirschke, J.S.; Feuerriegel, G.C.; Braun, P.; Schwaiger, B.J.; Karampinos, D.C.; et al. Longitudinal assessment of changes in muscle composition using proton density fat fraction and T2* in patients with and without incidental vertebral compression fractures. Front. Endocrinol. 2025, 16, 1549068. [Google Scholar] [CrossRef]
- Oranchuk, D.J.; Storey, A.G.; Nelson, A.R.; Cronin, J.B. Isometric training and long-term adaptations: Effects of muscle length, intensity, and intent: A systematic review. Scand. J. Med. Sci. Sports 2019, 29, 484–503. [Google Scholar] [CrossRef]
- Weineck, J. Sportbiologie; Spitta Verlag: Erlangen, Germany, 2010; Volume 10. [Google Scholar]
- Engelke, K.; Ghasemikaram, M.; Chaudry, O.; Uder, M.; Nagel, A.M.; Jakob, F.; Kemmler, W. The effect of ageing on fat infiltration of thigh and paraspinal muscles in men. Aging Clin. Exp. Res. 2022, 34, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, S.; Inhuber, S.; Rohrmeier, A.; Dieckmeyer, M.; Freitag, F.; Klupp, E.; Weidlich, D.; Feuerriegel, G.; Kreuzpointner, F.; Schwirtz, A.; et al. Association of paraspinal muscle water-fat MRI-based measurements with isometric strength measurements. Eur. Radiol. 2019, 29, 599–608. [Google Scholar] [CrossRef] [PubMed]
| Variable | SOG (n = 11) MV ± SD | CG (n = 10) MV ± SD | p |
|---|---|---|---|
| Age [years] | 77.6 ± 5.8 | 73.3 ± 8.3 | 0.477 |
| Body height [cm] 1 | 160.4 ± 4.4 | 162.9 ± 10.4 | 0.488 |
| Body mass [kg] 2 | 60.1 ± 10.5 | 59.8 ± 9.7 | 0.944 |
| Vertebral fractures [number] 3 | 2.45 ± 0.69 | 2.50 ± 0.85 | 0.894 |
| Vertebral fracture age [years] 4 | 3.10 ± 1.64 | 3.56 ± 1.99 | 0.576 |
| Osteoporosis Medication [n] 3 | 8 | 7 | 0.890 |
| Kyphosis-angle [°] 5 | 48.6 ± 6.6 | 46.1 ± 9.0 | 0.482 |
| Back Pain Intensity [Index] 6 | 2.8 ± 1.2 | 2.2 ± 1.4 | 0.305 |
| Back Extensor Strength [kg] 7 | 25.8 ± 7.6 | 29.6 ± 8.3 | 0.279 |
| Variable | SOG MV ± SD | CG MV ± SD | Difference MV (95% CI) | p |
|---|---|---|---|---|
| Erector spinae muscle volume [cm3] | ||||
| Baseline value | 10.4 ± 1.82 | 10.3 ± 1.43 | 0.962 | |
| Changes after 4 months | 0.133 ± 0.282 0.153 | 0.021 ± 0.295 0.829 | 0.115 (−0.161 to 0.391) | 0.393 |
| Erector spinae fat fraction [%] | ||||
| Baseline value | 34.8 ± 14.1 | 26.0 ± 6.0 | 0.084 | |
| Changes after 4 months | −0.23 ± 2.22 0.734 | 0.00 ± 2.32 0.999 | 0.09 (−2.12 to 2.31) | 0.930 |
| Erector spinae MT volume [cm3] | ||||
| Baseline value | 6.06 ± 1.89 | 7.42 ± 1.40 | 0.086 | |
| Changes after 4 months | 0.148 ± 0.398 0.231 | −0.027 ± 0.417 0.835 | 0.227 (−0.158 to 0.612) | 0.231 |
| Erector spinae MT fat fraction [%] | ||||
| Baseline value | 12.0 ± 3.9 | 8.8 ± 1.51 | 0.029 | |
| Changes after 4 months | −0.510 ± 1.09 0.503 | 0.332 ± 1.15 0.349 | 0.941 (−1.08 to 1.04) | 0.096 |
| Erector spinae IMAT volume [cm3] | ||||
| Baseline value | 4.31 ± 2.22 | 2.93 ± 0.091 | 0.085 | |
| Changes after 4 months | −0.064 ± 0.154 0.189 | 0.040 ± 0.162 0.422 | 0.084 (−0.068 to 0.237) | 0.646 |
| Psoas major muscle volume [cm3] | ||||
| Baseline value | 3.89 ± 0.95 | 3.71 ± 0.55 | 0.609 | |
| Changes after 4 months | 0.084 ± 0.177 0.257 | −0.156 ± 0.227 0.044 | 0.257 (0.004 to 0.470) | 0.021 |
| Psoas major fat fraction [%] | ||||
| Baseline value | 14.8 ± 3.1 | 13.2 ± 5.5 | 0.428 | |
| Changes after 4 months | −0.17 ± 1.56 0.716 | 0.32 ± 1.49 0.509 | 0.41 (−1.86 to 1.04) | 0.561 |
| Psoas major MT volume [cm3] | ||||
| Baseline value | 3.30 ± 0.79 | 3.22 ± 0.62 | 0.741 | |
| Changes after 4 months | 0.178 ± 0.171 0.003 | −0.127 ± 0.168 0.047 | 0.309 (0.150 to 0.467) | 0.001 |
| Psoas major MT fat fraction [%] | ||||
| Baseline value | 6.95 ± 0.98 | 6.25 ± 0.87 | 0.890 | |
| Changes after 4 months | −0.242 ± 1.04 0.503 | 0.171 ± 1.14 0.651 | 0.291 (−0.73 to 1.31) | 0.428 |
| Psoas major IMAT volume [cm3] | ||||
| Baseline value | 0.591 ± 0.251 | 0.500 ± 0.390 | 0.293 | |
| Changes after 4 months | −0.034 ± 0.094 0.253 | −0.044 ± 0.095 0.161 | 0.019 (−0.066 to 0.104) | 0.646 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiller, M.; Kohl, M.; Chaudry, O.; Engelke, K.; von Stengel, S.; Kemmler, W. Effects of Active Spinal Orthosis on Fatty Infiltration in Paraspinal Muscles in Kyphotic Women with Osteoporotic Vertebral Fracture—Sub-Analysis of a Randomized Controlled Trial. Healthcare 2025, 13, 1262. https://doi.org/10.3390/healthcare13111262
Hiller M, Kohl M, Chaudry O, Engelke K, von Stengel S, Kemmler W. Effects of Active Spinal Orthosis on Fatty Infiltration in Paraspinal Muscles in Kyphotic Women with Osteoporotic Vertebral Fracture—Sub-Analysis of a Randomized Controlled Trial. Healthcare. 2025; 13(11):1262. https://doi.org/10.3390/healthcare13111262
Chicago/Turabian StyleHiller, Marco, Matthias Kohl, Oliver Chaudry, Klaus Engelke, Simon von Stengel, and Wolfgang Kemmler. 2025. "Effects of Active Spinal Orthosis on Fatty Infiltration in Paraspinal Muscles in Kyphotic Women with Osteoporotic Vertebral Fracture—Sub-Analysis of a Randomized Controlled Trial" Healthcare 13, no. 11: 1262. https://doi.org/10.3390/healthcare13111262
APA StyleHiller, M., Kohl, M., Chaudry, O., Engelke, K., von Stengel, S., & Kemmler, W. (2025). Effects of Active Spinal Orthosis on Fatty Infiltration in Paraspinal Muscles in Kyphotic Women with Osteoporotic Vertebral Fracture—Sub-Analysis of a Randomized Controlled Trial. Healthcare, 13(11), 1262. https://doi.org/10.3390/healthcare13111262






