Respiratory Muscle Strength Training in Parkinson’s Disease—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Meta-Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Main Findings
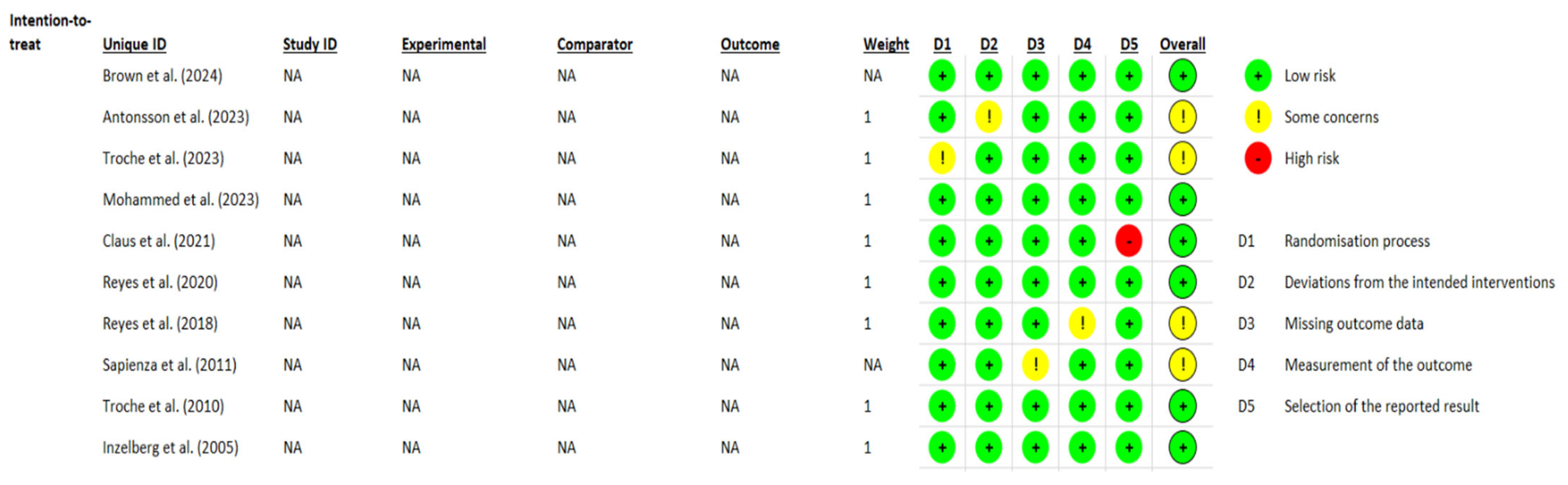
3.4. Methodological Quality and Risk of Bias Assessment
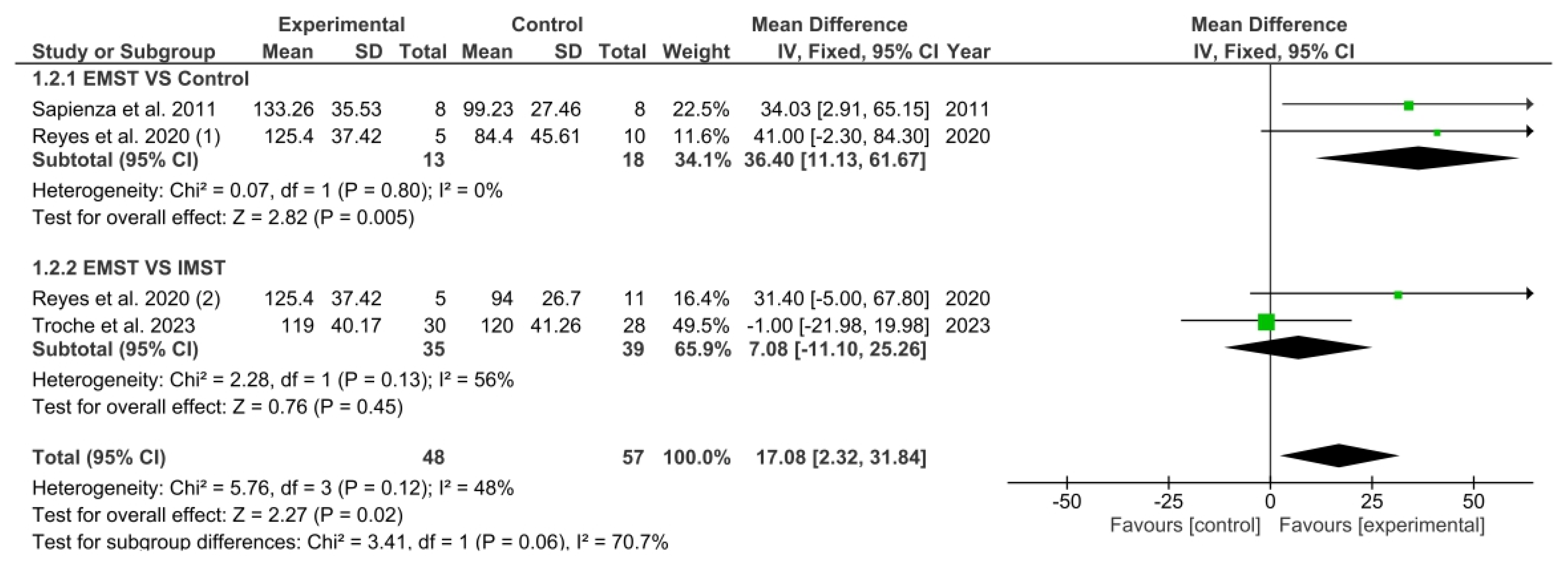
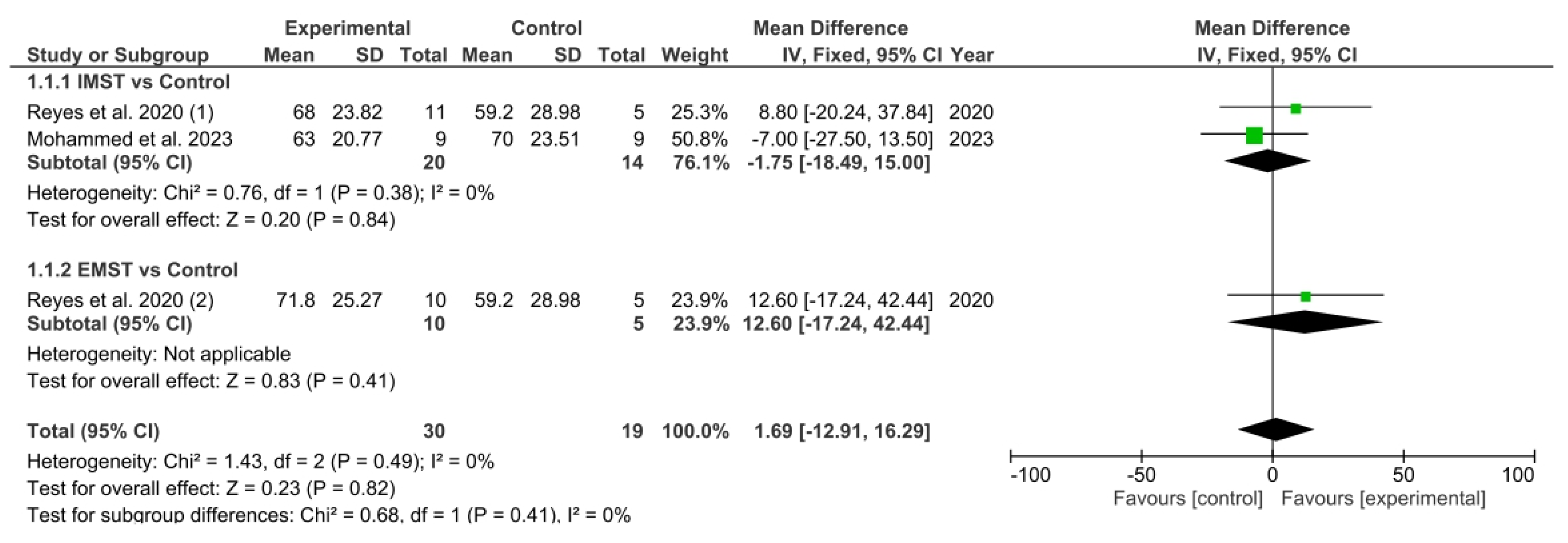
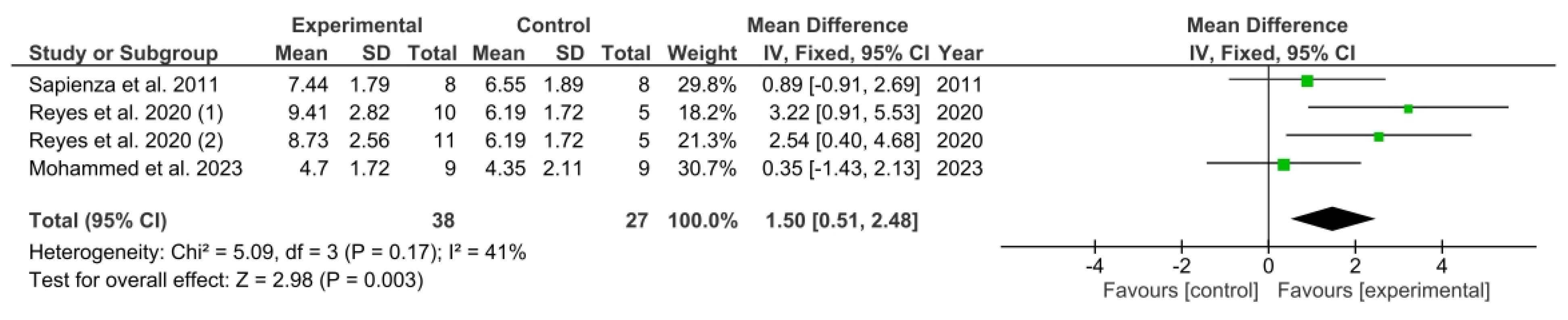
3.5. Results Obtained in Meta-Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balestrino, R.; Schapira, A. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO. Parkinson Disease. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (accessed on 17 May 2024).
- Dorsey, A.; Constantinescu, R.; Thompson, P.; Biglan, M.; Holloway, G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Wu, K.; Molloy, S.; Bain, P.; Chaudhuri, R.; Piccini, P. Parkinson’s disease symptoms: The patient’s perspective. Mov. Disord. 2010, 25, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.; Yarnall, J.; Duncan, W.; Coleman, S.; O’Brien, T.; Brooks, J.; Braker, R.A.; Burn, D.J. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013, 80, 276–281. [Google Scholar] [CrossRef]
- De Souza, S.; Dionísio, C.; Almeida, L. Multi-joint movements with reversal in Parkinson’s disease: Kinematics and electromyography. J. Electromyogr. Kinesiol. 2011, 21, 376–383. [Google Scholar] [CrossRef]
- Sathyaprabha, N.; Kapavarapu, K.; Pal, K.; Thennarasu, K.; Raju, R. Pulmonary functions in Parkinson’s disease. Indian J. Chest Dis. Allied Sci. 2005, 47, 251. [Google Scholar]
- Wang, Y.; Shao, W.-B.; Gao, L.; Lu, J.; Gu, H.; Sun, L.-H.; Tan, Y.; Zhang, Y.-D. Abnormal pulmonary function and respiratory muscle strength findings in Chinese patients with Parkinson’s disease and multiple system atrophy–comparison with normal elderly. PLoS ONE 2014, 9, e116123. [Google Scholar] [CrossRef]
- De Bruin, F.; de Bruin, M.; Lees, A.J.; Pride, N.B. Effects of treatment on airway dynamics and respiratory muscle strength in Parkinson’s disease. Am. Rev. Respir. Dis. 1993, 148 Pt 1, 1576–1580. [Google Scholar] [CrossRef]
- Polatli, M.; Akyol, A.; Çildaǧ, O.; Bayülkem, K. Pulmonary function tests in Parkinson’s disease. Eur. J. Neurol. 2001, 8, 341–345. [Google Scholar] [CrossRef]
- Ramig, L.O.; Fox, C.; Sapir, S. Speech treatment for Parkinson’s disease. Expert. Rev. Neurother. 2008, 8, 297–309. [Google Scholar] [CrossRef]
- Torsney, K.M.; Forsyth, D. Respiratory dysfunction in Parkinson’s disease. J. R. Coll. Phys. Edinb. 2017, 47, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Baille, G.; Perez, T.; Devos, D.; Deken, V.; Defebvre, L.; Moreau, C. Early occurrence of inspiratory muscle weakness in Parkinson’s disease. PLoS ONE 2018, 13, e0190400. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.M.; Trew, M.; Castle, P.C. Effects of respiratory muscle weakness on daily living function, quality of life, activity levels, and exercise capacity in mild to moderate Parkinson’s disease. Am. J. Phys. Med. Rehabil. 2004, 83, 601–607. [Google Scholar] [CrossRef]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R.A. Systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef]
- Illi, S.K.; Held, U.; Frank, I.; Spengler, C.M. Effect of respiratory muscle training on exercise performance in healthy individuals: A systematic review and meta-analysis. Sports Med. 2012, 42, 707–724. [Google Scholar] [CrossRef]
- Aliverti, A. Respiratory muscle training: Theory and practice. Breathe 2020, 16, e109–e110. [Google Scholar]
- Silva, I.S.; Pedrosa, R.; Azevedo, I.G.; Forbes, A.M.; Fregonezi, G.A.; Junior, M.E.D.; Lima, S.; Ferreira, G. Respiratory muscle training in children and adults with neuromuscular disease. Cochrane Database Syst. Rev. 2019, 9, CD011711. [Google Scholar] [CrossRef]
- Menezes, K.K.; Nascimento, L.R.; Ada, L.; Polese, J.C.; Avelino, P.R.; Teixeira-Salmela, L.F. Respiratory muscle training increases respiratory muscle strength and reduces respiratory complications after stroke: A systematic review. J. Physiother. 2016, 62, 138–144. [Google Scholar] [CrossRef]
- Zhuang, J.; Jia, J. Effects of respiratory muscle strength training on respiratory-related impairments of Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 929923. [Google Scholar] [CrossRef]
- Rodríguez, M.Á.; Crespo, I.; Del Valle, M.; Olmedillas, H. Should respiratory muscle training be part of the treatment of Parkinson’s disease? A systematic review of randomized controlled trials. Clin. Rehabil. 2020, 34, 429–437. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; McKenzie, J.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Centre for Reviews & Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Systematic Reviews in Healthcare; Centre for Reviews & Dissemination: York, UK, 2009. [Google Scholar]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health. 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Higgins, J.S.G. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Cochrane: London, UK, 2021. [Google Scholar]
- Brown, J.; Stegemöller, E.L. Therapeutic singing and expiratory muscle strength training in Parkinson’s disease: A mixed methods comparison. Front. Rehabil. Sci. 2024, 5, 1478490. [Google Scholar] [CrossRef]
- Antonsson, M.; Johansson, K.; Bonde-Dalemo, A.; Ivehorn-Axelsson, C.; Burge, Å.; Lesueur, U.; Hartelius, L. Effect of expiratory muscle strength training on voice and speech: An exploratory study in persons with Parkinson’s disease or multiple sclerosis. Int. J. Speech-Lang. Pathol. 2023, 26, 475–492. [Google Scholar] [CrossRef]
- Troche, M.S.; Curtis, J.A.; Sevitz, J.S.; Dakin, A.E.; Perry, S.E.; Borders, J.C.; Grande MPhil, A.A.; Mou, Y.; Vanegas-Arroyave, N.; Hegland, K.W. Rehabilitating cough dysfunction in Parkinson’s disease: A randomized controlled trial. Mov. Disord. 2023, 38, 201–211. [Google Scholar] [CrossRef]
- Mohammed-Yusuf, S.F.; Bhise, A.; Nuhmani, S.; Alghadir, A.H.; Khan, M. Effects of an incentive spirometer versus a threshold inspiratory muscle trainer on lung functions in Parkinson’s disease patients: A randomized trial. Sci. Rep. 2023, 13, 2516. [Google Scholar] [CrossRef]
- Claus, I.; Muhle, P.; Czechowski, J.; Ahring, S.; Labeit, B.; Suntrup-Krueger, S.; Wiendl, H.; Dziewas, R.; Warnecke, T. Expiratory muscle strength training for therapy of pharyngeal dysphagia in Parkinson’s disease. Mov. Disord. 2021, 36, 1815–1824. [Google Scholar] [CrossRef]
- Reyes, A.; Castillo, A.; Castillo, J.; Cornejo, I.; Cruickshank, T. The effects of respiratory muscle training on phonatory measures in individuals with Parkinson’s disease. J. Voice 2020, 34, 894–902. [Google Scholar] [CrossRef]
- Reyes, A.; Castillo, A.; Castillo, J.; Cornejo, I. The effects of respiratory muscle training on peak cough flow in patients with Parkinson’s disease: A randomized controlled study. Clin. Rehabil. 2018, 32, 1317–1327. [Google Scholar] [CrossRef]
- Sapienza, C.; Troche, M.; Pitts, T.; Davenport, P. Respiratory strength training: Concept and intervention outcomes. Semin. Speech Lang. 2011, 32, 21–30. [Google Scholar] [CrossRef]
- Troche, M.S.; Okun, M.S.; Rosenbek, J.C.; Musson, N.; Fernandez, H.H.; Rodriguez, R.; Sapienza, C.M. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurololgy 2010, 75, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Inzelberg, R.; Peleg, N.; Nisipeanu, P.; Magadle, R.; Carasso, R.L.; Weiner, P. Inspiratory muscle training and the perception of dyspnea in Parkinson’s disease. Can. J. Neurol. Sci. 2005, 32, 213–217. [Google Scholar] [CrossRef]
- Lauren, H.; Nathalie, J.; Alexandra, F.; Thomas, S.; Tamara, P. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar]
- Van de Wetering, V.; Kalf, J.G.; Van der Wees, P.J.; Bloem, B.R.; Nijkrake, M.J. The effects of respiratory training in Parkinson’s disease: A systematic review. J. Park. Dis. 2020, 10, 1315–1333. [Google Scholar] [CrossRef]
- Watson, K.; Egerton, T.; Sheers, N.; Retica, S.; McGaw, R.; Clohessy, T.; Webster, P.; Berlowitz, D.J. Respiratory muscle training in neuromuscular disease: A systematic review and meta-analysis. Eur. Respir. Rev. 2022, 31, 220065. [Google Scholar] [CrossRef]
- Gauld, L.M. Airway clearance in neuromuscular weakness. Dev. Med. Child. Neurol. 2009, 51, 350–355. [Google Scholar]
- Shapira, N.; Zabatino, S.M.; Ahmed, S.; Murphy, D.M.; Sullivan, D.; Lemole, G.M. Determinants of pulmonary function in patients undergoing coronary bypass operations. Ann. Thorac. Surg. 1990, 50, 268–273. [Google Scholar] [CrossRef]
- Romanini, W.; Muller, A.P.; Carvalho, D.; Olandoski, M.; Faria-Neto, J.R.; Mendes, F.L. The effects of intermittent positive pressure and incentive spirometry in the postoperative of myocardial revascularization. Arq. Bras. Cardiol. 2007, 89, 105–110. [Google Scholar] [CrossRef]
- Docu-Axelerad, A.; Stroe, A.Z.; Arghir, O.C.; Docu-Axelerad, D.; Gogu, A.E. Respiratory dysfunctions in Parkinson’s disease patients. Brain Sci. 2021, 11, 595. [Google Scholar] [CrossRef]
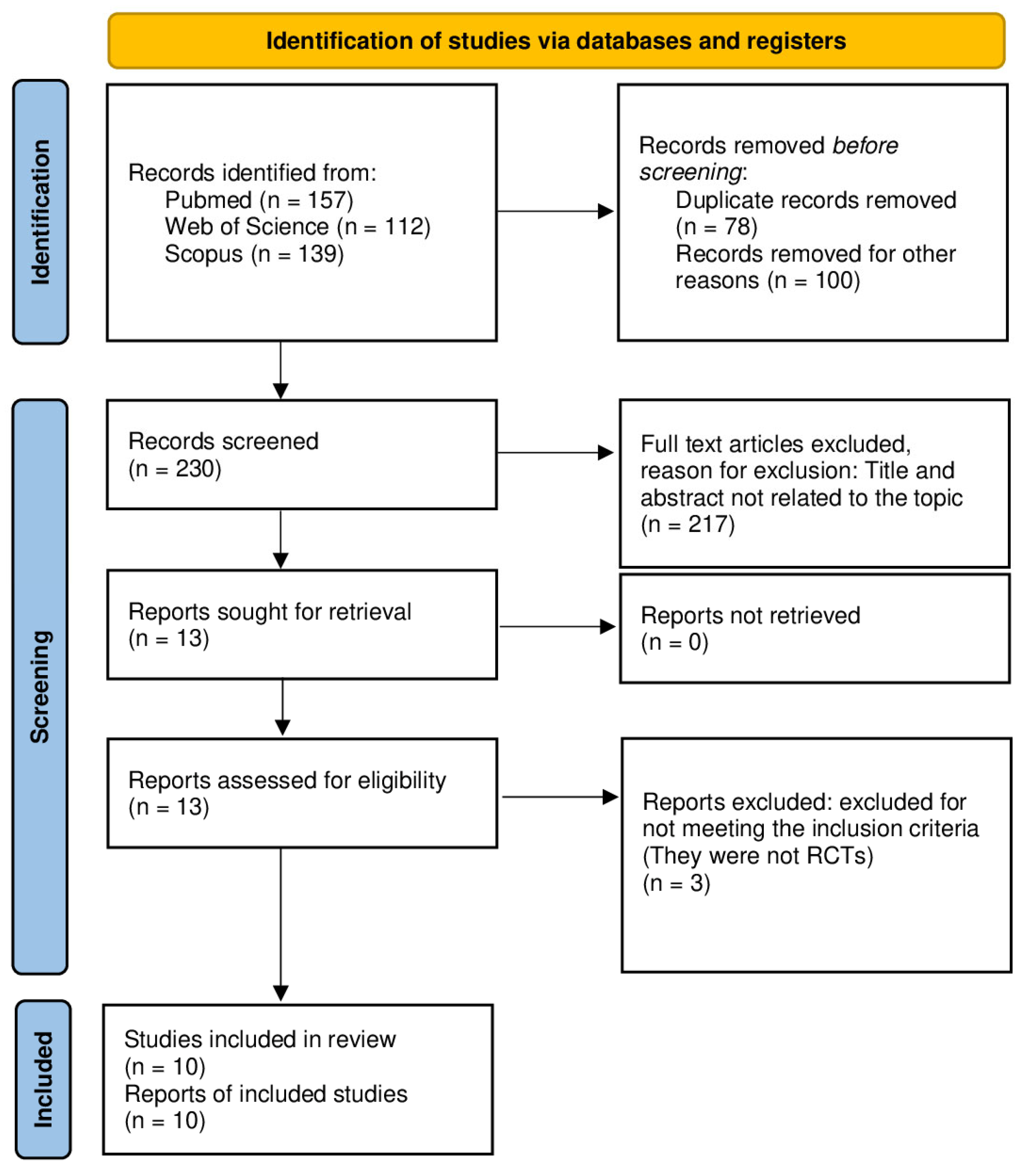
| Database | Search Equation | Results |
|---|---|---|
| PubMed | ((“respiratory training” OR “respiratory training intervention” OR “respiratory muscle strength training” OR “respiratory muscle strength programme” OR “respiratory muscle training programme” OR “respiratory training protocol” OR “respiratory muscle strengthening” OR “training of the respiratory musculature” OR “expiratory muscle strengthening” OR “inspiratory muscle strengthening” OR “inspiratory muscle training” OR “expiratory muscle training” OR “expiratory muscle strength training” OR “inspiratory muscle strength training” OR “programmes of expiratory and inspiratory muscle training” OR “breathing exercises” OR “inspiratory muscle rehabilitation” OR “expiratory muscle rehabilitation” OR “respiratory muscle rehabilitation”) AND (“parkinson disease” OR “parkinsonism” OR “parkinson’s disease”)) | 157 |
| Web of Science | TS = ((“respiratory training” OR “respiratory training intervention” OR “respiratory muscle strength training” OR “respiratory muscle strength programme” OR “respiratory muscle training programme” OR “respiratory training protocol” OR “respiratory muscle strengthening” OR “training of the respiratory musculature” OR “expiratory muscle strengthening” OR “inspiratory muscle strengthening” OR “inspiratory muscle training” OR “expiratory muscle training” OR “expiratory muscle strength training” OR “inspiratory muscle strength training” OR “programmes of expiratory and inspiratory muscle training” OR “breathing exercises” OR “inspiratory muscle rehabilitation” OR “expiratory muscle rehabilitation” OR “respiratory muscle rehabilitation”) AND (“parkinson disease” OR “parkinsonism” OR “parkinson’s disease”)) | 112 |
| Scopus | TITLE-ABS-KEY ((“respiratory training” OR “respiratory training intervention” OR “respiratory muscle strength training” OR “respiratory muscle strength programme” OR “respiratory muscle training programme” OR “respiratory training protocol” OR “respiratory muscle strengthening” OR “training of the respiratory musculature” OR “expiratory muscle strengthening” OR “inspiratory muscle strengthening” OR “inspiratory muscle training” OR “expiratory muscle training” OR “expiratory muscle strength training” OR “inspiratory muscle strength training” OR “programmes of expiratory and inspiratory muscle training” OR “breathing exercises” OR “inspiratory muscle rehabilitation” OR “expiratory muscle rehabilitation” OR “respiratory muscle rehabilitation”) AND (“parkinson disease” OR “parkinsonism” OR “parkinson’s disease”)) | 139 |
| Study (Year) | Participants | Interventions | On/Off | Training Protocol | Follow-Up | Measured Outcomes and Tools | Main Findings |
|---|---|---|---|---|---|---|---|
| Brown et al. (2024) [27] | n: 14 IG 1: 7 IG 2: 7 Mean age: IG 1: 70 ± 7 IG 2: 69 ± 7 Sex (M%): IG 1: 57% IG 2: 42% H&Y: I–III Mean DD: IG 1: 8 ± 8 IG 2: 9 ± 7 | IG 1: participants used a calibrated threshold for EMST IG 2: participants received therapeutic singing | On | IG 1 trained 5 days per week for 4 weeks They completed 5 sets × 5 reps IG 1 intensity was set at 75% MEP, and it was increased by a quarter each week IG 2 were given a homebased therapeutic singing protocol 5 days per week for 4 weeks IG 2 duration was 25 min | No | QoL: PDQ-39, PAS, GDS | QoL: No significant differences between groups |
| Antonsson et al. (2023) [28] | n: 19 (MS + PD) PD group: 9 IG: 5 CG: 4 Mean age (years (range)): 57.3 years Sex (M/F): 3/6 H&Y: I–III Mean DD: 5 years | IG: participants used a calibrated threshold for EMST CG: participants received sham treatment | NR | IG trained 5 days per week for 5 weeks They completed 25 reps and rest 15–30 s between breaths IG intensity was set at 75% MEP Homebased programme | No | Expiratory muscle strength: MEP Phonatory measures: MPT, DDK task, QASD | Expiratory muscle strength: Significant differences between baseline and post-EMST in IG. Phonatory measures: Significant differences between baseline and post-EMST for DDK. No significant differences between baseline and post-EMST for MPT and QASD |
| Troche et al. (2023) [29] | n: 58 IG 1: 30 IG 2: 28 Mean age (years (range)): IG 1: 70.5 IG 2: 69.1 Sex (M/F): IG 1: 21/13 IG 2: 22/9 H&Y: I–IV Mean DD: IG 1: 8 years IG 2: 7.6 years | IG 1: participants used a calibrated threshold for EMST IG 2: participants received a cough training approach called smTAP. They used a peak flow meter | On | All participants trained 5 days per week for 5 weeks They completed 5 sets × 5 reps IG: intensity was set at 75% MEP IG2: target set at 25% above baseline PEFR Homebased programme (1 supervised session once a week) | No | Expiratory muscle strength: MEP Cough volume: Voluntary CEV, Reflex CEV Peak flow: Voluntary PEFR, Reflex PEFR | Expiratory muscle strength: Significant differences between groups in favour of IG 1 Cough volume: Significant differences between groups in favour of IG2 Peak flow: Significant differences between groups in favour of IG 2 |
| Mohammed et al. (2023) [30] | n: 18 IG 1: 9 IG 2: 9 Mean age: IG 1: 70.22 ± 6.18 IG 2: 69.67 ± 5.89 Sex (M/F): NR H&Y: I–III Mean DD: NR | IG 1: participants used an incentive spirometer IG 2: participants used a calibrated threshold for IMST | NR | All participants trained 6 days per week, 15 min twice a day, during 6 weeks IG 2 intensity was set at 0% MIP 5% increase every week | No | Inspiratory muscle strength: MIP Pulmonary fuction: FVC, FEV1 Peak flow: PEFR Exercise capacity: 6-MWT | Inspiratory muscle strength: Significant differences between groups in favour of IG 2 Pulmonary function: No significant differences between groups Peak flow: No significant differences between groups Exercise capacity: Significant differences between groups in favour of IG 2 |
| Claus et al. (2021) [31] | n: 50 CG: 25 IG: 25 Mean age: CG: 67.1 ± 7.7 IG: 67.3 ± 9.5 Sex (M/F): CG: 19/5 IG: 18/5 H&Y: II–IV Mean DD: CG: 6.5 ± 7.7 IG: 6.6 ± 2.8 | CG: participants received sham training IG: participants used a calibrated threshold for EMST | NR | All participants trained 5 days per week for 4 weeks They did 5 sets × 5 reps per day IG: EMST intensity was set at 75% MEP | 3 months | Dysphagia symptoms: SDQ, FEES dysphagia score Cortical swallowing organisation: MEG Swallowing QoL: SWAL-QoL | Dysphagia symptoms: Significant differences between groups in favors to IG post-treatment as well as follow-up Cortical swallowing organisation: No significant differences between groups Swallowing QoL: No significant differences between groups. |
| Reyes et al. (2020) [32] | n: 31 CG: 10 IG 1: 10 IG 2: 11 Mean age: CG: 70.20 ± 6.69 IG 1: 70.45 ± 8.16 IG 2: 70.40 ± 6.81 Sex (M/F): CG: 4/6 IG 1: 6/5 IG 2: 7/3 H&Y: I–III Mean DD: NR | CG: participants used a threshold with fixed resistance (minimum pressure) IG 1: participants used a threshold with progressive resistance for EMST IG 2: participants used a threshold with progressive resistance for IMST | On | All participants trained 6 days per week for 8 weeks They did 5 sets × 5 reps per day Intensity from 50% to 75% MEP and MIP % adjusted every 2 weeks | No | Expiratory muscle strength: MEP Inspiratory muscle strength: MIP Phonatory measures: Mean SGP, MPT, Mean SPL Peak flow: Voluntary PCF | Expiratory muscle strength: Significant differences in effect size between IG1 and CG and also IG1 and IG2 Inspiratory muscle strength: Differences in effect size were moderate between IG2 and CG, small between IG2 and IG1 and also IG1 and CG Phonatory measures: Differences in effect size were trivial between IG2 and CG, small between IG1 and IG2 and moderate between IG1 and CG Peak flow: Differences in effect size were trivial between IG2 and CG, moderate between IG1 and IG2 and large between IG1 and CG |
| Reyes et al. (2018) [33] | n: 31 CG: 10 IG 1: 10 IG 2: 11 Mean age: CG: 70.20 ± 6.69 IG 1: 70.45 ± 8.16 IG 2: 70.40 ± 6.81 Sex (M/F): CG: 4/6 IG 1: 6/5 IG 2: 7/3 H&Y: I–III Mean DD: NR | CG: participants used a threshold with fixed resistance (minimum pressure) IG 1: threshold with progressive resistance for EMST IG 2: threshold with progressive resistance for IMST | On | All participants trained 6 days per week for 8 weeks They did 5 sets × 5 reps per day Intensity: from 50% to 75% MEP and MIP % adjusted every 2 weeks | No | Expiratory muscle strength: MEP Inspiratory muscle strength: MIP Pulmonary function: FVC, SVC Peak flow: Voluntary PCF, Reflex PCF | Expiratory muscle strength: Differences in effect size between IG2 and CG was moderate, large between IG1 and IG2 and between IG1. Inspiratory muscle strength: Differences in effect size between IG2 and CG was moderate, small between IG2 and IG1 and between IG1 and CG. Pulmonary function: Post-intervention effect size was very small in all groups. Peak flow: For Voluntary PCF, differences in effect size between IG2 and CG, moderate between IG1 and IG2, and large effect between IG1 and CG. Reflex peak cough flow had a trivial positive effect between IG2 and CG, a trivial negative effect between IG1 and IG2, and a moderate positive effect between IG1 and CG |
| Sapienza et al. (2011) [34] | n: 16 CG: 8 IG: 8 Mean age: CG: 68.50 ± 10.31 IG: 66.73 ± 8.90 Sex (M/F): CG: 22/8 IG: 25/5 H&Y: II–III Mean DD: NR | CG: participants received sham treatment IG: participants used a calibrated threshold for EMST | On | All participants trained 5 days per week for 4 weeks They did 5 sets × 5 reps IG intensity: NR Homebased programme | No | Expiratory muscle strength: MEP Pulmonary function: FVC, FEV1, FEV1/FVC Peak flow: PEFR | Expiratory muscle strength: Significant differences between groups in favour of IG Pulmonary function: No significant differences between groups Peak flow: No significant differences between groups |
| Troche et al. (2010) [35] | n: 60 CG: 30 IG: 30 Mean age: IG: 66.7 ± 8.9 CG: 68.5 ± 10.3 Sex (M/F): IG: 25/5 CG: 22/8 H&Y: II–IV Mean DD: NR | IG: participants used a calibrated threshold for EMST CG: participants received sham training | NR | All participants trained 5 days per week for 4 weeks They did 5 sets × 5 reps per day IG intensity was set at 75% MEP | No | Swallow safety: PA Score QoL: SWAL-QoL Duration of hyoid elevation: VFS Hyoid displacement: VFS | Sallow safety: Significant differences between groups in favour of IG Sallowing QoL: No significant differences between groups. Both groups improved. Duration of hyoid elevation: Significant impairment between baseline and post-sham in CG Hyoid displacement: Significant impairment between baseline and post-sham in CG |
| Inzelberg et al. (2005) [36] | n: 20 CG: 10 IG: 10 Mean age: CG: 65.2 ± 3.6 IG: 59.4 ± 2.4 Sex (M/F): IG: 9/1 CG: 9/1 H&Y: II–III Mean DD: IG: 8.58 ± 1.8 CG: 8.15 ± 2.0 | IG: participants used a calibrated trheshold for IMST CG: participants received sham training | On | All participants trained 6 days per week for 12 weeks They did 30 min per day IG intensity was set from 15% to 60% MIP % weekly adjusted | No | Inspiratory muscle strength: MIP Pulmonary function: FVC, FEV1 Inspiratory muscle endurance: PmPeak POD: Borg Scale QoL: SF-36 questionnaire | Inspiratory muscle strength: Significant differences between groups in favour of IG Pulmonary function: No significant differences between groups Inspiratory muscle endurance: Significant differences between groups in favour of IG POD: Significant differences between groups in favour of IG QoL: No significant differences between groups |
| Author (Year) | Reporting | External Validity | Internal Validity (Bias) | Confounding and Selection Bias | Power | Total |
|---|---|---|---|---|---|---|
| Brown et al. (2024) [27] | 8/11 | 1/3 | 6/7 | 3/6 | 0/1 | 18/28 |
| Antonsson et al. (2023) [28] | 10/11 | 3/3 | 6/7 | 6/6 | 0/1 | 25/28 |
| Troche et al. (2023) [29] | 9/11 | 2/3 | 7/7 | 5/6 | 1/1 | 24/28 |
| Mohammed et al. (2023) [30] | 8/11 | 1/3 | 5/7 | 3/6 | 1/1 | 18/28 |
| Clauss et al. (2021) [31] | 9/11 | 3/3 | 6/7 | 6/6 | 1/1 | 25/28 |
| Reyes et al. (2020) [32] | 9/11 | 3/3 | 6/7 | 4/6 | 1/1 | 23/28 |
| Reyes et al. (2018) [33] | 8/11 | 2/3 | 6/7 | 4/6 | 1/1 | 21/28 |
| Sapienza et al. (2011) [34] | 7/11 | 3/3 | 7/7 | 4/6 | 0/1 | 21/28 |
| Troche et al. (2010) [35] | 8/11 | 3/3 | 7/7 | 5/6 | 0/1 | 23/28 |
| Inzelberg et al. (2005) [36] | 8/11 | 0/3 | 7/7 | 3/6 | 0/1 | 18/28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navas-Garrido, I.; Martín-Núñez, J.; Raya-Benítez, J.; Granados-Santiago, M.; Navas-Otero, A.; López-López, L.; Valenza, M.C. Respiratory Muscle Strength Training in Parkinson’s Disease—A Systematic Review and Meta-Analysis. Healthcare 2025, 13, 1214. https://doi.org/10.3390/healthcare13101214
Navas-Garrido I, Martín-Núñez J, Raya-Benítez J, Granados-Santiago M, Navas-Otero A, López-López L, Valenza MC. Respiratory Muscle Strength Training in Parkinson’s Disease—A Systematic Review and Meta-Analysis. Healthcare. 2025; 13(10):1214. https://doi.org/10.3390/healthcare13101214
Chicago/Turabian StyleNavas-Garrido, Irene, Javier Martín-Núñez, Julia Raya-Benítez, María Granados-Santiago, Alba Navas-Otero, Laura López-López, and Marie Carmen Valenza. 2025. "Respiratory Muscle Strength Training in Parkinson’s Disease—A Systematic Review and Meta-Analysis" Healthcare 13, no. 10: 1214. https://doi.org/10.3390/healthcare13101214
APA StyleNavas-Garrido, I., Martín-Núñez, J., Raya-Benítez, J., Granados-Santiago, M., Navas-Otero, A., López-López, L., & Valenza, M. C. (2025). Respiratory Muscle Strength Training in Parkinson’s Disease—A Systematic Review and Meta-Analysis. Healthcare, 13(10), 1214. https://doi.org/10.3390/healthcare13101214







