A Comprehensive Literature Review on the Therapeutic Potential of Platelet-Rich Plasma for Diabetic Foot Management: Insights from a Case of a Neglected Deep Plantar Abscess
Abstract
1. Introduction
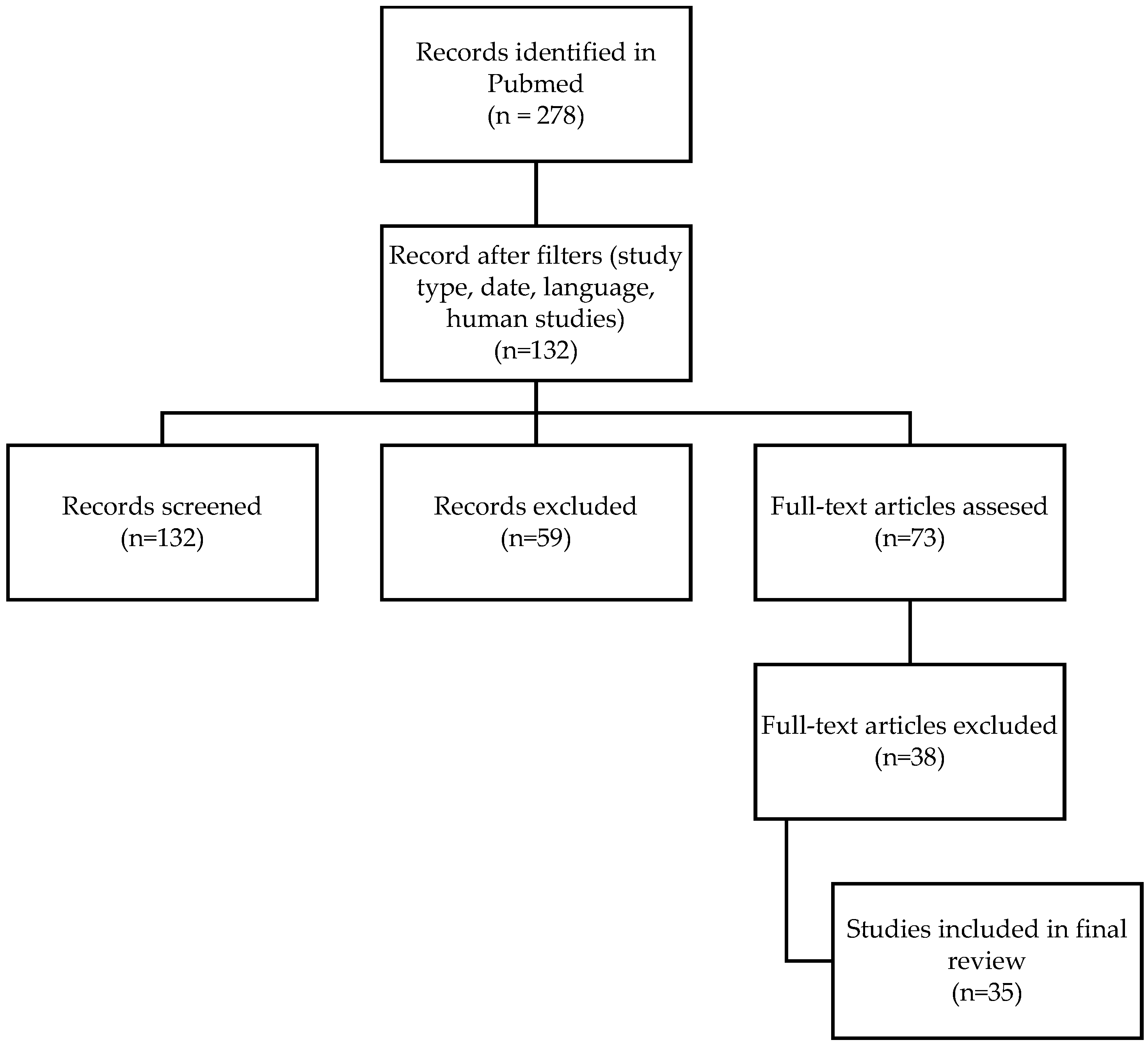
2. Materials and Methods
- Studies involving diabetic foot ulcer patients treated with PRP, either as a singular intervention or in combination with other therapies.
- Clinical outcomes related to wound healing, infection rates, granulation tissue formation, and amputation prevention.
- Studies on non-diabetic wounds or other conditions not related to DFUs.
- Studies with insufficient data on PRP preparation or application.
3. Results
- A.
- Outcome assessment in clinical trials
- B.
- Key findings in meta-analyses and reviews
- C.
- Limitations and gaps in research
- The efficacy of PRP requires further clinical validation, particularly in large-scale, well-designed trials.
- PRP treatment has a limited effect in certain patient populations, such as those with platelet dysfunction, thrombocytopenia, leukemia, or poor general health status.
- The systemic metabolic environment in T2DM—including elevated levels of glucose, reactive lipid byproducts, and nitric oxide (NO) metabolites—can lead to PRP cytotoxicity.
- The clinical application of PRP should be individualized; the etiology of the ulcer, as well as patient-specific factors, should be taken into consideration.
- The lack of standardized protocols for PRP preparation, concentration, and administration introduces significant variability in clinical outcomes.
- Many studies do not consistently report key details, such as PRP preparation methods, platelet concentrations, or leukocyte content, making it difficult to compare findings across studies.
- Differences in centrifugation methods, leukocyte content, platelet concentration, and delivery techniques (injection vs. topical application) make it challenging to compare results across studies.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2020, 236, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. International Working Group on the Diabetic Foot (IWGDF). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3280. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.J.; Lipsky, B.A. Diagnosis and management of infection in the diabetic foot. Med. Clin. N. Am. 2013, 97, 911–946. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Serrano, R.; Zimmermann, L.M.; Matos, L.L.; Baptista, M.S.; Pinhal, M.A.S.; Atallah, Á.N. Is surgical debridement necessary in the diabetic foot treated with photodynamic therapy? Diabet. Foot Ankle 2017, 8, 1373552. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wang, C.; Zhang, J.; Wang, P.; Xu, J.; Ding, M.; Li, X.; Hou, X.; Feng, S.; Li, X. Can we stop antibiotic therapy when signs and symptoms have resolved in diabetic foot infection patients? Int. J. Low Extrem. Wounds 2015, 14, 277–283. [Google Scholar] [CrossRef]
- Li, X.; Wen, S.; Dong, M.; Yuan, Y.; Gong, M.; Wang, C.; Yuan, X.; Jin, J.; Zhou, M.; Zhou, L. The Metabolic Characteristics of Patients at the Risk for Diabetic Foot Ulcer: A Comparative Study of Diabetic Patients with and without Diabetic Foot. Diabetes Metab. Syndr. Obes. 2023, 16, 3197–3211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meznerics, F.A.; Fehérvári, P.; Dembrovszky, F.; Kovács, K.D.; Kemény, L.V.; Csupor, D.; Hegyi, P.; Bánvölgyi, A. Platelet-Rich Plasma in Chronic Wound Management: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2022, 11, 7532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delwiche, F.; Raines, E.; Powell, J.; Ross, R.; Adamson, J. Platelet-derived growth factor enhances in vitro erythropoiesis via stimulation of mesenchymal cells. J. Clin. Investig. 1985, 76, 137–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bielecki, T.M.; Gazdzik, T.S.; Arendt, J.; Szczepanski, T.; Król, W.; Wielkoszynski, T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: An in vitro study. J. Bone Jt. Surg. Br. 2007, 89, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, D.; Zhou, L.; Ye, Y. Platelet-rich plasma promotes diabetic ulcer repair through inhibition of ferroptosis. Ann. Transl. Med. 2022, 10, 1121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohammadi, M.H.; Molavi, B.; Mohammadi, S.; Nikbakht, M.; Mohammadi, A.M.; Mostafaei, S.; Norooznezhad, A.H.; Ghorbani Abdegah, A.; Ghavamzadeh, A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Transfus. Apher. Sci. 2017, 56, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Kumar, V.; Pandey, A.; Pandey, P.; Gupta, V.; Verma, R. Role of platelet-rich plasma in healing diabetic foot ulcers: A prospective study. J. Wound Care 2018, 27, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, A.; El-Said, M.; Emile, S.; Youssef, M.; Khafagy, W.; Elshobaky, A. Randomized Controlled Trial on Autologous Platelet-Rich Plasma Versus Saline Dressing in Treatment of Non-healing Diabetic Foot Ulcers. World J. Surg. 2020, 44, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.J.; Leigh, R.; Kanapathy, M.; Macneal, P.; Jell, G.; Hachach-Haram, N.; Mann, H.; Mosahebi, A. Fat grafting and platelet-rich plasma for the treatment of diabetic foot ulcers: A feasibility-randomised controlled trial. Int. Wound J. 2020, 17, 1578–1594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alamdari, N.M.; Shafiee, A.; Mirmohseni, A.; Besharat, S. Evaluation of the efficacy of platelet-rich plasma on healing of clean diabetic foot ulcers: A randomized clinical trial in Tehran, Iran. Diabetes Metab. Syndr. 2021, 15, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Saeed Modaghegh, M.H.; Mostafavi-Pour, Z.; Azarpira, N.; Mousavian, A.; Bonakdaran, S.; Jarahi, L.; Samadi, A.; Peimani, M.; Hamidi Alamdari, D. The effect of platelet-rich plasma-fibrin glue dressing in combination with oral vitamin E and C for treatment of non-healing diabetic foot ulcers: A randomized, double-blind, parallel-group, clinical trial. Expert. Opin. Biol. Ther. 2021, 21, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Hossam, E.M.; Alserr, A.H.K.; Antonopoulos, C.N.; Zaki, A.; Eldaly, W. Autologous Platelet Rich Plasma Promotes the Healing of Non-Ischemic Diabetic Foot Ulcers. A Randomized Controlled Trial. Ann. Vasc. Surg. 2022, 82, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Alhawari, H.; Jafar, H.; Al Soudi, M.; Ameereh, L.A.; Fawaris, M.; Saleh, M.; Aladwan, S.; Younes, N.; Awidi, A. Perilesional injections of human platelet lysate versus platelet poor plasma for the treatment of diabetic foot ulcers: A double-blinded prospective clinical trial. Int. Wound J. 2023, 20, 3116–3122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohura, N.; Kimura, C.; Ando, H.; Yuzuriha, S.; Furukawa, M.; Higashita, R.; Ayabe, S.; Tsuji, Y.; Fujii, M.; Terabe, Y.; et al. Efficacy of autologous platelet-rich plasma gel in patients with hard-to-heal diabetic foot ulcers: A multicentre study in Japan. J. Wound Care 2024, 33, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-A.; Hsu, R.-J.; Jheng, W.-L.; Weng, F.-J.; Lee, J.-J. Comparative Efficacy of Regenerative Therapies for Diabetic Foot Ulcers: A Network Meta-analysis. Ann. Plast. Surg. 2025, 94 (Suppl. 1), S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Rai, V. Platelet-Rich Plasma in Diabetic Foot Ulcer Healing: Contemplating the Facts. Int. J. Mol. Sci. 2024, 25, 12864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, Y.; Wang, J.; Liu, X.; Zhou, Y.; Jia, S.; Xu, J.; Zheng, C. Efficacy of Platelet-Rich Plasma in the Treatment of Diabetic Foot Ulcers: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2024, 98, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-N.; Li, J.; Feng, D.-H.; Lu, R.-R.; Dong, G.-X.; Zhao, D.-Y. Efficacy and safety of autologous platelet-rich plasma for diabetic foot ulcers: A systematic review and meta-analysis. J. Wound Care 2023, 32, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Wang, Z.; Hunt, C.; Morrow, A.S.; Urtecho, M.; Amin, M.; Shah, S.; Hasan, B.; Abd-Rabu, R.; Ashmore, Z.; et al. The effectiveness and safety of platelet-rich plasma for chronic wounds: A systematic review and meta-analysis. Mayo Clin. Proc. 2021, 96, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Sivaramakrishnan, G. Growth factors for diabetic foot ulcers: Mixed treatment comparison analysis of randomized clinical trials. Br. J. Clin. Pharmacol. 2018, 84, 434–444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perussolo, J.; Calciolari, E.; Dereka, X.; Donos, N. Platelet-rich plasma and plasma rich in growth factors in extra-oral wound care. Periodontol 2000 2025, 97, 320–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izzo, P.; De Intinis, C.; Molle, M.; Polistena, A.; Sibio, S.; Codacci-Pisanelli, M.; Biacchi, D.; Di Cello, P.; Santini, D.; Izzo, L.; et al. Case report: The use of PRP in the treatment of diabetic foot: Case series and a review of the literature. Front. Endocrinol. 2023, 14, 1286907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, X.L.; Hu, L.; Li, T.; Zou, Y.; Li, H.L. A meta-analysis on the efficacy of vacuum sealing drainage combined with autologous platelet-rich plasma in the treatment of Grade 2 and Grade 3 diabetic foot ulcers. Int. Wound J. 2023, 20, 1033–1041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vicenti, G.; Bizzoca, D.; Caruso, I.; Nappi, V.S.; Giancaspro, G.; Carrozzo, M.; Moretti, B. New insights into the treatment of non-healing diabetic foot ulcers. J. Biol. Regul. Homeost. Agents 2018, 32, 15–21. [Google Scholar] [PubMed]
- Gomez, P.T.; Andrews, K.L.; Arthurs, J.R.; Bruce, A.J.; Wyles, S.P. Platelet-Rich Plasma in the Treatment of Diabetic Foot Ulcers. Adv. Skin Wound Care 2024, 37, 608–615. [Google Scholar] [CrossRef] [PubMed]
- OuYang, H.; Yang, J.; Wan, H.; Huang, J.; Yin, Y. Effects of different treatment measures on the efficacy of diabetic foot ulcers: A network meta-analysis. Front. Endocrinol. 2024, 15, 1452192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, A.Y.W.; Hooi, N.M.F.; Yeo, B.S.Y.; Sultana, R.; Bee, Y.M.; Lee, A.R.Y.B.; Tay, S.M. Improving Diabetic Wound-Healing Outcomes With Topical Growth Factor Therapies. J. Clin. Endocrinol. Metab. 2024, 109, e1642–e1651. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, M.; Zhang, X.; Zhang, H. Efficacy and safety of autologous platelet-rich plasma for diabetic foot ulcer healing: A systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2023, 18, 370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abd El-Mabood, E.A.; Ali, H.E. Platelet-rich plasma versus conventional dressing: Does this really affect diabetic foot wound-healing outcomes? Egypt. J. Surg. 2018, 37, 16–26. [Google Scholar] [CrossRef]
- Anitua, E.; Aguirre, J.J.; Algorta, J.; Ayerdi, E.; Cabezas, A.I.; Orive, G.; Andia, I. Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 415–421. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Ahmed, A.A.Q.; Lamboni, L.; Shi, Z.; Mukole, B.M.; Zheng, R.; Mbang, M.P.; Zhang, B.; Gauthier, M.; Yang, G. Engineering homologous platelet-rich plasma, platelet-rich plasma-derived exosomes, and mesenchymal stem cell-derived exosomes-based dual-crosslinked hydrogels as bioactive diabetic wound dressings. Bioact. Mater. 2023, 28, 74–94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, S.-C.; Tao, S.-C.; Yin, W.-J.; Qi, X.; Yuan, T.; Zhang, C.-Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef]
- Yang, L.; Gao, L.; Lv, Y.; Wang, J. Autologous platelet-rich gel for lower-extremity ischemic ulcers in patients with type 2 diabetes. Int. J. Clin. Exp. Med. 2017, 10, 13796–13801. [Google Scholar]
- Amato, B.; Farina, M.A.; Campisi, S.; Ciliberti, M.; Di Donna, V.; Florio, A.; Grasso, A.; Miranda, R.; Pompeo, F.; Farina, E.; et al. Cgf treatment of leg ulcers: A randomized controlled trial. Open Med. 2020, 14, 959–967. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Alimohamadi, Y.; Janani, M.; Hejazi, P.; Kamali, M.; Goodarzi, A. Platelet-rich plasma in the treatment of scars, to suggest or not to suggest? A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2022, 16, 875–899. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, Y.; Li, Y.; Lin, C.; Wang, S.; Wang, J.; Ma, C.; Wu, S. Comparison of Leukocyte-Rich and Leukocyte-Poor Platelet-Rich Plasma on Pressure Ulcer in a Rat Model. J. Burn. Care Res. 2023, 44, 860–868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, R.; Gu, Y.; Ran, J.; Hu, Y.; Zheng, Z.; Zeng, M.; Heng, B.C.; Chen, X.; Yin, Z.; Chen, W.; et al. Intratendon Delivery of Leukocyte-Poor Platelet-Rich Plasma Improves Healing Compared With Leukocyte-Rich Platelet-Rich Plasma in a Rabbit Achilles Tendinopathy Model. Am. J. Sports Med. 2017, 45, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Miroshnichenko, S.; Usynin, I.; Dudarev, A.; Nimaev, V.; Solovieva, A. Apolipoprotein A-I supports MSCs survival under stress conditions. Int. J. Mol. Sci. 2020, 21, 4062. [Google Scholar] [CrossRef]
- Adela, R.; Nethi, S.K.; Bagul, P.K.; Barui, A.K.; Mattapally, S.; Kuncha, M.; Patra, C.R.; Reddy, P.N.C.; Banerjee, S.K. Hyperglycaemia enhances nitric oxide production in diabetes: A study from South Indian patients. PLoS ONE 2015, 10, e0125270. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Shetty, S.; Orci, L.; Unger, R.H.; Scherer, P.E. Diabetes and apoptosis: Lipotoxicity. Apoptosis 2009, 14, 1484–1495. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, A.O.; Sitnikova, N.A.; Nimaev, V.V.; Koroleva, E.A.; Manakhov, A.M. PRP of T2DM Patient Immobilized on PCL Nanofibers Stimulate Endothelial Cells Proliferation. Int. J. Mol. Sci. 2023, 24, 8262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- OuYang, H.; Tang, Y.; Yang, F.; Ren, X.; Yang, J.; Cao, H.; Yin, Y. Platelet-rich plasma for the treatment of diabetic foot ulcer: A systematic review. Front. Endocrinol. 2023, 14, 1256081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
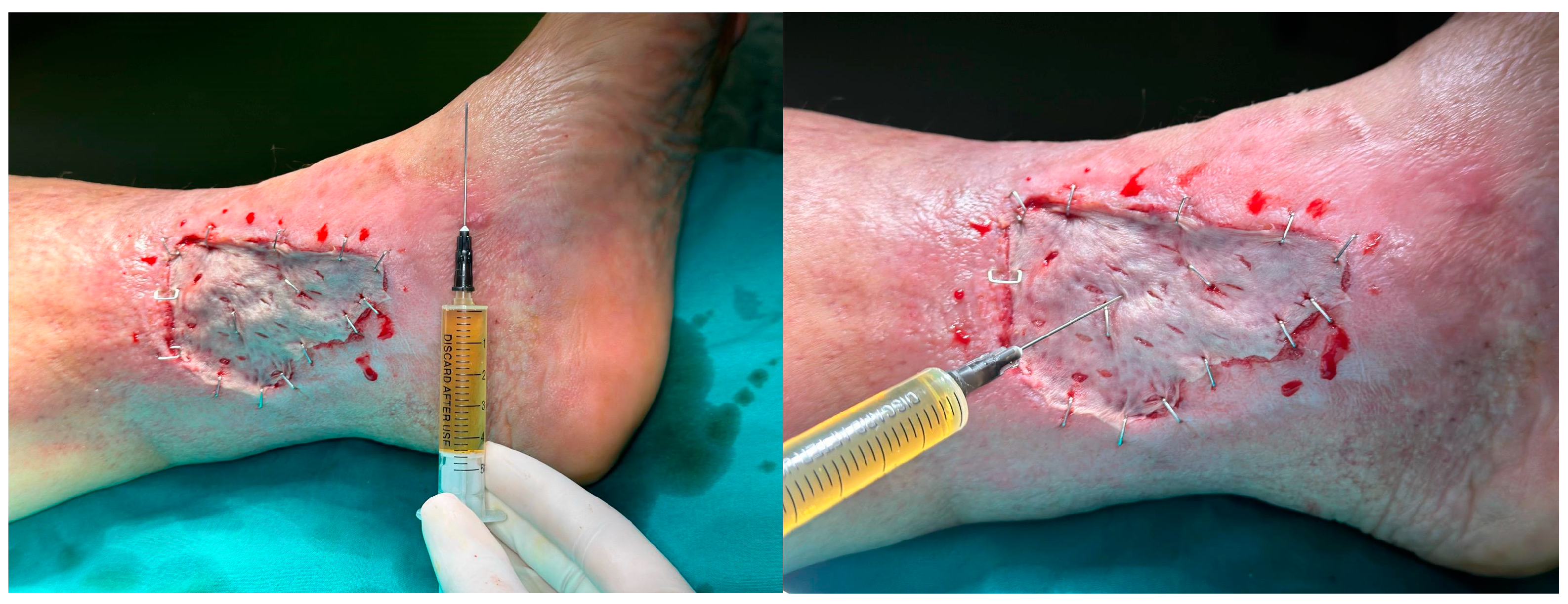
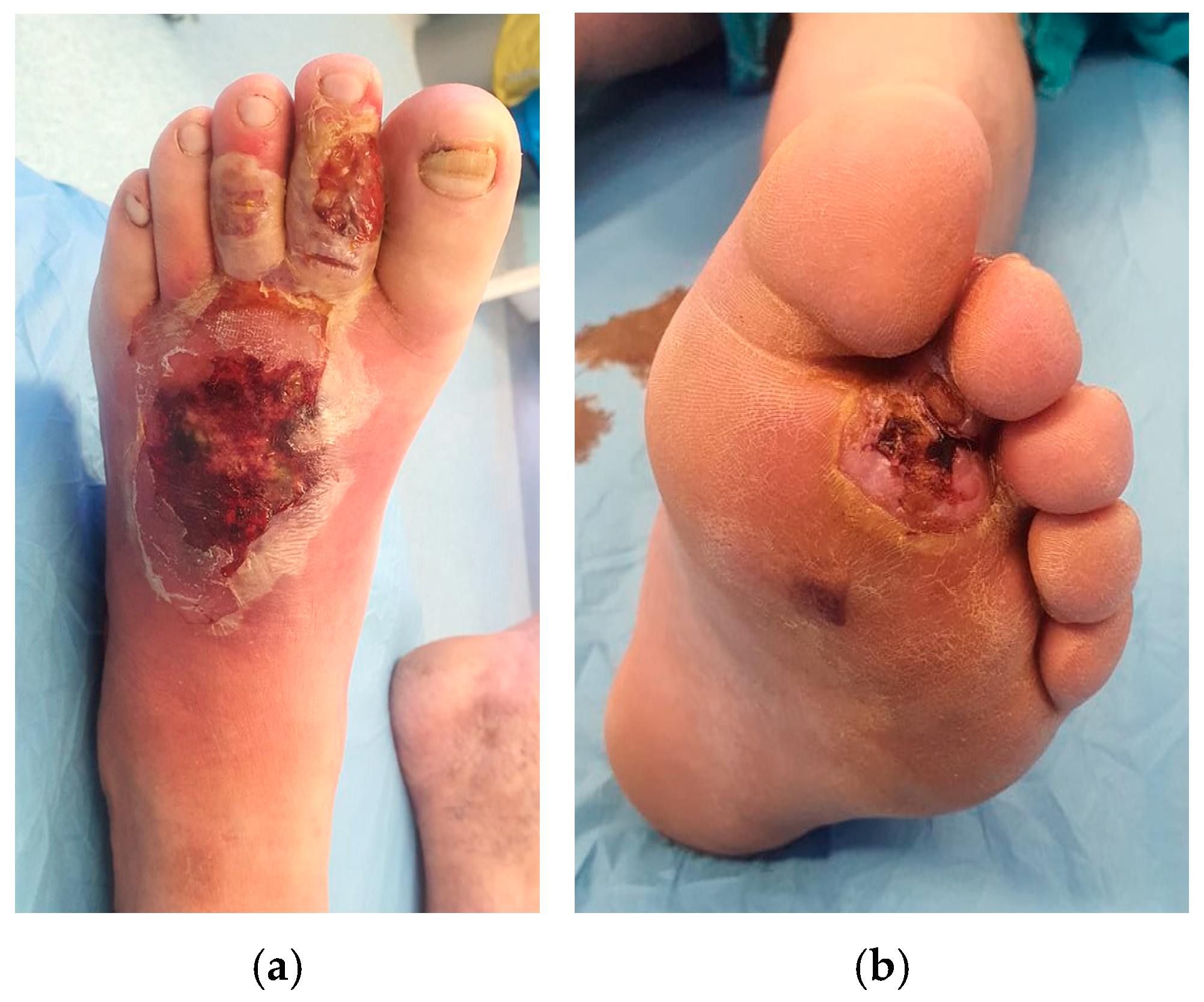
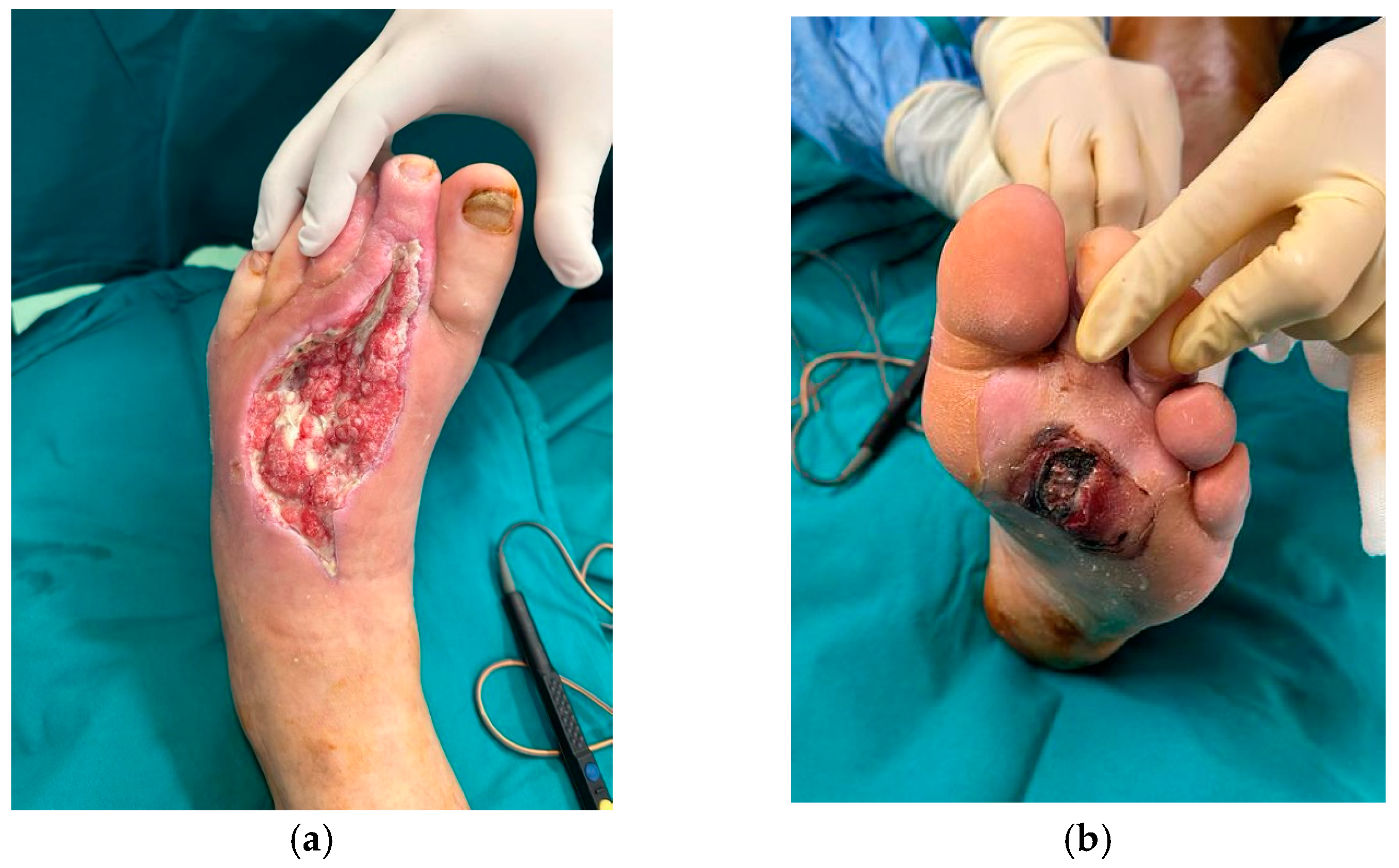
| Study | No. of Patients (Male/Female) | Mean Age | PRP Technique | Complete Wound Closure | Time to Healing | Reduction in Wound Size | Infection Rates | Amputation Rate |
|---|---|---|---|---|---|---|---|---|
| Mohammadi MH et al., 2017 [12] | 70 (58/12) | 53.8 years | gel | N/A | 8.7 weeks | 51.90% | N/A | N/A |
| Singh S. et al., 2018 [13] | 55 (34/21) | 53.76 years | injections | 96.30% | 9 weeks | 68.80% | N/A | N/A |
| Elsaid et al., 2020 [14] | 12 (8/4) | 54.7 years | gel | 25% | 6.3 weeks | 43.20% | N/A | N/A |
| Smith et al., 2020 [15] | 6 (5/1) | 57.5 years | injections | 33.30% | 10 weeks | N/A | N/A | N/A |
| Malekpour AN. et al., 2021 [16] | 47 (30/17) | 56.7 years | gel | N/A | 7.8 weeks | N/A | N/A | 11.6% |
| Yarahmadi A. et al., 2021 [17] | 25 (18/7) | 55.62 years | gel | 32% | N/A | N/A | N/A | N/A |
| Hossam EM et al., 2021 [18] | 40 (28/12) | 54.9 years | injections | 95% | 6 weeks | ≥50% | 10% | 0% |
| Alhawari H et al., 2023 [19] | 10 (6/4) | 61.4 years | injections | 90% | 12 weeks | N/A | 0% | 0% |
| Ohura N et al., 2024 [20] | 54 | N/A | gel | 57.40% | 8.1 weeks | 72.80% | N/A | N/A |
| Supporting Evidence | Key Findings | Notes |
|---|---|---|
| Yang HA et al. (2025) [21] | PRP + conventional treatment accelerates wound healing | Enhanced epithelialization and wound closure observed |
| Smith J. et al. (2024) [22] | ||
| Peng Y. et al. (2024) [23] | ||
| Su YN et al. (2023) [24] | ||
| Qu W et al. (2021) [25] | ||
| Sridharan K et al. (2018) [26] | ||
| Perussolo J. et al. (2025) [27] | PRP and PRGF reduce wound size and accelerate healing | PRGF also showed additional anti-inflammatory benefits |
| Izzo P et al. (2023) [28] | ||
| Yin XL et al. (2023) [29] | PRP is effective and safe for DFU healing | No serious adverse effects; favorable safety profile |
| Vicenti G et al. (2018) [30] | ||
| Gomez PT. et al. (2024) [31] | PRP + standard of care leads to superior wound healing vs. SOC alone | RCT; significant improvement in healing rate in PRP group |
| OuYang H et al. (2024) [32] | PRP reduces amputation rate and adverse reactions | Notable limb-sparing outcomes in high-risk patients |
| OuYang H et al. (2024) [32] | PRP + NPWT is more effective for complete ulcer healing vs. SOC | Combination therapy showed synergistic effect on healing |
| Wong AYW et al. (2024) [33] | PRP reduces ulcer-related adverse events (e.g., infections) | Lower infection rates and improved local wound environment |
| Deng J et al. (2023) [34] | PRP decreases the risk of amputations | Reported reduction in major amputation rate and prolonged limb salvage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riza, S.-M.; Porosnicu, A.-L.; Sinescu, R.D. A Comprehensive Literature Review on the Therapeutic Potential of Platelet-Rich Plasma for Diabetic Foot Management: Insights from a Case of a Neglected Deep Plantar Abscess. Healthcare 2025, 13, 1130. https://doi.org/10.3390/healthcare13101130
Riza S-M, Porosnicu A-L, Sinescu RD. A Comprehensive Literature Review on the Therapeutic Potential of Platelet-Rich Plasma for Diabetic Foot Management: Insights from a Case of a Neglected Deep Plantar Abscess. Healthcare. 2025; 13(10):1130. https://doi.org/10.3390/healthcare13101130
Chicago/Turabian StyleRiza, Stefania-Mihaela, Andrei-Ludovic Porosnicu, and Ruxandra Diana Sinescu. 2025. "A Comprehensive Literature Review on the Therapeutic Potential of Platelet-Rich Plasma for Diabetic Foot Management: Insights from a Case of a Neglected Deep Plantar Abscess" Healthcare 13, no. 10: 1130. https://doi.org/10.3390/healthcare13101130
APA StyleRiza, S.-M., Porosnicu, A.-L., & Sinescu, R. D. (2025). A Comprehensive Literature Review on the Therapeutic Potential of Platelet-Rich Plasma for Diabetic Foot Management: Insights from a Case of a Neglected Deep Plantar Abscess. Healthcare, 13(10), 1130. https://doi.org/10.3390/healthcare13101130






