ACTIVA-Senior: Study Design and Protocol for a Preliminary Multidomain Outdoor Intervention Promoting Healthy Aging and Mitigating Psycho-Physiological Decline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Ethical Considerations
2.3. Sample Size and Power Calculation
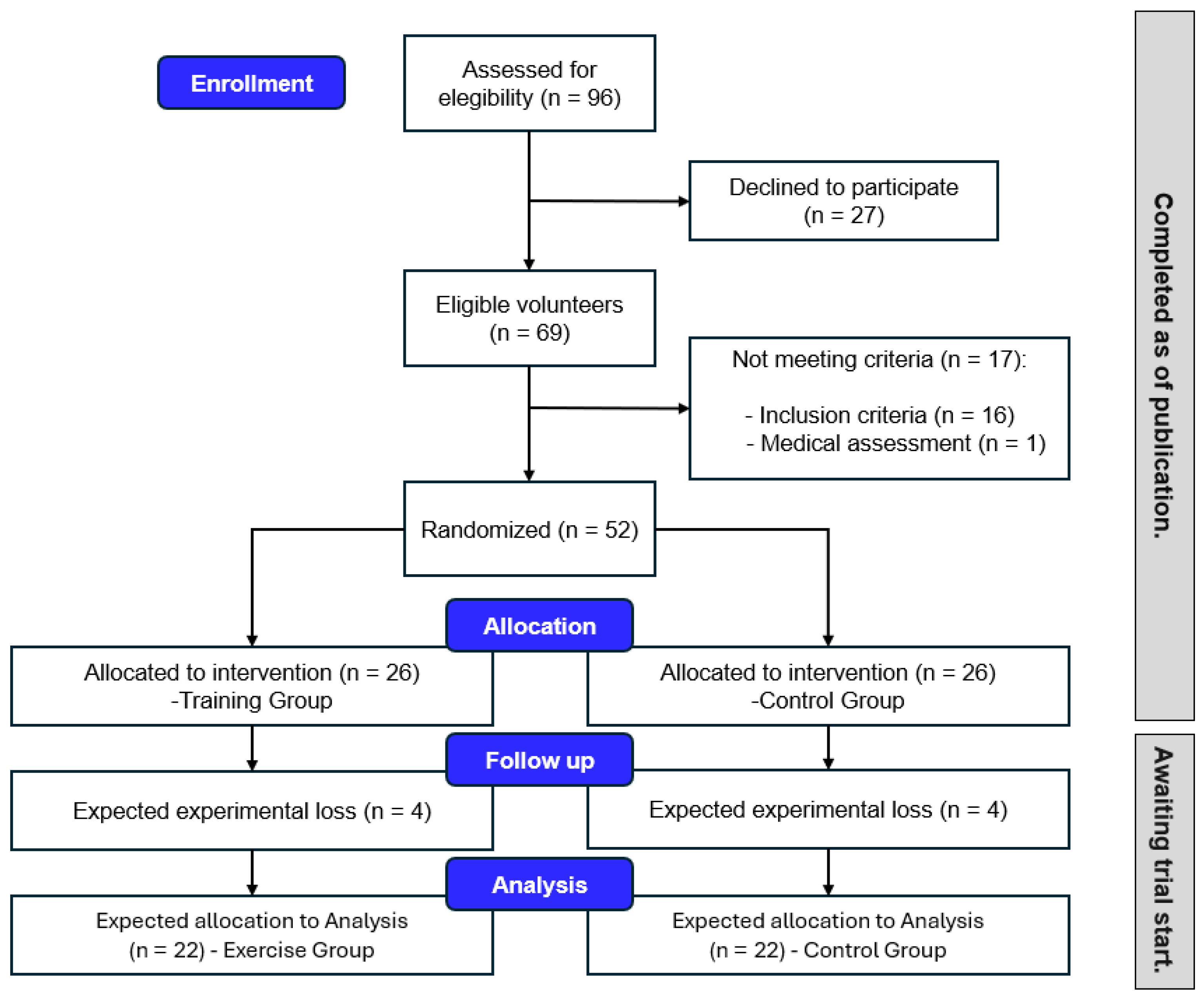
2.4. Participant Recruitment
2.5. Randomization and Blinding
2.6. Intervention
2.7. Instruments
2.8. Procedures
2.9. Statistical Analysis
2.10. Feasibility and Acceptability Assessment
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RCT | Randomized controlled trial |
| TG | Training group |
| WHO | World Health Organization |
| CG | Control group |
| CONSORT | Consolidated Standards for Reporting Trials |
| SPIRIT | Standard Protocol Items: Recommendations for Interventional Trials |
| SD | Standard deviation |
| CI | Confidence interval |
| EKG | Electrocardiogram |
| BMI | Body mass index |
| COPD | Chronic obstructive pulmonary disease |
| NSCA | National Strength and Conditioning Association |
| ACSM | American College of Sports Medicine |
| RIR | Repetitions in reserve |
| 10MWT | 10 m walking test |
| TUG | Time up and go |
| χ2 | Chi-square |
| ANOVA | Repeated measures analysis of variance |
References
- INE National Institute of Statistics 2018. Available online: https://www.ine.es/prodyser/espa_cifras/2018/ (accessed on 2 August 2022).
- World Health Organization. Ageing and Health; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- United Nations Department of Economic and Social Affairs. 2020. Available online: https://digitallibrary.un.org/record/3898412/files/undesa_pd-2020_world_population_ageing_highlights.pdf (accessed on 2 August 2022).
- Luo, Y.; Su, B.; Zheng, X. Trends and Challenges for Population and Health During Population Aging—China, 2015–2050. China CDC Wkly. 2021, 3, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Yue, L.; Dang, X.; Chen, F.; Gong, Y.; Lin, X.; Luo, Y. Rosenroot (Rhodiola): Potential Applications in Aging-Related Diseases. Aging Dis. 2019, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.; Steffens, D.C. What Are the Causes of Late-Life Depression? Psychiatr. Clin. N. Am. 2013, 36, 497–516. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, F.; Qin, T. Relationships between Chronic Diseases and Depression among Middle-Aged and Elderly People in China: A Prospective Study from CHARLS. Curr. Med. Sci. 2020, 40, 858–870. [Google Scholar] [CrossRef]
- Liu, J.Y.W.; Kor, P.P.K.; Lee, P.L.; Chien, W.T.; Siu, P.M.; Hill, K.D. Effects of an Individualized Exercise Program Plus Behavioral Change Enhancement Strategies for Managing Fatigue in Older People Who Are Frail: Protocol for a Cluster Randomized Controlled Trial. Phys. Ther. 2019, 99, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Rios-Romenets, S.; Acosta-Baena, N.; Lopez, L.; Madrigal-Zapata, L.; Street, H.; Jakimovich, L.; Langbaum, J.B.; Cho, W.; Reiman, E.M.; Tariot, P.N.; et al. Adherence/Retention Alzheimer’s Prevention Initiative Colombia Plan. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 283–287. [Google Scholar] [CrossRef]
- Brown, R.T.; Covinsky, K.E. Moving Prevention of Functional Impairment Upstream: Is Middle Age an Ideal Time for Intervention? Womens Midlife Health 2020, 6, 4. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Zhang, Y.; Liu, P.; Song, Y.; Zhou, Y.; Ma, L. Visceral Fat Obesity Correlates with Frailty in Middle-Aged and Older Adults. Diabetes Metab. Syndr. Obes. 2022, 15, 2877–2884. [Google Scholar] [CrossRef]
- Predovan, D.; Fraser, S.A.; Renaud, M.; Bherer, L. The Effect of Three Months of Aerobic Training on Stroop Performance in Older Adults. J. Aging Res. 2012, 2012, 269815. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; Orquin-Castrillón, F.J.; Gea-García, G.M.; Menayo-Antúnez, R.; González-Gálvez, N.; de Souza Vale, R.G.; Martínez-Rodríguez, A. Effects of a Moderate-to-High Intensity Resistance Circuit Training on Fat Mass, Functional Capacity, Muscular Strength, and Quality of Life in Elderly: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 7830. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Andreu-Caravaca, L.; Carrasco-Poyatos, M.; Benito, P.J.; Rubio-Arias, J.Á. Effects of Circuit Resistance Training on Body Composition, Strength, and Cardiorespiratory Fitness in Middle-Aged and Older Women: A Systematic Review and Meta-Analysis. J. Aging Phys. Act. 2022, 30, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Castaño, L.A.A.; de Lima, V.C.; Barbieri, J.F.; de Lucena, E.G.P.; Gáspari, A.F.; Arai, H.; Teixeira, C.V.L.; Coelho-Júnior, H.J.; Uchida, M.C. Resistance Training Combined With Cognitive Training Increases Brain Derived Neurotrophic Factor and Improves Cognitive Function in Healthy Older Adults. Front. Psychol. 2022, 13, 870561. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.A.; Pedisic, Z.; Suni, J.H.; Tokola, K.; Husu, P.; Biddle, S.J.H.; Vasankari, T. Self-reported Health-enhancing Physical Activity Recommendation Adherence among 64,380 Finnish Adults. Scand. J. Med. Sci. Sports 2017, 27, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Klimova, B.; Valis, M.; Kuca, K. Cognitive Decline in Normal Aging and Its Prevention: A Review on Non-Pharmacological Lifestyle Strategies. Clin. Interv. Aging 2017, 12, 903–910. [Google Scholar] [CrossRef]
- Roig-Coll, F.; Castells-Sánchez, A.; Lamonja-Vicente, N.; Torán-Monserrat, P.; Pera, G.; García-Molina, A.; Tormos, J.M.; Montero-Alía, P.; Alzamora, M.T.; Dacosta-Aguayo, R.; et al. Effects of Aerobic Exercise, Cognitive and Combined Training on Cognition in Physically Inactive Healthy Late-Middle-Aged Adults: The Projecte Moviment Randomized Controlled Trial. Front. Aging Neurosci. 2020, 12, 590168. [Google Scholar] [CrossRef]
- Rodriguez-Rodríguez, S.; Canet-Vintró, M.; Wee, S.O.; Rodríguez-Sanz, J.; López-de-Celis, C.; Oviedo, G.R.; Labata-Lezaun, N.; Pérez-Bellmunt, A. Cognitive Enhancement Strategies for Older Adults: An Evaluation of Different Training Modalities to Improve Executive Function—A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1301. [Google Scholar] [CrossRef]
- Kelly, M.E.; Loughrey, D.; Lawlor, B.A.; Robertson, I.H.; Walsh, C.; Brennan, S. The Impact of Cognitive Training and Mental Stimulation on Cognitive and Everyday Functioning of Healthy Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2014, 15, 28–43. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 Year Multidomain Intervention of Diet, Exercise, Cognitive Training, and Vascular Risk Monitoring versus Control to Prevent Cognitive Decline in at-Risk Elderly People (FINGER): A Randomised Controlled Trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Tirapu Ustárroz, J.; Rios Lago, M.; Maestú Unturbe, F. Manual de Neuropsicología 2.a Edición; Viguera Editores: Barcelona, Spain, 2011. [Google Scholar]
- García-Llorente, A.M.; Casimiro-Andújar, A.J.; Linhares, D.G.; Vale, R.G.D.S.; Marcos-Pardo, P.J. Multidomain Interventions for Sarcopenia and Cognitive Flexibility in Older Adults for Promoting Healthy Aging: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Aging Clin. Exp. Res. 2024, 36, 47. [Google Scholar] [CrossRef]
- Castro, C.B.; Costa, L.M.; Dias, C.B.; Chen, J.; Hillebrandt, H.; Gardener, S.L.; Brown, B.M.; Loo, R.L.; Garg, M.L.; Rainey-Smith, S.R.; et al. Multi-Domain Interventions for Dementia Prevention–A Systematic Review. J. Nutr. Health Aging 2023, 27, 1271–1280. [Google Scholar] [CrossRef]
- Ahmed, F.S.; McMillan, T.M.; Guenther, B.A.; Dearborn, P. Cognitive Performance Following Single- or Multi-Session Exercise Intervention in Middle Age: A Systematic Review. Exp. Aging Res. 2024, 50, 28–64. [Google Scholar] [CrossRef] [PubMed]
- Levinger, P.; Sales, M.; Polman, R.; Haines, T.; Dow, B.; Biddle, S.J.H.; Duque, G.; Hill, K.D. Outdoor physical activity for older people—The senior exercise park: Current research, challenges and future directions. Health Promot. J. Aust. 2018, 29, 353–359. [Google Scholar] [CrossRef]
- Roh, H.W.; Hong, C.H.; Lim, H.K.; Chang, K.J.; Kim, H.; Kim, N.R.; Choi, J.W.; Lee, K.S.; Cho, S.M.; Park, B.; et al. A 12-Week Multidomain Intervention for Late-Life Depression: A Community-Based Randomized Controlled Trial. J. Affect. Disord. 2020, 263, 437–444. [Google Scholar] [CrossRef]
- Salzman, T.; Sarquis-Adamson, Y.; Son, S.; Montero-Odasso, M.; Fraser, S. Associations of Multidomain Interventions With Improvements in Cognition in Mild Cognitive Impairment. JAMA Netw. Open 2022, 5, e226744. [Google Scholar] [CrossRef]
- Teixeira, C.V.L.; de Rezende, T.J.R.; Weiler, M.; Magalhães, T.N.C.; Carletti-Cassani, A.F.M.K.; Silva, T.Q.A.C.; Joaquim, H.P.G.; Talib, L.L.; Forlenza, O.V.; Franco, M.P.; et al. Cognitive and Structural Cerebral Changes in Amnestic Mild Cognitive Impairment Due to Alzheimer’s Disease after Multicomponent Training. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.L.; Ellis, J.; Otis, L.; Marchetti, G. A Multidimensional Exercise Program in the Home for Older Adults Designed to Improve Function. Home Health Care Manag. Pract. 2019, 31, 147–154. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise Interventions for Cognitive Function in Adults Older than 50: A Systematic Review with Meta-Analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Falvey, J.R.; Mangione, K.K.; Nordon-Craft, A.; Cumbler, E.; Burrows, K.L.; Forster, J.E.; Stevens-Lapsley, J.E. Progressive Multicomponent Intervention for Older Adults in Home Health Settings Following Acute Hospitalization: Randomized Clinical Trial Protocol. Phys. Ther. 2019, 99, 1141–1149. [Google Scholar] [CrossRef]
- Xu, X.; Pang, T.; Zhou, Y.; Zhang, H.; Ma, A.; Yuan, C.; Chen, H.; Wen, X.; Yang, Q.; Xu, X. The Multi-Domain Lifestyle Intervention for Cognitive Impairment in Community-Dwelling Older Adults in Hangzhou (The Heritage Study): Study Design and Protocol. J. Prev. Alzheimers Dis. 2024, 11, 601–611. [Google Scholar] [CrossRef]
- Yoon, J.; Isoda, H.; Ueda, T.; Okura, T. Cognitive and Physical Benefits of a Game-like Dual-task Exercise among the Oldest Nursing Home Residents in Japan. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12276. [Google Scholar] [CrossRef]
- Merchant, R.A.; Morley, J.E.; Izquierdo, M. Exercise, Aging and Frailty: Guidelines for Increasing Function. J. Nutr. Health Aging 2021, 25, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-K.; Lee, W.-J.; Chou, M.-Y.; Hwang, A.-C.; Lin, C.-S.; Peng, L.-N.; Hsiao, F.-Y.; Loh, C.-H.; Chen, L.-K. Roles of Baseline Intrinsic Capacity and Its Subdomains on the Overall Efficacy of Multidomain Intervention in Promoting Healthy Aging among Community-Dwelling Older Adults: Analysis from a Nationwide Cluster-Randomized Controlled Trial. J. Prev. Alzheimers Dis. 2024, 11, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Belleville, S.; Cuesta, M.; Bieler-Aeschlimann, M.; Giacomino, K.; Widmer, A.; Mittaz Hager, A.G.; Perez-Marcos, D.; Cardin, S.; Boller, B.; Bier, N.; et al. Pre-Frail Older Adults Show Improved Cognition with StayFitLonger Computerized Home–Based Training: A Randomized Controlled Trial. Geroscience 2022, 45, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hwang, A.; Lee, W.; Peng, L.; Lin, M.; Neil, D.L.; Shih, S.; Loh, C.; Chiou, S. Efficacy of Multidomain Interventions to Improve Physical Frailty, Depression and Cognition: Data from Cluster-randomized Controlled Trials. J. Cachexia Sarcopenia Muscle 2020, 11, 650–662. [Google Scholar] [CrossRef]
- Müller, P.; Achraf, A.; Zou, L.; Apfelbacher, C.; Erickson, K.I.; Müller, N.G. COVID-19, Physical (In-)Activity, and Dementia Prevention. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12091. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Orav, J.E.; Kanis, J.A.; Rizzoli, R.; Schlögl, M.; Staehelin, H.B.; Willett, W.C.; Dawson-Hughes, B. Comparative Performance of Current Definitions of Sarcopenia against the Prospective Incidence of Falls among Community-Dwelling Seniors Age 65 and Older. Osteoporos. Int. 2015, 26, 2793–2802. [Google Scholar] [CrossRef]
- Ansai, J.H.; Rebelatto, J.R. Effect of Two Physical Exercise Protocols on Cognition and Depressive Symptoms in Oldest-Old People: A Randomized Controlled Trial. Geriatr. Gerontol. Int. 2015, 15, 1127–1134. [Google Scholar] [CrossRef]
- Bae, S.; Lee, S.; Lee, S.; Jung, S.; Makino, K.; Harada, K.; Harada, K.; Shinkai, Y.; Chiba, I.; Shimada, H. The Effect of a Multicomponent Intervention to Promote Community Activity on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Complement. Ther. Med. 2019, 42, 164–169. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; González-Gálvez, N.; Gea-García, G.M.; López-Vivancos, A.; Espeso-García, A.; de Souza Vale, R.G. Sarcopenia as a Mediator of the Effect of a Gerontogymnastics Program on Cardiorespiratory Fitness of Overweight and Obese Older Women: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 7064. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; González-Gálvez, N.; Vaquero-Cristóbal, R.; Sagarra-Romero, L.; López-Vivancos, A.; Velázquez-Díaz, D.; García, G.M.G.; Ponce-González, J.G.; Esteban-Cornejo, I.; Jiménez-Pavón, D.; et al. Multidomain Healthy-Age Programme. Recomendations for Healthy Ageing: On Behalf of the Healthy-Age Network. Cult. Cienc. Deporte 2021, 16, 311–320. [Google Scholar] [CrossRef]
- Liguori, G. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Webster, A.L.; Aznar-Laín, S. Intensity of physical activity and the “‘talk test’” a brief review and practical application learning objective. ACSM’s Health Fit. J. 2008, 12, 13–17. [Google Scholar] [CrossRef]
- Williams, N. The Borg Rating of Perceived Exertion (RPE) Scale. Occup. Med. 2017, 67, 404–405. [Google Scholar] [CrossRef]
- Skou, S.T.; Nyberg, M.; Dideriksen, M.; Overgaard, J.A.; Bodilsen, C.; Soja, A.M.; Attarzadeh, A.P.; Bieder, M.J.; Dridi, N.P.; Heltberg, A.; et al. Study Protocol for a Multicenter Randomized Controlled Trial of Personalized Exercise Therapy and Self-Management Support for People with Multimorbidity: The MOBILIZE Study. J. Multimorb. Comorbidity 2023, 13, 26335565231154447. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent Validation of the OMNI Perceived Exertion Scale for Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; Espeso-García, A.; Vaquero-Cristóbal, R.; Abelleira-Lamela, T.; González-Gálvez, N. The Effect of Resistance Training with Outdoor Fitness Equipment on the Body Composition, Physical Fitness, and Physical Health of Middle-Aged and Older Adults: A Randomized Controlled Trial. Healthcare 2024, 12, 726. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Tang, P.-F. Factors Contributing to Single- and Dual-Task Timed “Up & Go” Test Performance in Middle-Aged and Older Adults Who Are Active and Dwell in the Community. Phys. Ther. 2016, 96, 284–292. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S. The Stroop Color and Word Test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Torrents Rodas, D.; Garre-Olmo, J. The Trail Making Test. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Mecherques-Carini, M.; Albaladejo-Saura, M.; Vaquero-Cristóbal, R.; Baglietto, N.; Esparza-Ros, F. Validity and Agreement between Dual-Energy X-Ray Absorptiometry, Anthropometry and Bioelectrical Impedance in the Estimation of Fat Mass in Young Adults. Front. Nutr. 2024, 11, 1421950. [Google Scholar] [CrossRef]
- Esparza-Ros, F.; Vaquero-Cristóbal, R.; Marfell-Jones, M. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry (ISAK): Murcia, Spain, 2019. [Google Scholar]
- Esparza-Ros, F.; Vaquero-Cristóbal, R. Antropometría: Fundamentos Para La Aplicación e Interpretación; Aula Magna: Ottignies-Louvain-la-Neuve, Belgium, 2023. [Google Scholar]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; González-Gálvez, N.; López-Vivancos, A.; Espeso-García, A.; Martínez-Aranda, L.M.; Gea-García, G.M.; Orquín-Castrillón, F.J.; Carbonell-Baeza, A.; Jiménez-García, J.D.; Velázquez-Díaz, D.; et al. Sarcopenia, Diet, Physical Activity and Obesity in European Middle-Aged and Older Adults: The LifeAge Study. Nutrients 2020, 13, 8. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; González-Gálvez, N.; Carbonell-Baeza, A.; Jiménez-Pavón, D.; Vaquero-Cristóbal, R. GDLAM and SPPB Batteries for Screening Sarcopenia in Community-Dwelling Spanish Older Adults: Healthy-Age Network Study. Exp. Gerontol. 2023, 172, 112044. [Google Scholar] [CrossRef]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for Blood Pressure Measurement in Humans and Experimental Animals. Hypertension 2005, 45, 142–161. [Google Scholar] [CrossRef]
- Correll, S.; Field, J.; Hutchinson, H.; Mickevicius, G.; Fitzsimmons, A.; Smoot, B. Reliability and validity of the halo digital goniometer for shoulder range of motion in healthy subjects. Int. J. Sports Phys. Ther. 2018, 13, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.J. Refining the Ten-Metre Walking Test for Use with Neurologically Impaired People. Physiotherapy 2002, 88, 386–397. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Vaquero-Cristóbal, R.; Martínez González-Moro, I.; Alacid Cárceles, F.; Ros Simón, E. Valoración de La Fuerza, La Flexibilidad, El Equilibrio, La Resistencia y La Agilidad En Función Del Índice de Masa Corporal En Mujeres Mayores Activas. Rev. Esp. Geriatr. Gerontol. 2013, 48, 171–176. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A Systematic Review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, T.; Kishimoto, H.; Susaki, Y.; Kumagai, S. Development of a Fried Frailty Phenotype Questionnaire for Use in Screening Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 272–276.e1. [Google Scholar] [CrossRef] [PubMed]
- Eaton, W.W.; Smith, C.; Ybarra, M.; Muntaner, C.; Tien, A. Center for Epidemiologic Studies Depression Scale—Revised. PsycTESTS Dataset 2014. [Google Scholar] [CrossRef]
- Sanz-Carrillo, C.; García-Campayo, J.; Rubio, A.; Santed, M.A.; Montoro, M. Validation of the Spanish Version of the Perceived Stress Questionnaire. J. Psychosom. Res. 2002, 52, 167–172. [Google Scholar] [CrossRef]
- Dominguez-Lara, S.A.; Merino-Soto, C.; Gutiérrez-Torres, A. Structural Study of a Brief Measure of Emotional Intelligence in Adults: The EQ-i-M20. Rev. Iberoam. Diagn. Evaluacion Psicologica 2018, 4, 5–21. [Google Scholar] [CrossRef]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The Satisfaction With Life Scale. J. Pers. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef]
- Cid, L.; Monteiro, D.; Teixeira, D.; Teques, P.; Alves, S.; Moutão, J.; Silva, M.; Palmeira, A. The Behavioral Regulation in Exercise Questionnaire (BREQ-3) Portuguese-Version: Evidence of Reliability, Validity and Invariance Across Gender. Front. Psychol. 2018, 9, 1940. [Google Scholar] [CrossRef]
- Kim, D.; Ko, Y.; Jung, A. Longitudinal Effects of Exercise According to the World Health Organization Guidelines on Cognitive Function in Middle-Aged and Older Adults. Front. Public Health 2022, 10, 1009775. [Google Scholar] [CrossRef]
- Duijvestijn, M.; de Wit, G.A.; van Gils, P.F.; Wendel-Vos, G.C.W. Impact of Physical Activity on Healthcare Costs: A Systematic Review. BMC Health Serv. Res. 2023, 23, 572. [Google Scholar] [CrossRef]
- Chang, L.; Chen, S.-C.; Lin, P.-Y.; Chen, M.-C.; Liao, L.-L.; Lin, H.-P.; Tsao, Y.-Y.; Chen, M.-C. Sustainable Participation in Community Health Programs to Promote a Healthy Lifestyle and Prevent and Protect against Dementia among Rural Taiwanese Middle-Aged and Older Adults. J. Prev. Alzheimers Dis. 2024, 11, 612–619. [Google Scholar] [CrossRef]
| Study Period | Enrolment | Pre-Intervention (Pre-Test) | Familiarization (2 Weeks) | Intervention (Weeks 1–18) | Post-Intervention (Post-Test) | 3-Month Follow-Up (Re-Test 1) | 6-Month Follow-Up (Re-Test 2) |
|---|---|---|---|---|---|---|---|
| Informed Consent | X | ||||||
| Eligibility Screening | X | ||||||
| Randomization | X | ||||||
| Baseline Assessments | X | ||||||
| Multidomain Intervention | X | X | |||||
| Outcome Assessments | X | X | X |
| Session Part | Content | Details | Monitoring | Cognitive Integration |
|---|---|---|---|---|
| Warm-up (10 min) | - Activation exercises - Joint mobility | Playful tasks and movement prep activities; progressive cardiorespiratory stimulus. | RPE (Borg), trainer supervision | Sensorimotor activities, coordination-based games |
| Main (40 min) | First weekly session Local muscle endurance (1) Balance and coordination | Circuit of 8 exercises (6 strength, 2 balance/coordination). 3–4 sets of 10–12 reps. 20–30 s rest between exercises. Equipment: dumbbells, bands, TRX. | OMNI-RES RPE (6–7/10), trainer diary | Dual tasks (e.g., movement + verbal/cognitive tasks) |
| Second weekly session Power training + local muscle endurance (2) Cardiorespiratory | Power block: 3 exercises, 2 sets of 6–8 reps. Load moved as fast as possible. Local muscle endurance block: 3 exercises, 3 sets of 10–12 reps. Cardio: 2 × 4 min intervals, moderate to vigorous using the talk test. | OMNI-RES RPE, Borg scale, trainer diary | Dual tasks, attention/memory components | |
| Cool-down (10 min) | - Flexibility - Relaxation - Emotional intelligence tasks | Static stretching (8 exercises, 10–30 s), body–mind exercises (Tai Chi, breathing), emotional intelligence games (collaboration, expression of feelings). | Trainer checklist | Breathing visualization, reflective and emotional tasks |
| Autonomous training | Brisk walking (2×/week) | 30+ min per session, moderate intensity. Monitored via self-reported training diaries and talk test. | Training diary (duration, distance, RPE) | — |
| Dimension | Test | Description | |
|---|---|---|---|
| Primary evaluations | Sociodemographic questionnaire | Ad hoc | An ad hoc questionnaire used in previous research [1] will be used to collect data on age, sex, physical independence, history of chronic diseases, and systematic exercise. |
| Cognitive state | Victoria Stroop Test (VST) | The Stroop test assesses attention, inhibition, and processing speed, observing the Stroop effect where reading interferes with color identification. The test involves three phases and aims to distinguish between colors and words accurately without errors [2]. | |
| Trail Making Test (TMT) | TMT test assesses thought flexibility and visuospatial ability. It involves connecting numbers and words in a numerical sequence without making errors. | ||
| Body composition and anthropometric variables | Bioimpedance + Anthropometry variables + Proportionality variables | Regarding body composition, fat mass, fat-free mass, lean mass, and muscle mass will be assessed with Inbody 120. The measurements shall be carried out according to the manufacturer’s standardization conditions and previous studies [3]. Body mass, with Inbody 120; height with SECA 217 scale; and waist and hips with Lufkin tape; will be assessed according to the guidelines of the International Society for the Advancement of Anthropometry (ISAK) [4]. Subsequently, BMI (kg/m2), as well as waist/hip and waist/height ratio, will be calculated [5] | |
| Sarcopenia | Handgrip test and chair stand test (CST) (5-times sit-to-stand) | The probability of sarcopenia will be assessed using the handgrip test with a Takei tkk5401 dynamometer (P & A Medical Ltd., Duxbury, UK) and CST (5 times sit-to-stand), following the indications of the European consensus on definition and diagnosis [6]. The tests will be performed according to the protocol of previous research [7]. | |
| Muscle mass | For the diagnosis of sarcopenia, muscle mass will be assessed using Inbody 120, following the indications of the European consensus on definition and diagnosis [6]. The tests will be performed according to the protocol of previous research [3]. | ||
| Timed Up and Go test (TUG) + Gait speed test (4 m walk test—4MWT) + Short Physical Performance Battery (SPPB) | The severity of sarcopenia (functional performance) will be assessed using TUG, gait speed, and the SPPB test, following the indications of the European consensus on definition and diagnosis [6]. The tests will be performed according to the protocol of previous research [7]. Photovoltaic cells from Microgate (Bolzano, Italy) will be used for TUG and 4MWT. The SPPB battery includes the balance test, gait speed test, and chair stand test. The Total SPPB score, ranging from 0 to 12, will be calculated by adding the scores from the three individual tests, following previous studies [8]. | ||
| Cardiovascular state | Blood pressure and resting heart rate | Blood pressure and resting heart rate will be measured using a calibrated and automated device (Omron M6W, Omron Healthcare Ltd. Hoffman Estates, IL, USA), following the protocol of previous studies [9] | |
| Physical fitness | Upper and lower limbs muscle strength | Upper and lower limb isometric muscle strength will be evaluated using the functional electromechanical dynamometer Dinasystem. For this purpose, the following exercises will be performed randomly, maintaining the isometric phase for 6 s during evaluation: bilateral rowing; bilateral push from the seated position with shoulder abduction at 45° and elbow flexion at 90°; and the sit-to-stand test. All of this will follow the methodology of previous research [10]. | |
| Velocity | The 10 m walk test (10MWT) will be used for the assessment of velocity. Photovoltaic cells from Microgate (Bolzano, Italy) will be used for evaluation. This test will be conducted following the guidelines of previous research [11]. | ||
| Cardiorespiratory fitness | The 6-minute walk test will be used for the assessment of cardiorespiratory fitness. The distance covered, heart rate at the end, and heart rate recovery at 1 and 3 min will be recorded, following previous studies [12]. | ||
| Flexibility | Sit-and-reach test with a box (Acuflex, Psymtec, Madrid, Spain) and back scratch test will be used for the assessment of flexibility, following the protocol of previous studies [13] | ||
| Secondary evaluations | Physical activity (PA) | International Physical Activity Questionnaire (IPAQ-SF) | The short version of the IPAQ-SF for adults will be used to analyze levels of PA [14] |
| Frailty | Fried questionnaire | The Fried questionnaire evaluates five criteria related to frailty: involuntary weight loss, exhaustion, weakness, slow gait speed, and low physical activity. The number of criteria met is classified as a three-level variable representing robustness (0 criteria met), pre-frailty (1 or 2 criteria met), and frailty (3 or more criteria met) [15]. | |
| Emotional state | Centre for Epidemiological Studies Depression Scale-Revised (CESD-R) | The CESD-R is a 20-item questionnaire that measures depressive symptoms among older participants. Participants indicate the number of days on which they experienced depressive symptoms during the previous week using a standard four-point Likert scale. Higher scores indicate more depressive symptoms [16]. | |
| Perceived Stress Questionnaire (PSQ) | The Perceived Stress Questionnaire (PSQ) is a psychological tool used to assess an individual’s subjective perception of stress in their daily life, focusing on feelings, thoughts, and reactions to stressful situations [17]. | ||
| Emotional intelligence in adults (EQ-i-M20) | The EQ-i-M20 is a 20-item questionnaire with subcategories such as intrapersonal, interpersonal, stress management, adaptability, and general mood [18]. | ||
| Satisfaction with life | Satisfaction with Life Scale (SWL) | The scale consists of four items rated on a Likert-type scale, ranging from 1 to 7, which assesses global subjective happiness [19]. | |
| (e) Motivation | Behavioral Regulation during Exercise Questionnaire-3 (BREQ-3) | The BREQ-3 will be used to assess motivational regulation related to physical exercise. The questionnaire consists of twenty-three items distributed across three dimensions based on the Self-Determination Theory (SDT), which distinguishes between autonomous motivation, controlled motivation, and amotivation [20]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Llorente, A.M.; Vaquero-Cristóbal, R.; Casimiro-Andújar, A.J.; Abraldes, J.A.; Marcos-Pardo, P.J. ACTIVA-Senior: Study Design and Protocol for a Preliminary Multidomain Outdoor Intervention Promoting Healthy Aging and Mitigating Psycho-Physiological Decline. Healthcare 2025, 13, 1110. https://doi.org/10.3390/healthcare13101110
García-Llorente AM, Vaquero-Cristóbal R, Casimiro-Andújar AJ, Abraldes JA, Marcos-Pardo PJ. ACTIVA-Senior: Study Design and Protocol for a Preliminary Multidomain Outdoor Intervention Promoting Healthy Aging and Mitigating Psycho-Physiological Decline. Healthcare. 2025; 13(10):1110. https://doi.org/10.3390/healthcare13101110
Chicago/Turabian StyleGarcía-Llorente, Antonio Manuel, Raquel Vaquero-Cristóbal, Antonio J. Casimiro-Andújar, J. Arturo Abraldes, and Pablo J. Marcos-Pardo. 2025. "ACTIVA-Senior: Study Design and Protocol for a Preliminary Multidomain Outdoor Intervention Promoting Healthy Aging and Mitigating Psycho-Physiological Decline" Healthcare 13, no. 10: 1110. https://doi.org/10.3390/healthcare13101110
APA StyleGarcía-Llorente, A. M., Vaquero-Cristóbal, R., Casimiro-Andújar, A. J., Abraldes, J. A., & Marcos-Pardo, P. J. (2025). ACTIVA-Senior: Study Design and Protocol for a Preliminary Multidomain Outdoor Intervention Promoting Healthy Aging and Mitigating Psycho-Physiological Decline. Healthcare, 13(10), 1110. https://doi.org/10.3390/healthcare13101110










