A Possible Missing Link Between Obstructive Sleep Apnea Syndrome (OSA) Associated with Tobacco Use and Inflammation Biomarkers
Abstract
1. Introduction
1.1. Inflammation in OSA—Still an Incompletely Elucidated Issue?
1.2. Study Design, Participants, and Data Collection
1.3. Sleep Test in Our Study
1.4. Inflammation Assessment
1.5. Statistical Analysis
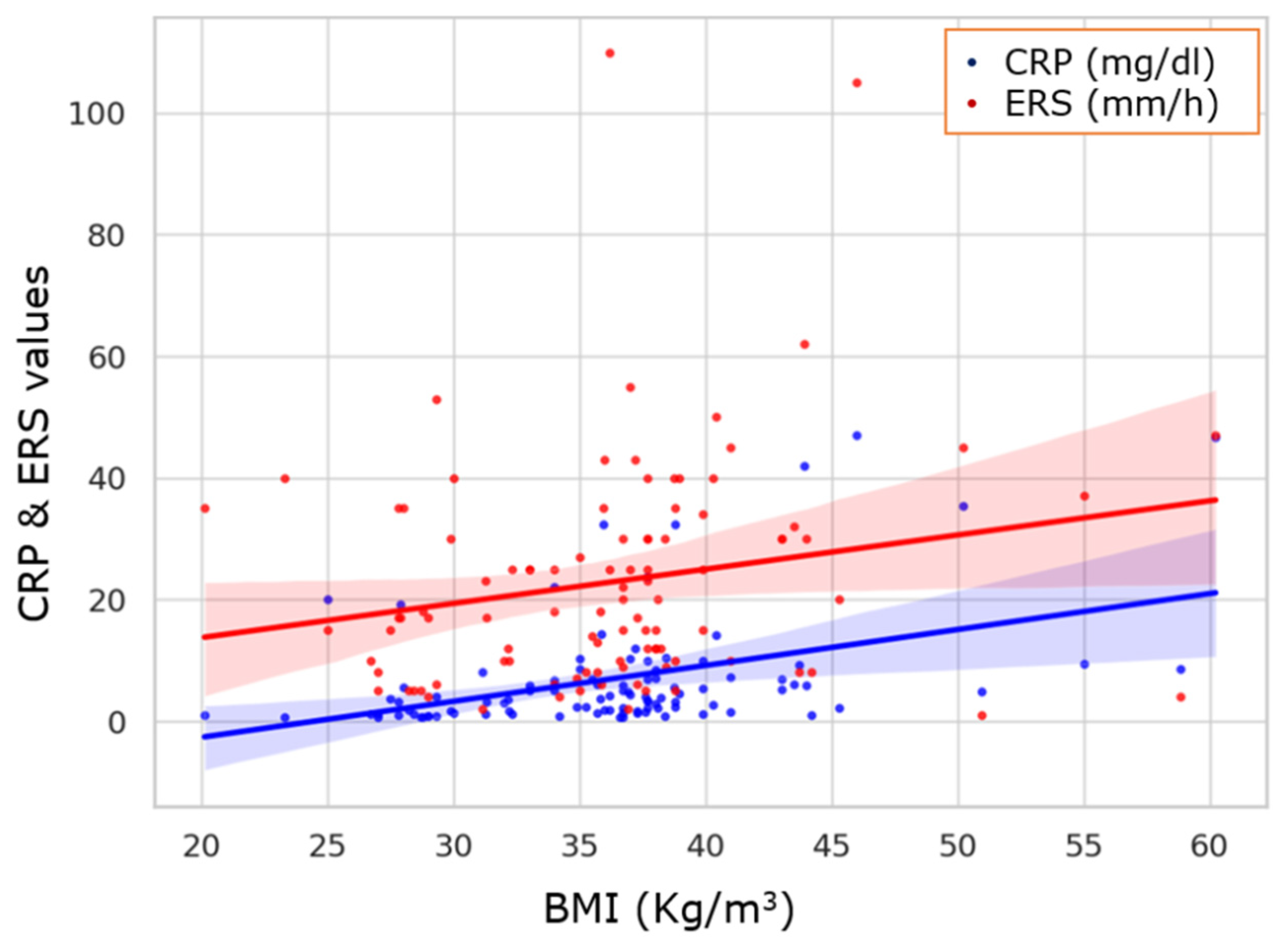
2. Results
3. Conclusions
3.1. Study Limitations
3.2. Further Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; In, K.; Kim, J.; You, S.; Kang, K.; Shim, J.; Lee, S.; Lee, J.; Lee, S.; Park, C.; et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am. J. Respir. Crit. Care Med. 2004, 170, 1108–1113. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pepin, J.-L. Estimation of the global prevalence and burden of obstructive sleep apnea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Gölen, M.K.; Isik, S.M.; Arikan, V. Is there a relationship between the severity of obstructive sleep apnea syndrome and the systemic immune-inflammation index? Eur. Arch. Otorhinolaryngol. 2024, 281, 5007–5013. [Google Scholar] [CrossRef]
- Labarca, G.; Gower, J.; Lampeti, L.; Dreyse, J.; Jorquera, J. Chronic intermittent hypoxia in obstructive sleep apnea: A narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath. 2020, 24, 751–760. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Lee, W.H.; Wee, J.H.; Rhee, C.S.; Yoon, I.-Y.; Kim, J.-W. Erythrocyte sedimentation rate may help predict severity of obstructive sleep apnea. Sleep Breath. 2016, 20, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Azofra, A.; Gu, W.; Masso-Silva, J.A.; Sanz-Rubio, D.; Marin-Oto, M.; Cubero, P.; Gil, A.V.; Moya, E.A.; Barnes, L.A.; Mesarwi, O.A. Inflammation biomarkers in OSA, chronic obstructive pulmonary disease, and chronic obstructive pulmonary disease/OSA overlap syndrome. J. Clin. Sleep Med. 2023, 19, 1447–1456. [Google Scholar] [CrossRef]
- Al-Mughales, J.; Wali, S.O.; Manzar, D.; Alhejaili, F.; Gozal, D. Pro-inflammatory markers in patients with obstructive sleep apnea and the effect of continuous positive airway pressure therapy. Sleep Sci. 2022, 15, 20–27. [Google Scholar] [CrossRef]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.P.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J. Cigarette smoking and variations in systemic immune and inflammation markers. J. Natl. Cancer Inst. 2014, 106, dju294. [Google Scholar] [CrossRef]
- Lavie, L.; Lavie, P. Smoking interacts with sleep apnea to increase cardiovascular risk. Sleep Med. 2008, 9, 247–253. [Google Scholar] [CrossRef]
- Ioannidou, D.; Kalamaras, G.; Kotoulas, S.-C.; Pataka, A. Smoking and obstructive sleep apnea: Is there an association between these cardiometabolic risk factors?—Gender analysis. Medicina 2021, 57, 1137. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, T.U.; Kokturk, O.; Bukan, N.; Bilgihan, A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 2004, 28, 87–91. [Google Scholar] [CrossRef]
- Phillips, B.G.; Kato, M.; Narkiewicz, K.; Choe, I.; Somers, V.K. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H234–H237. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yin, K.S.; Wang, H.; Xi, L.; Zhang, K.; Yin, S.; Su, S. Plasma adiponectin levels in obstructive sleep apnea–hypopnea syndrome. Chest 2006, 129, 405–409. [Google Scholar]
- Pataka, A.; Kotoulas, S.; Kalamaras, G.; Tzinas, A.; Grigoriou, I.; Kasnaki, N.; Argyropolou, P. Does smoking affect OSA? What about smoking cessation? J. Clin. Med. 2022, 11, 5164. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.H.; Park, S.Y.; Won, H.R.; Lee, H.J.; Yang, H.S.; Kim, H.J. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J. Clin. Sleep Med. 2012, 8, 367–374. [Google Scholar] [CrossRef]
- Conway, S.G.; Roizenblatt, S.S.; Palombini, L.; Castro, L.S.; Bittencourt, L.R.A.; Silva, R.S.; Tufik, S. Effect of smoking habits on sleep. Braz. J. Med. Biol. Res. 2008, 41, 722–727. [Google Scholar] [CrossRef]
- Lacedonia, D.; Salerno, F.G.; Carpagnano, G.E.; Sabato, R.; Depalo, A.; Foschino-Barbaro, M.P. Effect of CPAP-therapy on bronchial and nasal inflammation in patients affected by obstructive sleep apnea syndrome. Rhinology 2011, 49, 232–237. [Google Scholar] [CrossRef]
- Liu, K.; Li, K.; Zang, C.; Wang, J.; Liu, J.; Chen, Z.; He, M.; Liu, B.; Su, X.; Zhang, Y.; et al. Smoking and obstructive sleep apnea: A Mendelian randomization study. Sleep Med. X. 2023, 6, 100085. [Google Scholar]
- Jang, Y.S.; Nerobkova, N.; Hurh, K.; Park, E.-C.; Shin, J. Association between smoking and obstructive sleep apnea based on the STOP-Bang index: A nationwide cross-sectional study. Sci. Rep. 2023, 13, 9085. [Google Scholar] [CrossRef]
- Zeng, X.; Ren, Y.; Wu, K.; Yang, Q.; Zhang, S.; Wang, D.; Luo, Y.; Zhang, N. Association between smoking behavior and obstructive sleep apnea: A systematic review and meta-analysis. Nicotine Tob. Res. 2022, 25, 364–372. [Google Scholar] [CrossRef]
- Damiani, M.F.; Zito, A.; Carratu, P.; Falcone, V.A.; Bega, E.; Scicchitano, P.; Ciccone, M.M.; Resta, O. Obstructive sleep apnea, hypertension, and their additive effects on atherosclerosis. Biochem. Res. Int. 2015, 2015, 984193. [Google Scholar] [CrossRef]
- Wang, X.; Guan, L.; Wu, C.; Zhao, Y.; Zhao, G. Continuous positive airway pressure may improve hypertension in patients with obstructive sleep apnea–hypopnea syndrome by inhibiting inflammation and oxidative stress. Arch. Med. Sci. 2023, 19, 237–241. [Google Scholar] [CrossRef]
- Turnbull, C.D.; Rossi, V.A.; Santer, P.; Schwarz, E.I.; Stradling, J.R.; Petousi, N.; Kohler, M. Effect of OSA on hypoxic and inflammatory markers during CPAP withdrawal: Further evidence from three randomized control trials. Respirology 2017, 22, 793–799. [Google Scholar] [CrossRef]
- Msaad, S.; Chaabouni, A.; Marrakchi, R.; Boudaya, M.; Kotti, A.; Feki, W.; Jamoussi, K.; Kammoun, S. Nocturnal continuous positive airway pressure (nCPAP) decreases high-sensitivity C-reactive protein (hs-CRP) in obstructive sleep apnea–hypopnea syndrome. Sleep Disord. 2020, 2020, 8913247. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Perčić, M.; Vidović, D.; Štajduhar, A.; Galić, E. Impact of CPAP therapy on new inflammation biomarkers. J. Clin. Med. 2022, 11, 6113. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, P.; Nohlert, E.; Tegelberg, Å. Effect of oral appliance treatment on inflammatory biomarkers in obstructive sleep apnea: A randomized controlled trial. J. Sleep Res. 2021, 30, e13253. [Google Scholar] [CrossRef]
- Kundel, V.; Cohen, O.; Khan, S.; Patel, M.; Kim-Schulze, S.; Kovacic, J.; Suares-Farinas, M.; Shah, N.A. Advanced proteomics and cluster analysis for identifying novel obstructive sleep apnea subtypes before and after continuous positive airway pressure therapy. Ann. Am. Thorac. Soc. 2023, 20, 1038–1047. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 18 years | Chronic pulmonary disease (COPD, asthma) |
| CRP and ESR levels available at the time of diagnosis | Active infections (respiratory, urinary, or other) |
| AHI ≥ 5 events/hour | Known autoimmune or inflammatory disease affecting CRP/ESR |
| Documented smoking history | Prior OSA diagnosis or CPAP treatment |
| Incomplete data (missing CRP or ESR values, smoking history) |
| Variable | n | Mean ± SD | Median | Range |
|---|---|---|---|---|
| Age (years) | 104 | 58.27 ± 13.36 | 60.5 (52.0–68.0) | 4–80 |
| BMI (kg/m2) | 104 | 36.33 ± 6.63 | 36.7 (32.2–38.8) | 20.1–60.2 |
| Neck Circumference (cm) | 104 | 41.74 ± 4.20 | 42.0 (40.1–43.2) | 14.0–53.1 |
| Systolic BP (mmHg) | 104 | 72.92 ± 8.15 | 74.0 (67.0–80.0) | 51–90 |
| Diastolic BP (mmHg) | 104 | 129.98 ± 14.42 | 128.0 (120.0–142.3) | 107–160 |
| AHI (events/h) | 104 | 42.05 ± 20.62 | 37.3 (26.1–55.6) | 7.7–100.0 |
| ODI (events/h) | 104 | 42.28 ± 20.52 | 37.6 (28.2–56.1) | 8.3–100.0 |
| CRP (mg/dL) | 104 | 6.86 ± 9.38 | 3.75 (1.58–7.13) | 0.6–47.0 |
| ERS (mm/h) | 104 | 22.62 ± 18.45 | 18.0 (9.0–30.5) | 1–110 |
| ESS Score | 104 | 11.10 ± 5.31 | 11.0 (6.75–14.0) | 3–24 |
| AIS Score | 104 | 4.32 ± 1.66 | 4.0 (3.0–5.0) | 1–8 |
| FTND (Smokers only) | 55 | 7.80 ± 1.32 | 8.0 (7.0–9.0) | 4–10 |
| Nocturnal O2 Saturation (%) | 104 | 3.49 ± 15.09 | 0.92 (0.90–0.94) | 0.63–94.2 |
| BMI (Mean ± SD) | CRP (Mean ± SD) (mg/dL) | ERS (Mean ± SD) (mm/h) | AHI (Mean ± SD) (Events/h) | ODI (Mean ± SD) (Events/h) | ||
|---|---|---|---|---|---|---|
| OSA Severity | Mild OSA (5 ≤ AHI < 15) | 26.95 ± 2.57 | 0.73 ± 0.25 | 17.00 ± 16.51 | 10.75 ± 2.73 | |
| Moderate OSA (15 ≤ AHI < 30) | 35.09 ± 5.56 | 7.52 ± 10.34 | 23.16 ± 17.22 | 23.68 ± 5.01 | ||
| Severe OSA (AHI ≥ 30) | 37.47 ± 6.77 | 6.91 ± 9.13 | 22.70 ± 19.29 | 52.88 ± 17.22 | ||
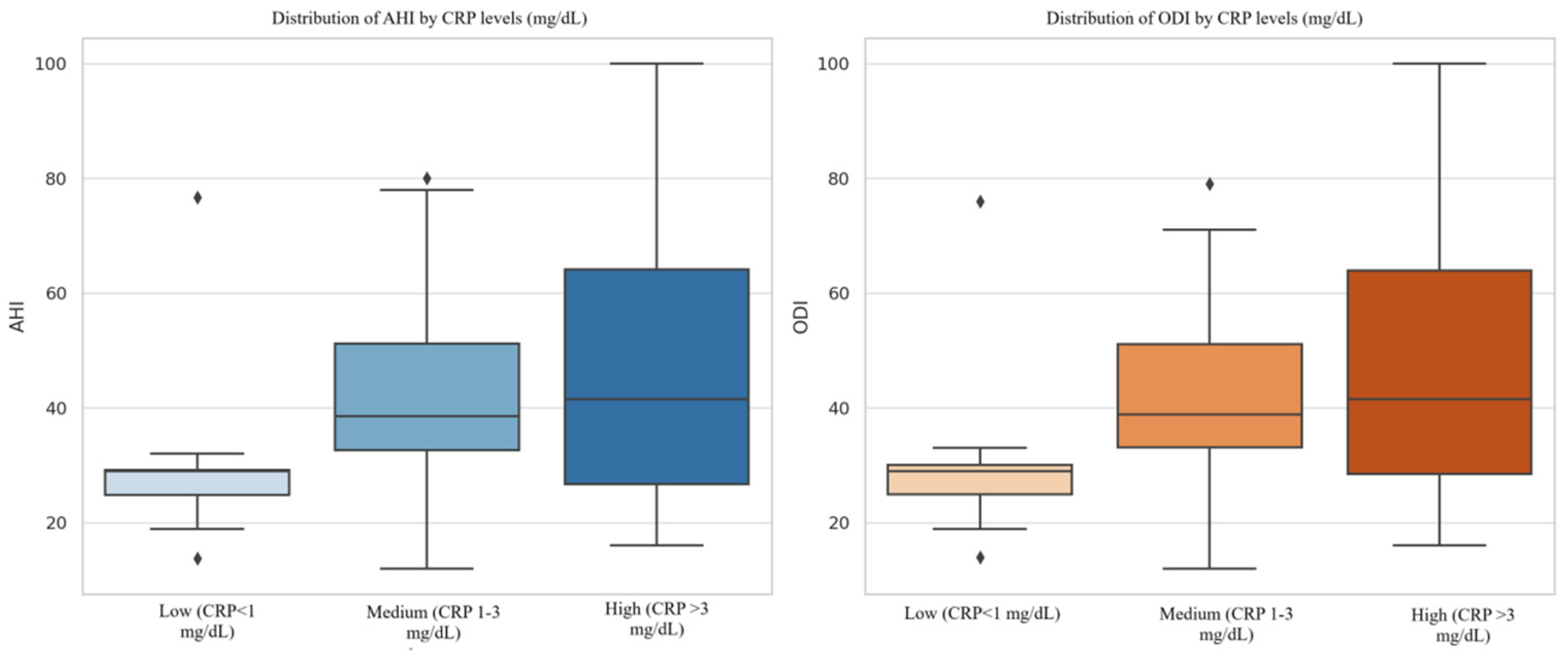
| CRP Group | CRP < 1 mg/dL | 30.20 ± 17.19 | 30.46 ± 17.52 | |||
| CRP 1–3 mg/dL | 41.38 ± 20.52 | 41.34 ± 20.06 | ||||
| CRP > 3 mg/dL | 45.87 ± 19.92 | 46.23 ± 20.05 | ||||
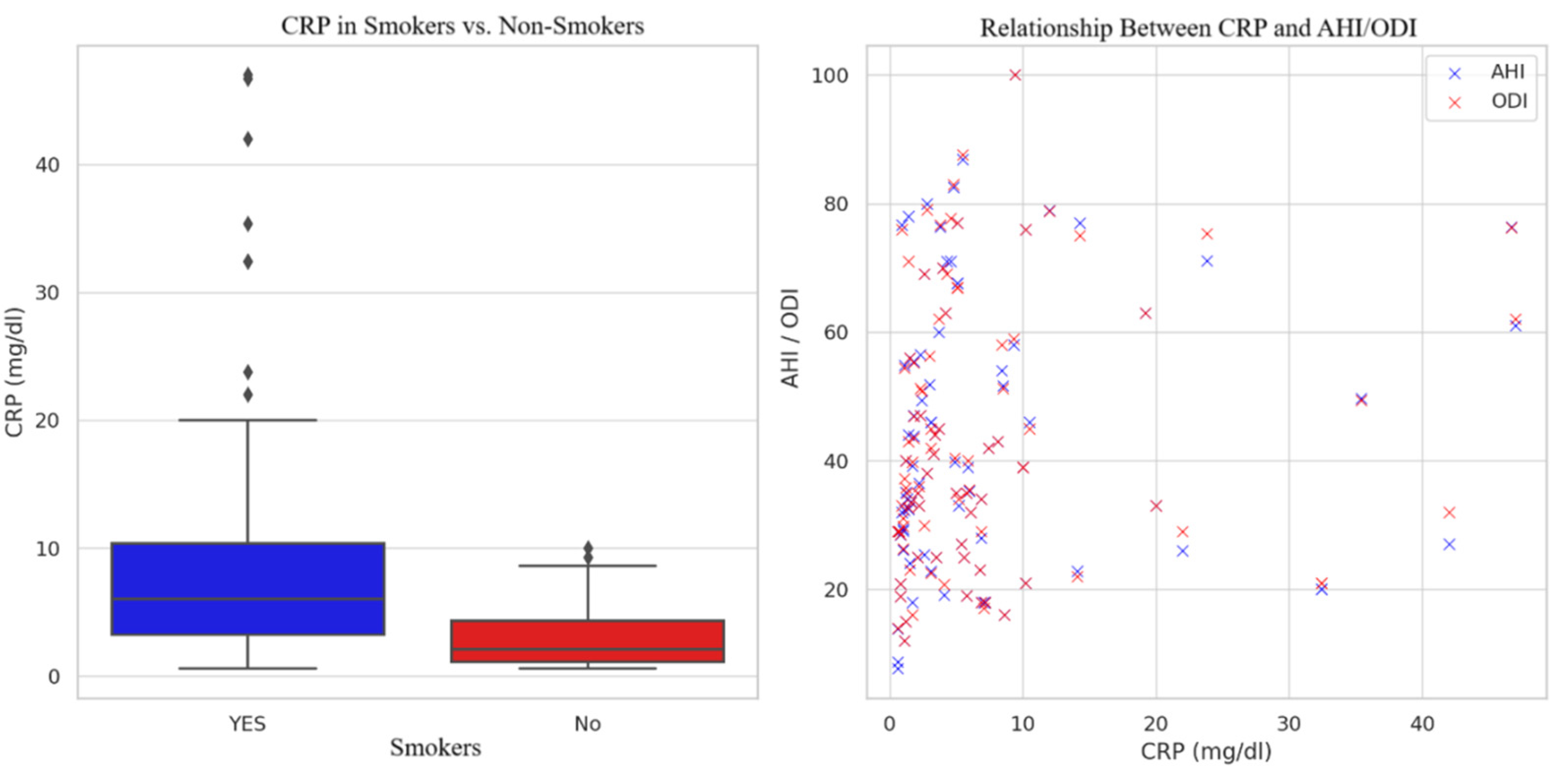
| Smoking Status | Smokers | 10.32 ± 11.69 | 26.20 ± 19.55 | 45.29 ± 20.94 | 45.69 ± 21.05 | |
| Non-Smokers | 2.97 ± 2.45 | 18.66 ± 16.41 | 38.40 ± 19.84 | 38.44 ± 19.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintilie, A.-L.; Zabara Antal, A.; Ciuntu, B.-M.; Toma, D.; Tiron, R.; Stirbu, R.; Zabara, M.L.; Crisan Dabija, R. A Possible Missing Link Between Obstructive Sleep Apnea Syndrome (OSA) Associated with Tobacco Use and Inflammation Biomarkers. Healthcare 2025, 13, 1177. https://doi.org/10.3390/healthcare13101177
Pintilie A-L, Zabara Antal A, Ciuntu B-M, Toma D, Tiron R, Stirbu R, Zabara ML, Crisan Dabija R. A Possible Missing Link Between Obstructive Sleep Apnea Syndrome (OSA) Associated with Tobacco Use and Inflammation Biomarkers. Healthcare. 2025; 13(10):1177. https://doi.org/10.3390/healthcare13101177
Chicago/Turabian StylePintilie, Adriana-Loredana, Andreea Zabara Antal, Bogdan-Mihnea Ciuntu, David Toma, Raluca Tiron, Ruxandra Stirbu, Mihai Lucian Zabara, and Radu Crisan Dabija. 2025. "A Possible Missing Link Between Obstructive Sleep Apnea Syndrome (OSA) Associated with Tobacco Use and Inflammation Biomarkers" Healthcare 13, no. 10: 1177. https://doi.org/10.3390/healthcare13101177
APA StylePintilie, A.-L., Zabara Antal, A., Ciuntu, B.-M., Toma, D., Tiron, R., Stirbu, R., Zabara, M. L., & Crisan Dabija, R. (2025). A Possible Missing Link Between Obstructive Sleep Apnea Syndrome (OSA) Associated with Tobacco Use and Inflammation Biomarkers. Healthcare, 13(10), 1177. https://doi.org/10.3390/healthcare13101177







