Effectiveness of Frequency-Specific Microcurrent (FSM) Therapy and Relaxation in Adults with Distress: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Interventions
2.3.1. Experimental Group 1 (EG1)
2.3.2. Experimental Group 2 (EG2)
2.3.3. Active Control Group (ACG)
2.3.4. Passive Control Group (PCG)
2.4. Outcomes
2.4.1. Primary Outcome Measures
2.4.2. Secondary Outcome Measures
2.5. Sample Size
2.6. Randomization
2.7. Ethical Considerations
2.8. Data Analysis
3. Results
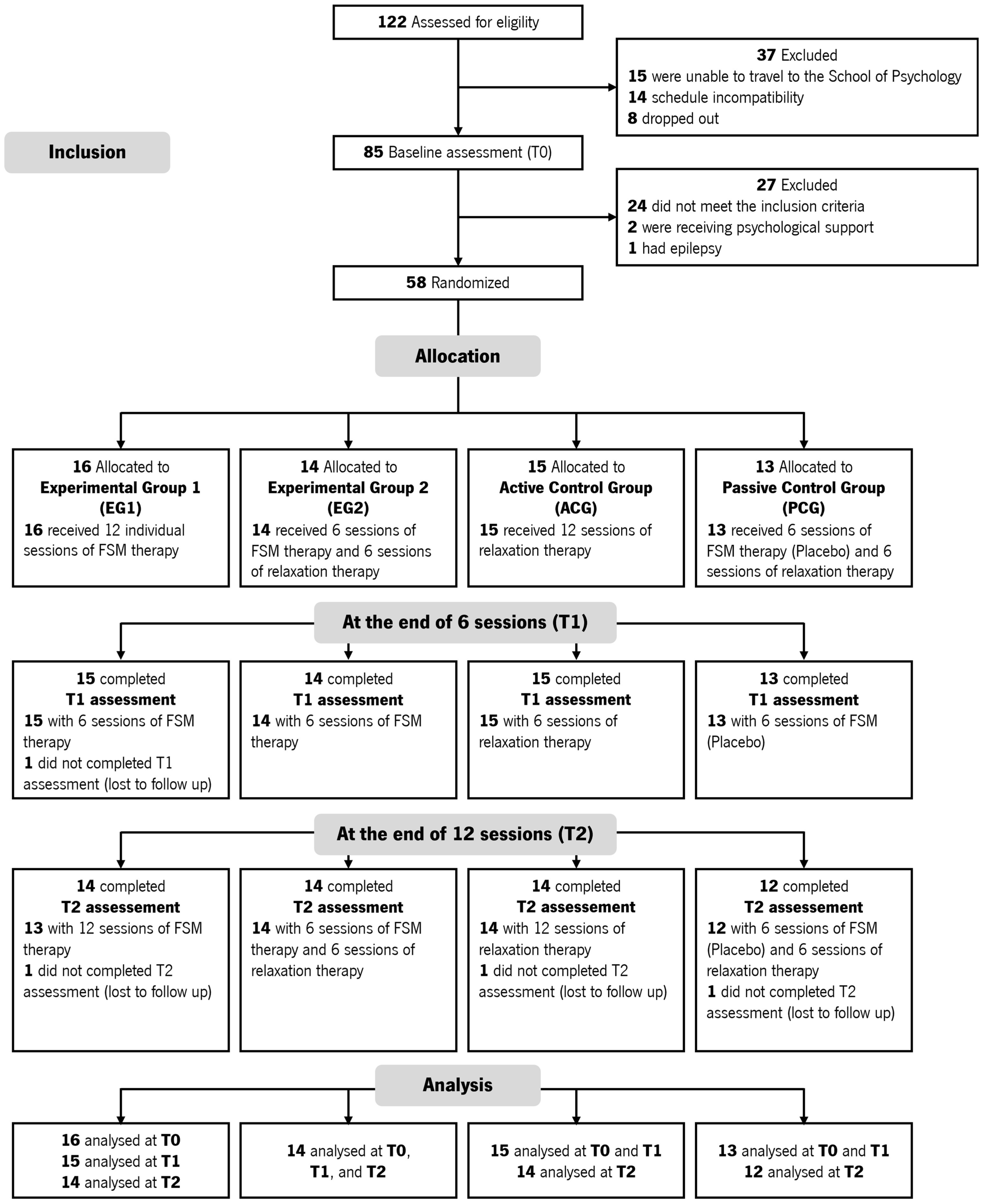
3.1. Participant Recruitment
3.2. Baseline Data
3.3. Outcomes and Estimation
3.4. Differences Between Groups over Time
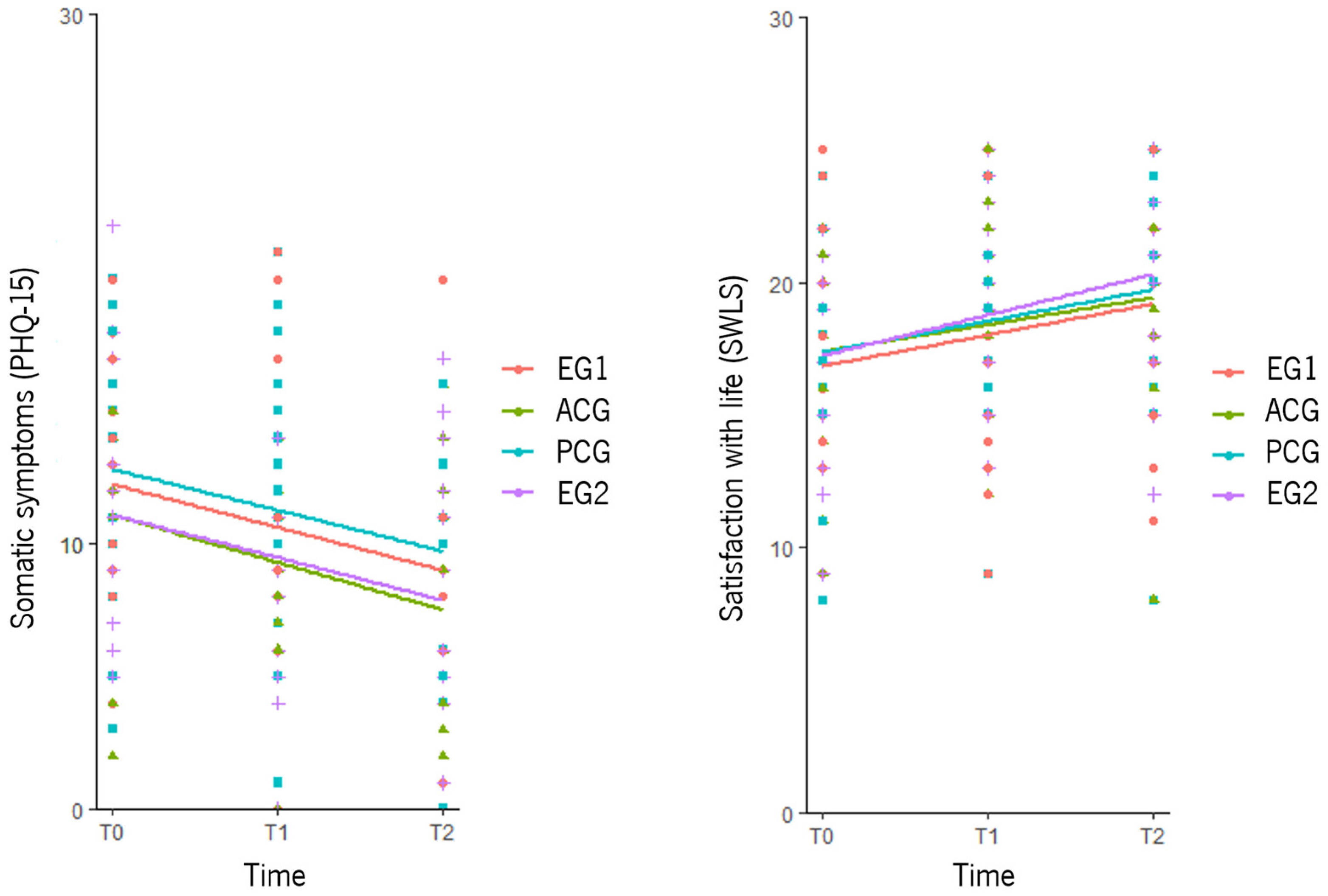
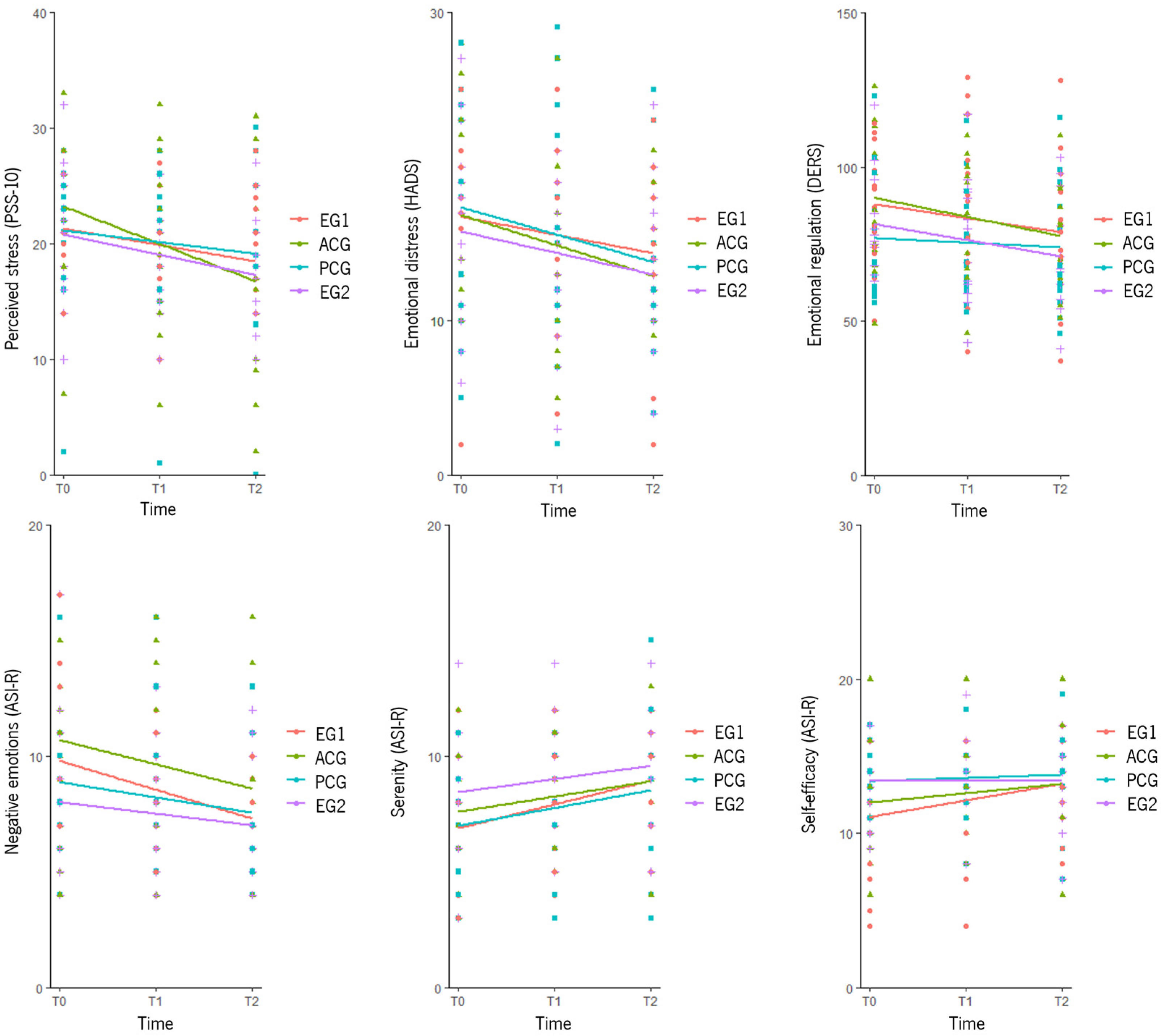
3.4.1. Primary Outcomes
3.4.2. Secondary Outcomes
3.5. Differences Within Groups over Time
3.5.1. Primary Outcomes
3.5.2. Secondary Outcomes
4. Discussion
Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spink, G.L.; Jorgensen, R.S.; Cristiano, S. Cognitive and affective factors predicting daily somatic complaints in college students. J. Couns. Psychol. 2018, 65, 110–119. [Google Scholar] [CrossRef]
- World Health Organization. Mental Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-health-strengthening-our-response (accessed on 1 July 2024).
- McNealy, K.R.; Lombardero, A. Somatic presentation of mental health concerns, stigma, and mental health treatment engagement among college students. J. Am. Coll. Health 2019, 68, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Wall, A.; Bruen, A.; Whittington, R. Is the global prevalence rate of adult mental illness increasing over time? Systematic review and meta-analysis of repeated cross-sectional population surveys. Acta Psychiatr. Scand. 2019, 140, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.; Prinstein, M.J.; Clark, L.A.; Rottenberg, J.; Abramowitz, J.S.; Albano, A.M.; Aldao, A.; Borelli, J.L.; Chung, T.; Davila, J.; et al. Mental health and clinical psychological science in the time of COVID-19: Challenges, opportunities, and a call to action. Am. Psychol. 2021, 76, 409–426. [Google Scholar] [CrossRef]
- World Health Organization. World Mental Health Report: Transforming Mental Health for All. 2022. Available online: https://www.who.int/teams/mental-health-and-substance-use/world-mental-health-report (accessed on 1 July 2024).
- González-Sanguino, C.; Ausín, B.; Castellanos, M.A.; Saiz, J.; Muñoz, M. Mental health consequences of the Covid-19 outbreak in Spain. A longitudinal study of the alarm situation and return to the new normality. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 107, 110219. [Google Scholar] [CrossRef]
- Ooi, P.B.; Khor, K.S.; Tan, C.C.; Ong, D.L.T. Depression, anxiety, stress, and satisfaction with life: Moderating role of interpersonal needs among university students. Front. Public Health 2022, 10, 958884. [Google Scholar] [CrossRef]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef]
- Sperling, E.L.; Hulett, J.M.; Sherwin, L.B.; Thompson, S.; Bettencourt, B.A. Prevalence, characteristics and measurement of somatic symptoms related to mental health in medical students: A scoping review. Ann. Med. 2023, 55, 2242781. [Google Scholar] [CrossRef]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef]
- Cole, K.R.; Shields, R.K. Age and cognitive stress influences motor skill acquisition, consolidation, and dual-task effect in Humans. J. Mot. Behav. 2019, 51, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Sperling, E.L.; Hulett, J.M.; Sherwin, L.B.; Thompson, S.; Bettencourt, B.A. The effect of mindfulness interventions on stress in medical students: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0286387. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimäki, M. Stress and Cardiovascular Disease: An Update on Current Knowledge. Annu. Rev. Public Health 2013, 34, 337–354. [Google Scholar] [CrossRef]
- Lisco, G.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Jirillo, E.; Vitale, E.; Triggiani, V. Chronic Stress as a Risk Factor for Type 2 Diabetes: Endocrine, Metabolic, and Immune Implications. Endocr. Metab. Immune Disord. Drug Targets 2024, 24, 321–332. [Google Scholar] [CrossRef]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Hennemann, S.; Wenzel, M.; Van den Bergh, O.; Wessels, M.; Witthöft, M. Emotion dynamics and somatic symptoms in everyday life: Ecological momentary assessment in somatic symptom disorder and healthy controls. J. Psychosom. Res. 2023, 172, 111429. [Google Scholar] [CrossRef]
- Klaus, K.; Rief, W.; Brähler, E.; Martin, A.; Glaesmer, H.; Mewes, R. Validating psychological classification criteria in the context of somatoform disorders: A one- and four-year follow-up. J. Abnorm. Psychol. 2015, 124, 1092–1101. [Google Scholar] [CrossRef]
- Schnabel, K.; Schulz, S.M.; Witthöft, M. Emotional reactivity, emotion regulation, and regulatory choice in somatic symptom disorder. Psychosom. Med. 2022, 84, 1077–1086. [Google Scholar] [CrossRef]
- Gross, J.J. The extended process model of emotion regulation: Elaborations, applications, and future directions. Psychol. Inq. 2015, 26, 130–137. [Google Scholar] [CrossRef]
- Okur Güney, Z.E.; Sattel, H.; Witthöft, M.; Henningsen, P. Emotion regulation in patients with somatic symptom and related disorders: A systematic review. PLoS ONE 2019, 14, e0217277. [Google Scholar] [CrossRef]
- Petzke, T.M.; Witthöft, M. The association of emotion regulation and somatic symptoms. Psychosom. Med. 2024, 86, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, S.U.; Huma, Z.-E.; Zafar, S.W.; Suleman, N.; Baneen, U.-U.; Waqas, A.; Rahman, A. Effectiveness of relaxation techniques ‘as an active ingredient of psychological interventions’ to reduce distress, anxiety and depression in adolescents: A systematic review and meta-analysis. Int. J. Ment. Health Syst. 2022, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.; Roth, W.T. Muscle relaxation therapy for anxiety disorders: It works but how? J. Anxiety Disord. 2007, 21, 243–264. [Google Scholar] [CrossRef]
- Rainforth, M.V.; Schneider, R.H.; Nidich, S.I.; Gaylord-King, C.; Salerno, J.W.; Anderson, J.W. Stress reduction programs in patients with elevated blood pressure: A systematic review and meta-analysis. Curr. Hypertens. Rep. 2007, 9, 520–528. [Google Scholar] [CrossRef]
- Muhammad Khir, S.; Wan Mohd Yunus, W.M.A.; Mahmud, N.; Wang, R.; Panatik, S.A.; Mohd Sukor, M.S.; Nordin, N.A. Efficacy of Progressive Muscle Relaxation in adults for stress, anxiety, and depression: A systematic review. Psychol. Res. Behav. Manag. 2024, 17, 345–365. [Google Scholar] [CrossRef]
- Torales, J.; O’Higgins, M.; Barrios, I.; González, I.; Almirón, M. An overview of Jacobson’s progressive muscle relaxation in managing anxiety. Rev. Argent. Clin. Psicol. 2020, 29, 17–23. [Google Scholar] [CrossRef]
- Breznoscakova, D.; Kovanicova, M.; Sedlakova, E.; Pallayova, M. Autogenic Training in mental disorders: What can we expect? Int. J. Environ. Res. Public Health 2023, 20, 4344. [Google Scholar] [CrossRef]
- Aivazyan, T.A.; Zaitsev, V.P. The effectiveness of autogenic training in the psycho-corrective treatment of the patients presenting with chronic somatic diseases. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2018, 95, 11–15. [Google Scholar] [CrossRef]
- Schultz, J.H. O Treinamento Autógeno [The Autogenic Training]; Editora Mestre Jou: Sao Paulo, Brazil, 1967. [Google Scholar]
- Shetty, G.M.; Rawat, P.; Sharma, A. Effect of adjuvant frequency-specific microcurrents on pain and disability in patients treated with physical rehabilitation for neck and low back pain. J. Bodyw. Mov. Ther. 2020, 24, 168–175. [Google Scholar] [CrossRef]
- McMakin, C.R.; Oschman, J.L. Visceral and somatic disorders: Tissue softening with frequency-specific microcurrent. J. Altern. Complement. Med. 2013, 19, 170–177. [Google Scholar] [CrossRef]
- McMakin, C.R. The Resonance Effect: How Frequency-Specific Microcurrent Is Changing Medicine; North Atlantic Books: Berkeley, CA, USA, 2004. [Google Scholar]
- Sharp, S.J.; Huynh, M.T.; Filart, R. Frequency-Specific Microcurrent as Adjunctive Therapy for Three Wounded Warriors. Med. Acupunct. 2019, 31, 189–192. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Mannion, H.; Sezgin, D.; O’Donovan, M.R.; Liew, A.; Molloy, D.W. Non-pharmacological treatments for sleep disturbance in mild cognitive impairment and dementia: A systematic review and meta-analysis. Maturitas 2019, 127, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Lee, W.S.; Lee, J.H.; Kwon, D.R. Microcurrent therapy as the nonpharmacological new protocol against Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1344072. [Google Scholar] [CrossRef]
- Rajfur, J.; Pasternok, M.; Rajfur, K.; Walewicz, K.; Fras, B.; Bolach, B.; Dymarek, R.; Rosinczuk, J.; Halski, T.; Taradaj, J. Efficacy of Selected Electrical Therapies on Chronic Low Back Pain: A Comparative Clinical Pilot Study. Med. Sci. Monit. 2017, 23, 85–100. [Google Scholar] [CrossRef]
- Barclay, T.H.; Barclay, R.D. A clinical trial of cranial electrotherapy stimulation for anxiety and comorbid depression. J. Affect. Disord. 2014, 164, 171–177. [Google Scholar] [CrossRef]
- Bystritsky, A.; Kerwin, L.; Feusner, J. A pilot study of cranial electrotherapy stimulation for Generalized Anxiety Disorder. J. Clin. Psychiatry 2008, 69, 412–417. [Google Scholar] [CrossRef]
- Hein, E.; Nowak, M.; Kiess, O.; Biermann, T.; Bayerlein, K.; Kornhuber, J.; Kraus, T. Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. J. Neural Transm. 2012, 120, 821–827. [Google Scholar] [CrossRef]
- McClure, D.; Greenman, S.C.; Koppolu, S.S.; Varvara, M.; Yaseen, Z.S.; Galynker, I.I. A pilot study of safety and efficacy of cranial electrotherapy stimulation in treatment of Bipolar II Depression. J. Nerv. Ment. Dis. 2015, 203, 827–835. [Google Scholar] [CrossRef]
- Stößlein, B.A.C.; Kuypers, K.P.C. Self-Rated Recovery and Mood Before and After Resistance Training and Muscle Microcurrent Application. Front. Psychol. 2022, 13, 836695. [Google Scholar] [CrossRef]
- Kolimechkov, S.; Seijo, M.; Swaine, I.; Thirkell, J.; Colado, J.C.; Naclerio, F. Physiological effects of microcurrent and its application for maximising acute responses and chronic adaptations to exercise. Eur. J. Appl. Physiol. 2023, 123, 451–465. [Google Scholar] [CrossRef]
- Duke, G.; Yotter, C.N.; Sharifian, B.; Duke, G.; Petersen, S. The effectiveness of microcurrent neurofeedback on depression, anxiety, post-traumatic stress disorder, and quality of life. J. Am. Acad. Nurse Pract. 2024, 36, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Marmann, P.; Wiatrek, W. Observational Study to Assesses the Efficacy and Safety of Microcurrent Therapy with a Portable Device in Patients Suffering from Chronic Back Pain, Skeletal System Pain, Fibromyalgia, Migraine or Depression. Med. Devices 2023, 16, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Trigo, M.; Canudo, N.; Branco, F.; Silva, D. Estudo das propriedades psicométricas da Perceived Stress Scale (PSS) na população portuguesa. Psychologica 2010, 53, 353–378. [Google Scholar] [CrossRef]
- Schemieke, M.; Kasraei, S.; Seydlitz, J. Manual Timewaver Frequency; Timewaver Home GmbH: Märkisch Linden, Germany, 2015; Available online: www.timewaver.de (accessed on 10 January 2025).
- Wongsarnpigoon, A.; Grill, W.M. Energy-efficient waveform shapes for neural stimulation revealed with a genetic algorithm. J. Neural Eng. 2010, 7, 046009. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Mohapatra, A.K. Threshold optimization in diagnostic tests: Balancing sensitivity and specificity. Stat. Med. 2016, 35, 1205–1215. [Google Scholar]
- Proença, J.; Paixão, R.; Quartilho, M.J. Propriedades psicométricas e estrutura fatorial da versão Portuguesa do questionário da saúde do paciente-15 (PHQ-15) [Psychometric Properties and Factor Structure of the Portuguese Version of the Patient Health Questionnaire-15 (PHQ-15)]. Rev. Iberoam. Diagn. Eval. Psicol. 2023, 69, 43–51. [Google Scholar] [CrossRef]
- Neto, F.; Barros, J.; Barros, A. Satisfação com a vida [Satisfaction with life]. In A Acção Educativa; Almeida, L., Santiago, R., Silva, P., Caetano, O., Marques, J., Eds.; ESEL/APPORT: Lisboa, Portugal, 1990; pp. 91–100. [Google Scholar]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–237. [Google Scholar] [CrossRef]
- Coutinho, J.; Ribeiro, E.; Ferreirinha, R.; Dias, P. Versão portuguesa da escala de dificuldades de regulação emocional e sua relação com sintomas psicopatológicos. Arch. Clin. Psychiatry 2010, 37, 145–151. [Google Scholar] [CrossRef]
- Moreira, J.M.; Gamboa, P. Inventário de estados afetivos-reduzido: Uma medida multidimensional breve de indicadores emocionais de ajustamento [The Affective State Inventory–Reduced: A Brief Multidimensional Measure of Emotional Indicators of Adjustment]. Rev. Iberoam. Diagn. Eval. Psicol. 2016, 1, 132–144. [Google Scholar]
- Cocks, K.; Torgerson, D.J. Sample size calculations for pilot randomized trials: A confidence interval approach. J. Clin. Epidemiol. 2013, 66, 197–201. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Hoffman, L. Longitudinal Analysis: Modeling Within-Person Fluctuation and Change; Routledge: Oxfordshire, UK, 2015. [Google Scholar] [CrossRef]
- Ligeza, T.S.; Kałamała, P.; Tarnawczyk, O.; Maciejczyk, M.; Wyczesany, M. Frequent physical exercise is associated with better ability to regulate negative emotions in adult women: The electrophysiological evidence. Ment. Health Phys. Act. 2019, 17, 100294. [Google Scholar] [CrossRef]
- Yoon, E.S.; So, W.-Y.; Jang, S. Association between Perceived Psychological Stress and Exercise Behaviors: A Cross-Sectional Study Using the Survey of National Physical Fitness. Life 2023, 13, 2059. [Google Scholar] [CrossRef] [PubMed]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The Satisfaction with Life Scale. J. Pers. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef]
- Litwic-Kaminska, K.; Kotyśko, M.; Pracki, T.; Wiłkość-Dębczyńska, M.; Stankiewicz, B. The effect of Autogenic Training in a form of audio recording on sleep quality and physiological stress reactions of University Athletes—Pilot study. Int. J. Environ. Res. Public Health 2022, 19, 16043. [Google Scholar] [CrossRef]
- Piras, A.; Zini, L.; Trofè, A.; Campa, F.; Raffi, M. Effects of acute microcurrent electrical stimulation on muscle function and subsequent recovery strategy. Int. J. Environ. Res. Public Health 2021, 18, 4597. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Tang, J.; Chen, C.; Tan, L.; Wu, Z.; Yu, F.; Wang, X. Progressive muscle relaxation improves anxiety and depression of pulmonary arterial hypertension patients. J. Evid. Based Complement. Altern. Med. 2015, 2015, 792895. [Google Scholar] [CrossRef]
- Toussaint, L.; Nguyen, Q.A.; Roettger, C.; Dixon, K.; Offenbächer, M.; Kohls, N.; Hirsch, J.; Sirois, F. Effectiveness of progressive muscle relaxation, deep breathing, and guided imagery in promoting psychological and physiological states of relaxation. J. Evid. Based Complement. Altern. Med. 2021, 2021, 5924040. [Google Scholar] [CrossRef]
| Session | EG1: FSM Only | EG2: FSM + Relaxation | ACG: Relaxation Only | PCG: Placebo FSM + Relaxation |
|---|---|---|---|---|
| 1–6 | FSM | FSM | Relaxation | Placebo FSM |
| 7–12 | FSM | Relaxation | Relaxation | Relaxation |
| Total | 12 FSM sessions | 6 FSM + 6 relaxation sessions | 12 relaxation sessions | 6 placebo FSM + 6 relaxation sessions |
| Baseline (T0) | ||||||
|---|---|---|---|---|---|---|
| EG1 (n = 16) | EG2 (n = 14) | ACG (n = 15) | PCG (n = 13) | Total (n = 58) | ||
| Categorical Variables | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Gender | ||||||
| Men | 5 (31.3) | 1 (7.1) | 1 (6.7) | 2 (15.4) | 9 (15.5) | |
| Women | 11 (68.8) | 13 (92.9) | 14 (93.3) | 11 (84.6) | 49 (84.5) | |
| Marital status | ||||||
| Single | 13 (81.3) | 14 (100) | 14 (93.3) | 11 (84.6) | 52 (89.7) | |
| Married | 1 (6.3) | 0 (0) | 1 (6.7) | 1 (7.7) | 3 (5.2) | |
| Living with a partner | 1 (6.3) | 0 (0) | 0 (0) | 0 (0) | 1 (1.7) | |
| Divorced or separated | 1 (6.3) | 0 (0) | 0 (0) | 1 (7.7) | 2 (3.4) | |
| University student | ||||||
| No | 2 (12.5) | 0 (0) | 2 (13.3) | 2 (15.4) | 6 (10.3) | |
| Yes | 14 (87.5) | 14 (100) | 13 (86.7) | 11 (84.6) | 52 (89.7) | |
| Physical activity | ||||||
| No | 6 (37.5) | 4 (28.6) | 7 (46.7) | 6 (46.2) | 23 (39.7) | |
| Yes | 10 (62.5) | 10 (71.4) | 8 (53.3) | 7 (53.8) | 35 (60.3) | |
| Tobacco consumption | ||||||
| No | 15 (93.8) | 14 (100) | 14 (93.3) | 12 (92.3) | 55 (94.8) | |
| Yes | 1 (6.3) | 0 (0) | 1 (6.7) | 1 (7.7) | 3 (5.2) | |
| Alcohol consumption | ||||||
| No | 7 (43.8) | 8 (57.1) | 4 (26.7) | 8 (61.5) | 27 (46.6) | |
| Yes | 9 (56.3) | 6 (42.9) | 11 (73.3) | 5 (38.5) | 31 (53.4) | |
| Medication a | ||||||
| No | 8 (50) | 13 (92.9) | 13 (86.7) | 11 (84.6) | 45 (77.6) | |
| Yes | 8 (50) | 1 (7.1) | 2 (13.3) | 2 (15.4) | 13 (22.4) | |
| Continuous Variables | Min–Max | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| Age | 18–62 | 28.94 (11.85) | 21.50 (5.11) | 24.33 (10.90) | 26.00 (12.53) | 25.29 (10.62) |
| Educational level (in years) | 12–25 | 15.92 (4.77) | 14.33 (2.93) | 13.50 (1.45) | 13.45 (1.37) | 14.35 (3.13) |
| T0 (N = 58) | T1 (N = 57) | T2 (N = 54) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EG1 (n = 16) | EG2 (n = 14) | ACG (n = 15) | PCG (n = 13) | EG1 (n = 15) | EG2 (n = 14) | ACG (n = 15) | PCG (n = 13) | EG1 (n = 14) | EG2 (n = 14) | ACG (n = 14) | PCG (n = 12) | |||||
| Continuous Variables | Min–Max | M (SD) | M (SD) | M (SD) | ||||||||||||
| EG1 | EG2 | ACG | PCG | |||||||||||||
| Emotional distress (HADS) | 5–28 | 6–27 | 4–29 | 2–25 | 17.13 (5.23) | 16.14 (6.19) | 17.07 (7.09) | 16.46 (5.98) | 13.67 (5.70) | 13.64 (4.88) | 16.13 (7.61) | 16.08 (5.80) | 13.14 (3.94) | 13.36 (5.24) | 13.50 (6.11) | 14.08 (6.24) |
| Perceived stress (PSS-10) | 8–28 | 10–32 | 2–33 | 0–30 | 21.44 (3.71) | 21.36 (5.87) | 23.40 (6.12) | 21.23 (6.86) | 18.00 (4.78) | 17.86 (4.74) | 21.13 (7.19) | 19.85 (6.97) | 18.64 (4.88) | 17.86 (5.04) | 17.29 (8.88) | 19.25 (8.07) |
| Emotion regulation (DERS) | 46–135 | 54–120 | 37–129 | 46–123 | 90.50 (23.89) | 81.57 (15.49) | 86.67 (17.92) | 76.77 (20.84) | 85.80 (19.99) | 75.86 (20.32) | 85.67 (25.00) | 75.46 (18.72) | 77.93 (19.65) | 71.14 (19.60) | 77.50 (23.72) | 73.75 (21.59) |
| Negative emotions (ASI-R NE) | 4–17 | 4–17 | 4–16 | 4–16 | 9.81 (2.88) | 8.00 (3.44) | 10.40 (3.60) | 8.46 (2.96) | 8.27 (2.37) | 7.50 (2.65) | 10.20 (4.07) | 8.69 (3.75) | 7.57 (1.56) | 7.00 (2.35) | 8.29 (3.38) | 7.42 (3.18) |
| Euphoric arousal (ASI-R EA) | 4–15 | 4–11 | 4–14 | 4–13 | 8.38 (3.10) | 7.50 (2.38) | 7.60 (2.59) | 8.23 (2.28) | 8.87 (2.77) | 8.43 (2.82) | 8.07 (2.76) | 8.77 (3.09) | 9.29 (2.92) | 8.14 (2.69) | 8.79 (2.81) | 8.58 (2.91) |
| Self-efficacy (ASI-R SE) | 4–17 | 7–19 | 6–20 | 7–19 | 11.25 (3.47) | 13.21 (2.42) | 12.07 (3.62) | 13.62 (2.02) | 12.00 (3.30) | 13.79 (2.58) | 12.40 (3.33) | 13.62 (2.40) | 13.36 (2.74) | 13.21 (2.61) | 13.29 (3.69) | 13.67 (3.14) |
| Warmth (ASI-R W) | 5–19 | 10–20 | 7–20 | 7–18 | 12.94 (3.64) | 14.86 (3.46) | 13.67 (3.02) | 14.62 (1.94) | 13.60 (2.64) | 14.79 (2.05) | 14.20 (2.46) | 13.69 (2.50) | 14.07 (3.58) | 15.00 (2.18) | 14.21 (3.04) | 15.08 (2.71) |
| Serenity (ASI-R S) | 3–13 | 3–14 | 3–12 | 3–15 | 7.50 (2.58) | 8.43 (3.41) | 6.80 (2.65) | 6.85 (2.64) | 8.53 (1.96) | 9.00 (2.29) | 8.07 (2.76) | 7.77 (2.62) | 8.79 (2.81) | 9.57 (2.65) | 8.86 (2.14) | 8.25 (3.33) |
| Somatic symptoms (PHQ-15) | 0–16 | 4–22 | 0–21 | 0–21 | 11.06 (4.74) | 12.14 (5.76) | 12.27 (5.35) | 12.46 (4.56) | 8.80 (4.09) | 7.36 (3.41) | 12.33 (5.85) | 10.15 (5.97) | 7.21 (5.03) | 8.93 (4.76) | 9.14 (4.75) | 9.25 (4.33) |
| Satisfaction with life (SWLS) | 8–25 | 9–25 | 9–25 | 8–25 | 17.00 (3.88) | 17.00 (4.08) | 17.20 (4.41) | 17.46 (4.39) | 18.79 (3.49) | 19.21 (3.75) | 17.27 (4.98) | 18.31 (4.03) | 18.86 (4.29) | 20.07 (3.67) | 19.57 (4.38) | 19.83 (5.10) |
| Response Variable | HADS | PSS-10 | DERS | ASI-R NE | ASI-R EA | ASI-R SE | ASI-R W | ASI-R S | PHQ-15 | SWLS |
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) |
| Intercept | 16.363 (1.1697) *** | 23.291 (1.806) *** | 84.340 (5.793) *** | 10.093 (0.851) *** | 8.124 (0.764) *** | 12.584 (0.858) *** | 13.878 (0.796) *** | 6.876 (0.755) *** | 12.340 (1.395) *** | 17.468 (1.225) *** |
| T1 | −0.933 (1.004) | −2.267 (1.165) | −1.000 (2.808) | −0.200 (0.645) | 0.467 (0.654) | 0.333 (0.599) | 0.533 (0.734) | 1.267 (0.628) * | 0.067 (0.991) | 0.067 (0.653) |
| T2 | −2.775 (1.136) * | −5.742 (1.527) *** | −6.988 (2.989) * | −1.857 (0.875) * | 1.098 (0.608) | 0.949 (0.635) | 0.661 (0.735) | 1.980 (0.624) ** | −2.803 (1.218) * | 2.032 (0.751) ** |
| EG1 | 0.225 (2.187) | −1.759 (2.343) | 1.233 (7.435) | −0.533 (1.097) | 0.670 (0.985) | −0.946 (1.110) | −0.626 (1.041) | 0.663 (0.985) | −1.126 (1.812) | −0.293 (1.597) |
| PCG | −0.903 (2.225) | −3.122 (2.385) | −10.833 (7.560) | −1.850 (1.134) | 0.866 (1.017) | 1.399 (1.129) | 0.706 (1.058) | 0.205 (1.002) | −0.119 (1.844) | 0.303 (1.630) |
| EG2 | −0.651 (2.241) | −2.000 (2.401) | −4.193 (7.617) | −2.281 (1.141) | −0.303 (1.023) | 0.947 (1.137) | 1.108 (1.065) | 1.599 (1.008) | −0.512 (1.856) | −0.304 (1.610) |
| PA | 1.507 (1.500) | 0.234 (1.540) | 4.986 (5.240) | 0.657 (0.687) | −1.124 (0.623) | −1.108 (0.747) | −0.453 (0.654) | −0.162 (0.627) | −0.157 (1.185) | −0.575 (1.112) |
| T1*EG1 | −2.234 (1.1.431) | −0.593 (1.663) | −2.987 (4.014) | −1.334 (0.904) | −0.056 (0.922) | 0.440 (0.854) | 0.303 (1.046) | −0.139 (0.899) | −1.871 (1.409) | 1.790 (0.940) |
| T2*EG1 | −0.183 (1.616) | 3.100 (2.160) | −3.750 (4.272) | −0.186 (1.214) | 0.046 (0.860) | 0.796 (0.904) | 0.527 (1.048) | −0.658 (0.895) | −0.779 (1.723) | −0.117 (1.081) |
| T1*PCG | 0.576 (1.445) | 1.410 (1.676) | 0.429 (4.042) | 0.414 (0.929) | −0.181 (0.941) | −0.333 (0.863) | −1.176 (1.056) | −0.552 (0.904) | −1.995 (1.427) | 0.779 (0.958) |
| T2*PCG | 0.124 (1.635) | 3.825 (2.199) | 2.204 (4.305) | 0.381 (1.261) | −0.501 (0.877) | −0.570 (0.914) | 0.148 (1.058) | −0.346 (0.899) | −0.523 (1.901) | 0.190 (1.103) |
| T1*EG2 | −1.567 (1.445) | −1.233 (1.676) | −4.714 (4.042) | −0.300 (0.929) | 0.462 (0.941) | 0.238 (0.863) | −0.605 (1.056) | −0.695 (0.904) | −3.981 (1.586) | 2.148 (0.940) |
| T2*EG2 | −0.011 (1.619) | 2.242 (2.172) | −3.441 (4.254) | 0.857 (1.247) | −0.455 (0.863) | −0.949 (0.906) | −0.518 (1.049) | −0.837 (0.887) | −0.097 (1.878) | 1.039 (1.072) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.G.; Machado, A.M.; Vilaça, M.; Faria, S.; Monteiro, I.; Santos, M. Effectiveness of Frequency-Specific Microcurrent (FSM) Therapy and Relaxation in Adults with Distress: A Pilot Randomized Controlled Trial. Healthcare 2025, 13, 1151. https://doi.org/10.3390/healthcare13101151
Pereira MG, Machado AM, Vilaça M, Faria S, Monteiro I, Santos M. Effectiveness of Frequency-Specific Microcurrent (FSM) Therapy and Relaxation in Adults with Distress: A Pilot Randomized Controlled Trial. Healthcare. 2025; 13(10):1151. https://doi.org/10.3390/healthcare13101151
Chicago/Turabian StylePereira, M. Graça, Ana Mónica Machado, Margarida Vilaça, Susana Faria, Isabela Monteiro, and Martim Santos. 2025. "Effectiveness of Frequency-Specific Microcurrent (FSM) Therapy and Relaxation in Adults with Distress: A Pilot Randomized Controlled Trial" Healthcare 13, no. 10: 1151. https://doi.org/10.3390/healthcare13101151
APA StylePereira, M. G., Machado, A. M., Vilaça, M., Faria, S., Monteiro, I., & Santos, M. (2025). Effectiveness of Frequency-Specific Microcurrent (FSM) Therapy and Relaxation in Adults with Distress: A Pilot Randomized Controlled Trial. Healthcare, 13(10), 1151. https://doi.org/10.3390/healthcare13101151






