Impact of Multiple Sclerosis on Load Distribution, Plantar Pressures, and Ankle Dorsiflexion Range of Motion in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size
2.3. Study Population
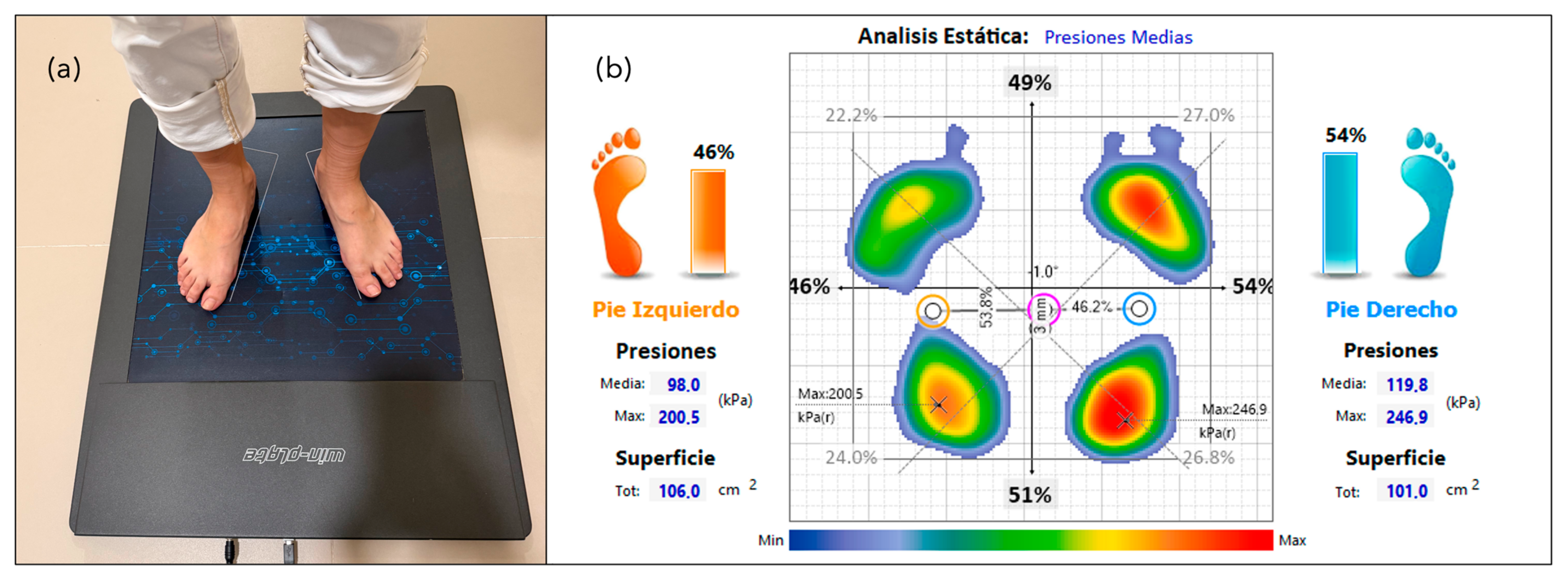
2.4. Data Collection
2.5. Statistical Analysis
3. Results
4. Discussion
Implications for Clinical Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| EDSS | Expanded Disability Status Scale |
| BMI | Body Mass Index |
| DF | Dorsiflexion |
References
- Lorenzut, S.; Negro, I.D.; Pauletto, G.; Verriello, L.; Spadea, L.; Salati, C.; Musa, M.; Gagliano, C.; Zeppieri, M. Exploring the Pathophysiology, Diagnosis, and Treatment Options of Multiple Sclerosis. J. Integr. Neurosci. 2025, 24, 25081. [Google Scholar] [CrossRef] [PubMed]
- Charabati, M.; Wheeler, M.A.; Weiner, H.L.; Quintana, F.J. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell 2023, 186, 1309–1327. [Google Scholar] [CrossRef]
- Cayuela, L.; García-Muñoz, C.; Sainz de la Maza, S.; Cayuela, A. Prevalence of multiple sclerosis in Spain. Estimates from the Primary Care Clinical Database (BDCAP). Neurologia 2024, in press. [Google Scholar] [CrossRef]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef]
- Bove, R.; Chitnis, T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult. Scler. 2014, 20, 520–526. [Google Scholar] [CrossRef]
- Moura, J.A.; Teixeira, L.A.D.C.; Tanor, W.; Lacerda, A.C.R.; Mezzarane, R.A. Prevalence of multiple sclerosis in Brazil: An updated systematic review with meta-analysis. Clin. Neurol. Neurosurg. 2025, 249, 108741. [Google Scholar] [CrossRef]
- Ponzio, M.; Monti, M.C.; Mallucci, G.; Borrelli, P.; Fusco, S.; Tacchino, A.; Brichetto, G.; Tronconi, L.; Montomoli, C.; Bergamaschi, R. The economic impact of comorbidity in multiple sclerosis. Neurol. Sci. 2023, 44, 999–1008. [Google Scholar] [CrossRef]
- Cameron, M.H.; Wagner, J.M. Gait abnormalities in multiple sclerosis: Pathogenesis, evaluation, and advances in treatment. Curr. Neurol. Neurosci. Rep. 2011, 11, 507–515. [Google Scholar] [CrossRef]
- Sosnoff, J.J.; Socie, M.J.; Boes, M.K.; Sandroff, B.M.; Pula, J.H.; Suh, Y.; Weikert, M.; Balantrapu, S.; Morrison, S.; Motl, R.W. Mobility, balance and falls in persons with multiple sclerosis. PLoS ONE 2011, 6, e28021. [Google Scholar] [CrossRef]
- Massot, C.; Guyot, M.A.; Donze, C.; Simoneau, E.; Gillet, C.; Leteneur, S. Ankle dysfunction in multiple sclerosis and the effects on walking. Disabil. Rehabil. 2021, 43, 2454–2463. [Google Scholar] [CrossRef]
- Hoang, P.D.; Psarakis, M.; Kwah, L.K.; Clarke, J.L.; Gandevia, S.C.; Diong, J. Brief report: Passive mechanical properties of gastrocnemius in multiple sclerosis and ankle contracture. Clin. Biomech. 2021, 84, 105338. [Google Scholar] [CrossRef] [PubMed]
- Mecagni, C.; Smith, J.P.; Roberts, K.E.; O’Sullivan, S.B. Balance and ankle range of motion in community-dwelling women aged 64 to 87 years: A correlational study. Phys. Ther. 2000, 80, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guillén, D.; Tolsada-Velasco, C.; Roig-Casasús, S.; Costa-Moreno, E.; Borja-de-Fuentes, I.; Blasco, J.M. Association ankle function and balance in community-dwelling older adults. PLoS ONE 2021, 16, e0247885. [Google Scholar] [CrossRef]
- Searle, A.; Spink, M.J.; Ho, A.; Chuter, V.H. Association between ankle equinus and plantar pressures in people with diabetes. A systematic review and meta-analysis. Clin. Biomech. 2017, 43, 8–14. [Google Scholar] [CrossRef]
- McNab, B.; Sadler, S.; Lanting, S.; Chuter, V. The relationship between foot and ankle joint flexibility measures and barefoot plantar pressures in healthy older adults: A cross-sectional study. BMC Musculoskelet. Disord. 2022, 23, 729. [Google Scholar] [CrossRef]
- Keklicek, H.; Cetin, B.; Salci, Y.; Balkan, A.F.; Altinkaynak, U.; Armutlu, K. Investigating the dynamic plantar pressure distribution and loading pattern in subjects with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 20, 186–191. [Google Scholar] [CrossRef]
- Kaya, M.; Karakuş, S.; Tuncer, S.A. Detection of ataxia with hybrid convolutional neural network using static plantar pressure distribution model in patients with multiple sclerosis. Comput. Methods Programs Biomed. 2022, 214, 106525. [Google Scholar] [CrossRef]
- Jones, S.L.; van Emmerik, R.E.A. Impaired foot vibration sensitivity is related to altered plantar pressures during walking in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 75, 104767. [Google Scholar] [CrossRef]
- Medina McKeon, J.M.; Hertel, J. Sex differences and representative values for 6 lower extremity alignment measures. J. Athl. Train. 2009, 44, 249–255. [Google Scholar] [CrossRef]
- Nakagawa, T.H.; Moriya, E.T.; Maciel, C.D.; Serrão, F.V. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single-leg squat in males and females with and without patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2012, 42, 491–501. [Google Scholar] [CrossRef]
- Wilkerson, R.D.; Mason, M.A. Differences in men’s and women’s mean ankle ligamentous laxity. Iowa Orthop. J. 2000, 20, 46–48. [Google Scholar]
- Wunderlich, R.E.; Cavanagh, P.R. Gender differences in adult foot shape: Implications for shoe design. Med. Sci. Sports Exerc. 2001, 33, 605–611. [Google Scholar] [CrossRef]
- Jallouli, S.; Ghroubi, S.; Ben Dhia, I.; Sakka, S.; Damak, M.; Jaafar, B.; Yahia, A.; Elleuch, M.H.; Mhiri, C.; Hammouda, O. Static postural balance and manual dexterity are sex-dependent among a sample of relapsing-remitting multiple sclerosis patients. Neurol. Res. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Erdeo, F.; Salcı, Y.; Uca, A.U.; Armutlu, K. Examination of the effects of coordination and balance problems on gait in ataxic multiple sclerosis patients. Neurosciences 2019, 24, 269–277. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Martin, R.L.; McPoil, T.G. Reliability of ankle goniometric measurements: A literature review. J. Am. Podiatr. Med. Assoc. 2005, 95, 564–572. [Google Scholar] [CrossRef]
- Balgetir, F.; Bilek, F.; Kakakus, S.; Arslan-Tuncer, S.; Demir, C.F. Detection of ataxia in low disability MS patients by hybrid convolutional neural networks based on images of plantar pressure distribution. Mult. Scler. Relat. Disord. 2021, 56, 103261. [Google Scholar] [CrossRef]
- Norbye, A.D.; Midgard, R.; Thrane, G. Spasticity, gait, and balance in patients with multiple sclerosis: A cross-sectional study. Physiother. Res. Int. 2020, 25, e1799. [Google Scholar] [CrossRef]
- Baumbach, S.F.; Brumann, M.; Binder, J.; Mutschler, W.; Regauer, M.; Polzer, H. The influence of knee position on ankle dorsiflexion—A biometric study. BMC Musculoskelet. Disord. 2014, 15, 246. [Google Scholar] [CrossRef]
- Feldman, R.; Schreiber, S.; Pick, C.G.; Been, E. Gait, balance, mobility and muscle strength in people with anxiety compared to healthy individuals. Hum. Mov. Sci. 2019, 67, 102513. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Martinez, G.; Cereatti, A.; Della Croce, U.; Ventura, L.; Dvir, Z.; Deriu, F. Isokinetic predictors of gait speed increase following high-intensity resistance training of the ankle dorsiflexors in people with multiple sclerosis: A pilot study. Clin. Biomech. 2019, 67, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ramdharry, G.M.; Marsden, J.F.; Day, B.L.; Thompson, A.J. De-stabilizing and training effects of foot orthoses in multiple sclerosis. Mult. Scler. J. 2006, 12, 219–226. [Google Scholar] [CrossRef]
- Ashkar, M.; Razeghinezhad, R.; Moghadasi Chevinlee, H.; Tavakoli, B.; Bagherzadeh Cham, M. The effects of orthotics device on the balance control in multiple sclerosis patients: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022, 66, 104005. [Google Scholar] [CrossRef]
- Monaghan, A.S.; Monaghan, P.G.; Richmond, S.B.; Roper, J.A.; Fling, B.W. The effect of shoe cushioning on gait and balance in females with multiple sclerosis. Exp. Brain Res. 2021, 239, 2593–2603. [Google Scholar] [CrossRef]
- Lee, Y.; Chen, K.; Ren, Y.; Son, J.; Cohen, B.A.; Sliwa, J.A.; Zhang, L.-Q. Robot-guided ankle sensorimotor rehabilitation of patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 11, 65–70. [Google Scholar] [CrossRef]
- Searle, A.; Spink, M.J.; Chuter, V.H. Weight bearing versus non-weight bearing ankle dorsiflexion measurement in people with diabetes: A cross sectional study. BMC Musculoskelet. Disord. 2018, 19, 183. [Google Scholar] [CrossRef]
| Sociodemographic Characteristics | Patients with MS n = 42 | Patients Without MS n = 42 | p Value |
|---|---|---|---|
| Mean ± SD n (%) | Mean ± SD n (%) | ||
| Age (years) | 55.31 ± 8.27 | 55.21 ± 8.07 | 0.911 a |
| BMI (kg/m2) | 0.224 | ||
| Underweight (BMI < 18.5) | 2 (4.8) | 0 (0) | |
| Normal weight (BMI 18.5–24.9) | 17 (40.5) | 24 (57.1) | |
| Overweight (BMI 25–29.9) | 14 (33.3) | 13 (31) | |
| Obesity (BMI ≥ 30) | 9 (21.4) | 5 (11.9) | |
| BMI (kg/m2) | 24.65 ± 3.89 | 26.09 ± 5.80 | 0.357 a |
| Educational level | 0.114 | ||
| No formal education | 1 (2.4) | 1 (2.4) | |
| Primary education | 10 (23.8) | 6 (14.3) | |
| Secondary education | 18 (42.9) | 11 (26.2) | |
| University education | 13 (31) | 24 (57.1) | |
| Marital status | 0.757 | ||
| Single | 9 (21.4) | 7 (16.7) | |
| Married | 24 (57.1) | 27 (64.3) | |
| Separated or divorced | 5 (11.9) | 6 (14.3) | |
| Widowed | 4 (9.5) | 2 (4.8) | |
| Employment status | 0.093 | ||
| Employed | 19 (45.2) | 30 (71.4) | |
| Unemployed | 6 (14.3) | 2 (4.8) | |
| On medical leave | 4 (9.5) | 3 (7.1) | |
| Retired or receiving a pension | 13 (31) | 7 (16.7) | |
| Smoking habits | 0.030 * | ||
| Smoker | 8 (19) | 5 (11.9) | |
| Former smoker | 22 (52.4) | 13 (31) | |
| Non-smoker | 12 (28.6) | 24 (57.1) | |
| Physical activity | 0.794 | ||
| Sedentary | 10 (23.8) | 9 (21.4) | |
| Physically active | 32 (76.2) | 33 (78.6) | |
| Comorbidities | |||
| Diabetes mellitus | 4 (9.5) | 1 (2.4) | 0.316 |
| Hypothyroidism | 7 (16.7) | 6 (14.3) | 0.763 |
| Arterial hypertension | 6 (14.3) | 8 (19) | 0.558 |
| Hypercholesterolemia | 13 (31) | 11 (26.2) | 0.629 |
| Irritable bowel syndrome | 1 (2.4) | 3 (7.1) | 0.616 |
| Depression | 14 (33.3) | 3 (7.1) | 0.003 * |
| Anxiety | 16 (38.1) | 5 (11.9) | 0.006 * |
| Pressure Platform | Patients with MS n = 42 | Patients Without MS n = 42 | p Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Mean pressure of the right foot (kPa) | 109.28 ± 9.18 | 111.12 ± 8.63 | 0.345 b |
| Mean pressure of the left foot (kPa) | 111.72 ± 9.34 | 111.72 ± 7.24 | 0.999 b |
| Maximum pressure of the right foot (kPa) | 232.87 ± 14.64 | 234.77 ± 13.46 | 0.390 a |
| Maximum pressure of the left foot (kPa) | 240.18 ± 13.19 | 242.21 ± 9.62 | 0.750 a |
| Surface area of the right foot (cm2) | 115.43 ± 24.38 | 111.07 ± 22.42 | 0.198 b |
| Surface area of the left foot (cm2) | 117.81 ± 21.50 | 114.43 ± 22.70 | 0.485 b |
| Load on the right foot (%) | 48.88 ± 4.99 | 49.31 ± 2.78 | 0.589 a |
| Load on the left foot (%) | 51.10 ± 4.97 | 50.69 ± 2.78 | 0.611 a |
| Load on the right forefoot (%) | 25.75 ± 5.21 | 23.41 ± 5.56 | 0.021 a* |
| Load on the left forefoot (%) | 26.57 ± 5.80 | 25.16 ± 5.89 | 0.136 b |
| Load on the right rearfoot (%) | 23.09 ± 5.47 | 26.01 ± 4.22 | 0.004 b* |
| Load on the left rearfoot (%) | 24.59 ± 4.88 | 26.60 ± 4.34 | 0.025 a* |
| Load on the forefoot (%) | 52.33 ± 8.66 | 46.40 ± 8.16 | <0.001 b* |
| Load on the rearfoot (%) | 47.64 ± 8.67 | 52.42 ± 8.27 | 0.006 b* |
| Measurement | Patients with MS n = 42 | Patients Without MS n = 42 | p Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| DF with knee flexed (right) | 5.95° ± 4.50 | 15.45° ± 5.04 | <0.001 b* |
| DF with knee flexed (left) | 6.76° ± 4.69 | 14.90° ± 5.43 | <0.001 b* |
| DF with knee extended (right) | 2.69° ± 3.69 | 8.17° ± 3.41 | <0.001 a* |
| DF with knee extended (left) | 3.12° ± 3.83 | 8.60° ± 3.31 | <0.001 a* |
| B | Standard Error | Wald | gl | Sig. | Exp (B) | 95% C.I. for Exp (B) | |
|---|---|---|---|---|---|---|---|
| Anxiety | 2.223 | 1.016 | 4.785 | 1 | 0.029 | 9.238 | 1.260–67.721 |
| Load on the forefoot | 0.157 | 0.068 | 5.353 | 1 | 0.021 | 1.170 | 1.024–1.336 |
| DF with knee flexed (right) | −0.535 | 0.162 | 10.969 | 1 | <0.001 | 0.586 | 0.427–0.804 |
| DF with knee extended (right) | 0.509 | 0.255 | 3.998 | 1 | 0.046 | 1.664 | 1.010–2.742 |
| DF with knee extended (left) | −0.407 | 0.200 | 4.114 | 1 | 0.043 | 0.666 | 0.450–0.986 |
| Constant | −2.925 | 2.928 | 0.998 | 1 | 0.318 | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zúnica-García, S.; Chicharro-Luna, E.; Gracia-Sánchez, A.; Jiménez-Trujillo, I.; García-Campos, J.; Sempere, Á.P. Impact of Multiple Sclerosis on Load Distribution, Plantar Pressures, and Ankle Dorsiflexion Range of Motion in Women. Healthcare 2025, 13, 1231. https://doi.org/10.3390/healthcare13111231
Zúnica-García S, Chicharro-Luna E, Gracia-Sánchez A, Jiménez-Trujillo I, García-Campos J, Sempere ÁP. Impact of Multiple Sclerosis on Load Distribution, Plantar Pressures, and Ankle Dorsiflexion Range of Motion in Women. Healthcare. 2025; 13(11):1231. https://doi.org/10.3390/healthcare13111231
Chicago/Turabian StyleZúnica-García, Sara, Esther Chicharro-Luna, Alba Gracia-Sánchez, Isabel Jiménez-Trujillo, Jonatan García-Campos, and Ángel P. Sempere. 2025. "Impact of Multiple Sclerosis on Load Distribution, Plantar Pressures, and Ankle Dorsiflexion Range of Motion in Women" Healthcare 13, no. 11: 1231. https://doi.org/10.3390/healthcare13111231
APA StyleZúnica-García, S., Chicharro-Luna, E., Gracia-Sánchez, A., Jiménez-Trujillo, I., García-Campos, J., & Sempere, Á. P. (2025). Impact of Multiple Sclerosis on Load Distribution, Plantar Pressures, and Ankle Dorsiflexion Range of Motion in Women. Healthcare, 13(11), 1231. https://doi.org/10.3390/healthcare13111231






