A Low-Cost Early Warning Method for Infectious Diseases with Asymptomatic Carriers
Abstract
1. Introduction
2. Methods
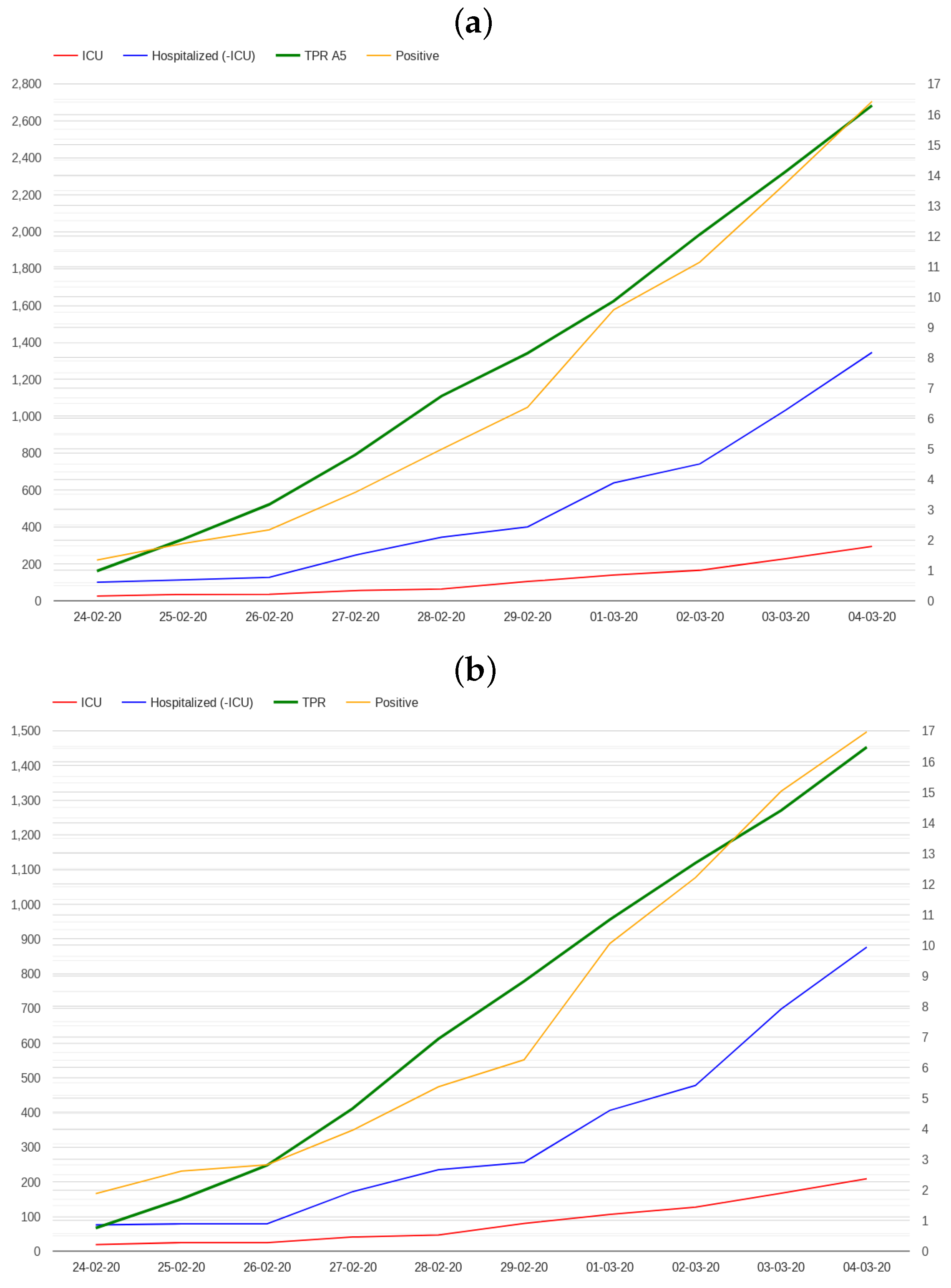
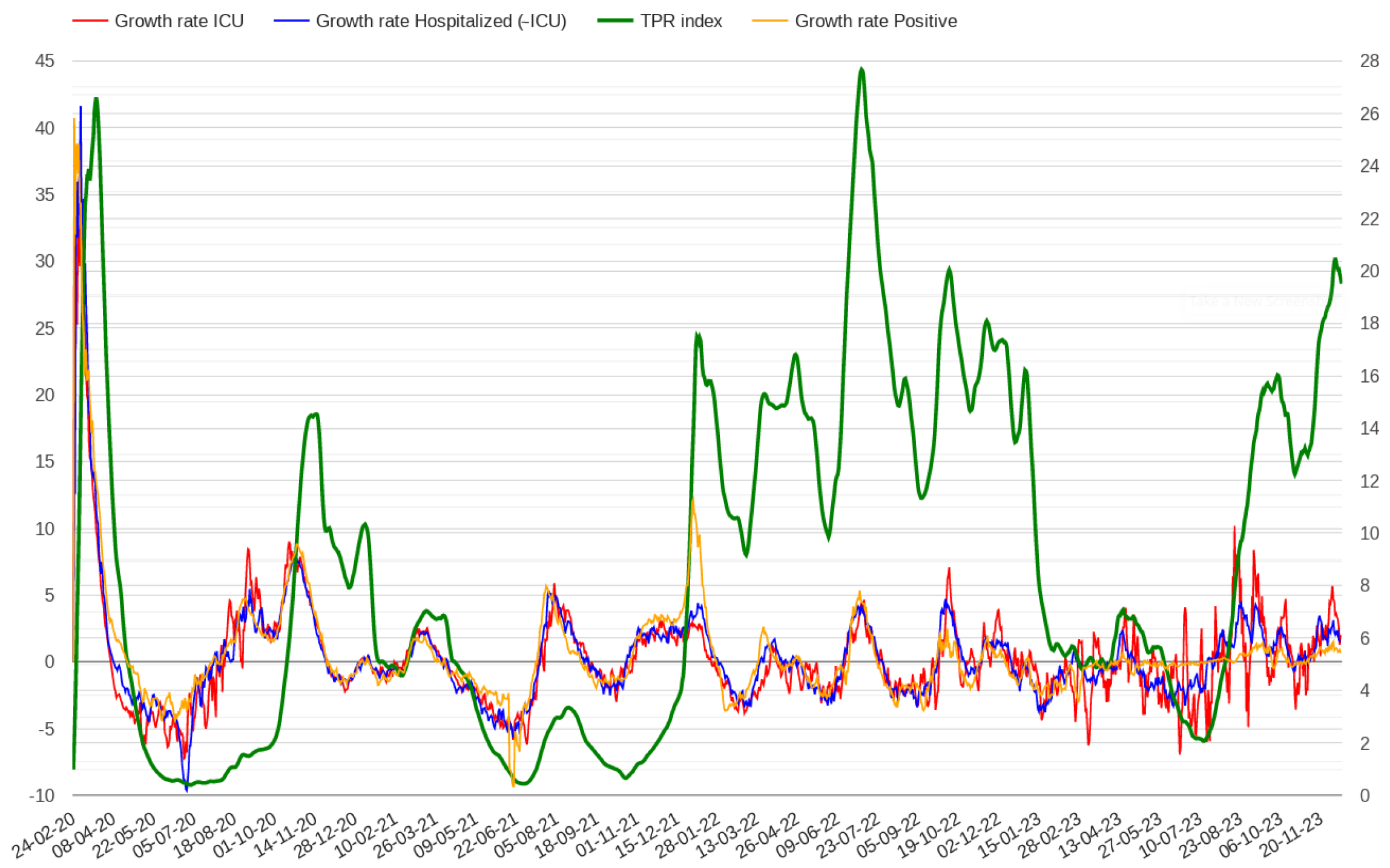
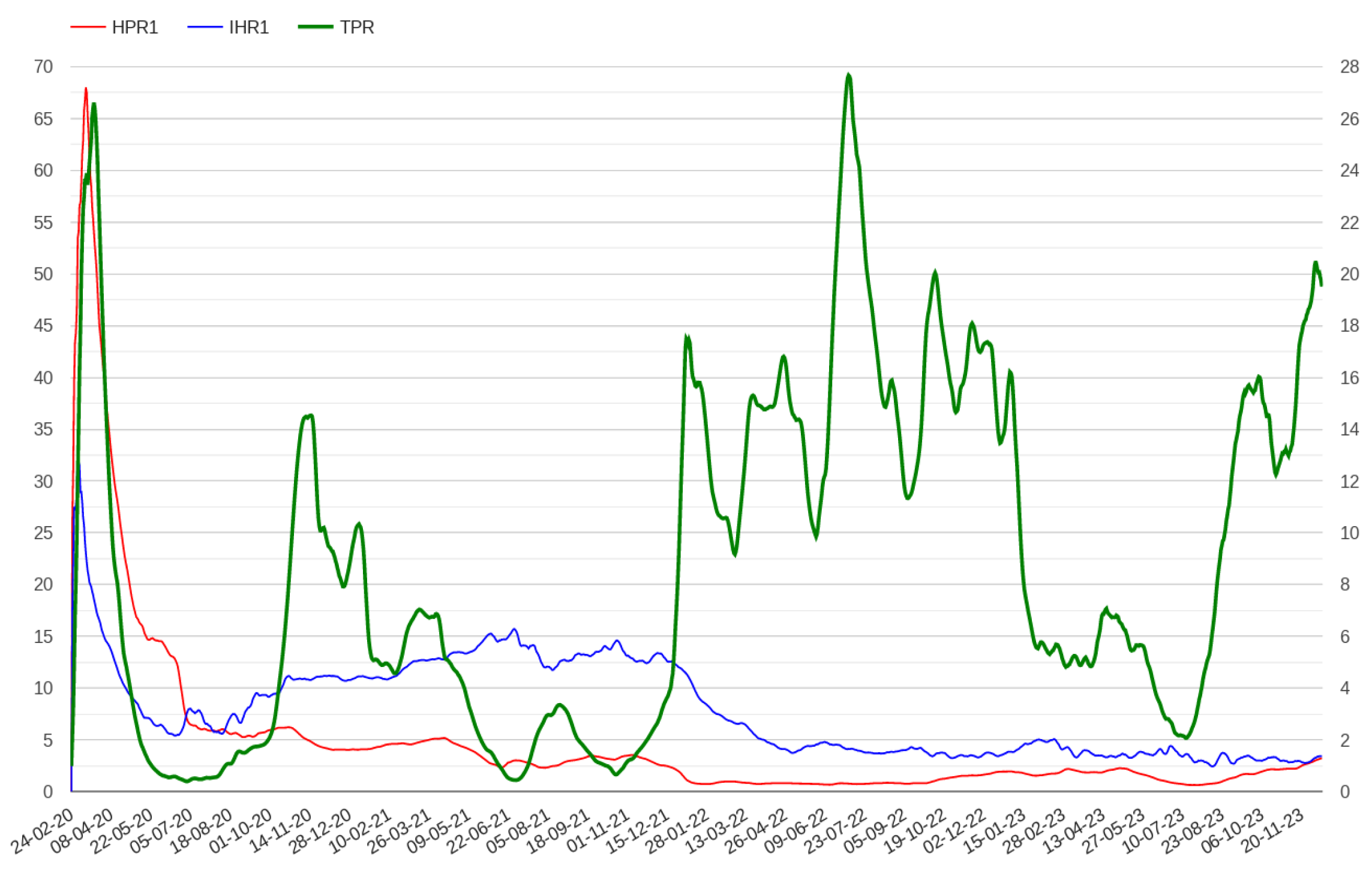
2.1. Modelling Test Positivity
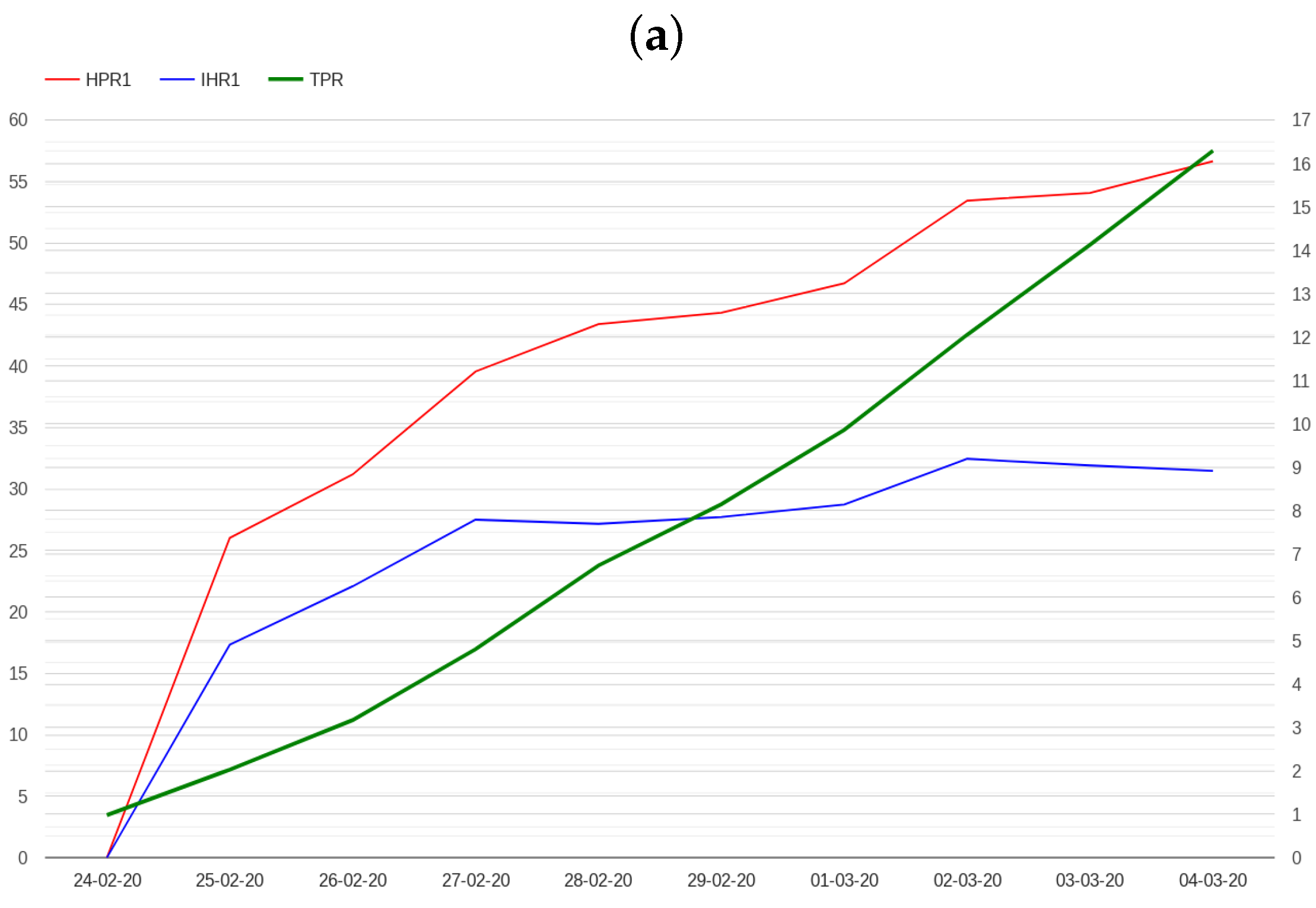
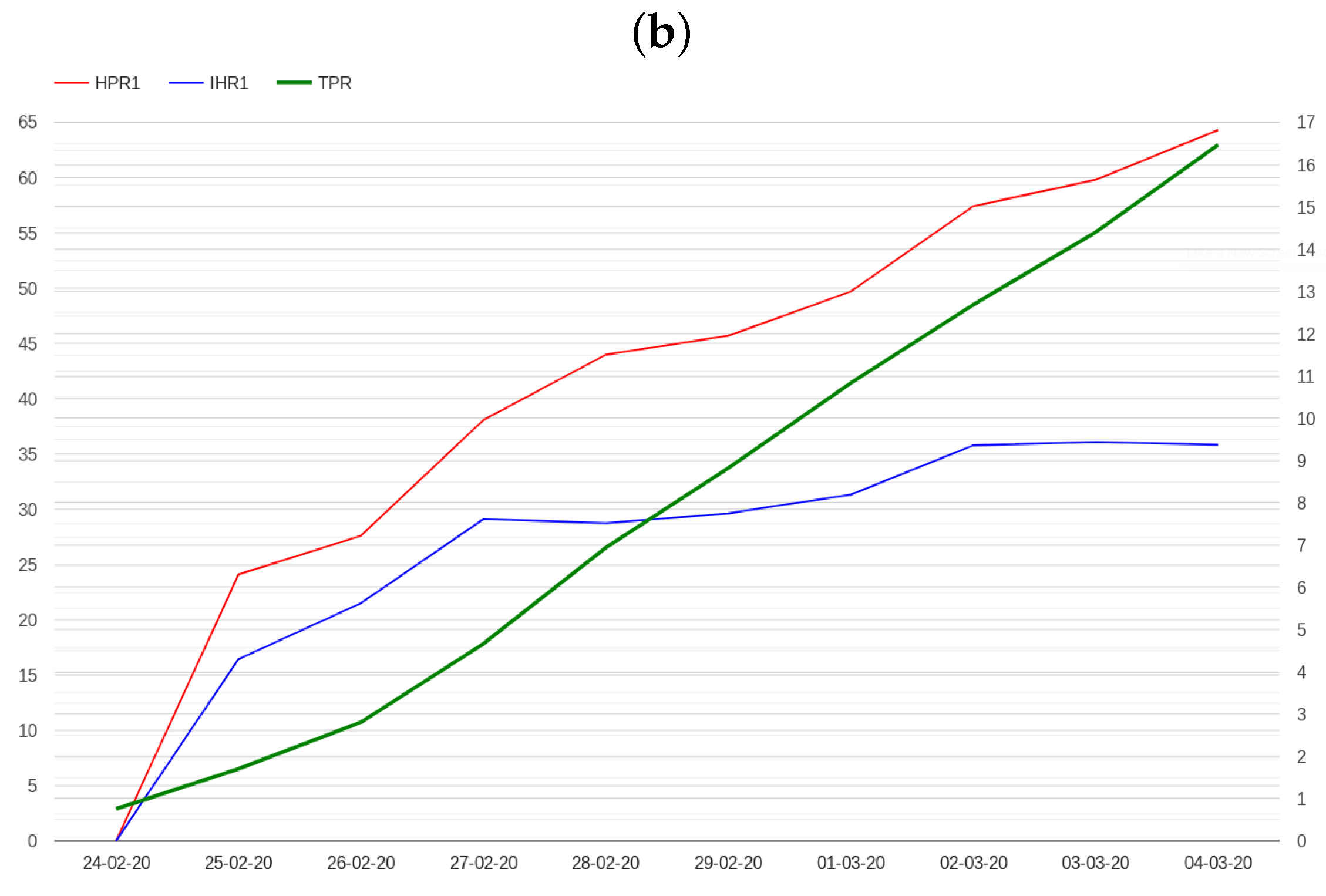
2.2. Modelling Admissions in Hospitals and Intensive Care Units
- The N hospital/positive ratio (HPRN), which is defined as the ratio between hospitalized patients and the number of positive cases N days before.
- The N ICU/hospital ratio (IHRN), which is defined as the ratio between patients admitted in ICU and hospitalized patients N days before.
2.3. Defining an Early Warning Method
- IF TPR grows more than 5 points
- AND the TPR is monotone
- AND the growth rate of the HPR1 is greather than 5
- AND the growth rate of the IHR1 is greather than 5
- AND The average value of IHR1 is greather than 15
- THEN an aberration signal is detected
- IF the number of patients admitted in ICU is 0 for one day in the week.
- AND TPR grows more than 8 points
- AND the TPR is monotone
- AND The average value of HPR1 is greather than 20
- AND The average value of IHR1 is greather than 10
- THEN an aberration signal is detected
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, T.W.; Golding, N.; Hellewell, J.; Abbott, S.; Wright, L.; Pearson, C.A.; van Zandvoort, K.; Jarvis, C.I.; Gibbs, H.; Liu, Y.; et al. Reconstructing the early global dynamics of under-ascertained COVID-19 cases and infections. BMC Med. 2020, 18, 332. [Google Scholar] [CrossRef]
- Beatrice, F.; Calleja, N. Early warning indicators of COVID-19 burden for a prosilient European pandemic response. Eur. J. Public Health 2021, 31, iv21–iv26. [Google Scholar] [CrossRef] [PubMed]
- Meckawy, R.; Stuckler, D.; Mehta, A.; Al-Ahdal, T.; Doebbeling, B.N. Effectiveness of early warning systems in the detection of infectious diseases outbreaks: A systematic review. BMC Public Health 2022, 22, 2216. [Google Scholar] [CrossRef]
- Yu, X.; Yang, R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir. Viruses 2020, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lu, X.; Deng, Y.; Tang, Y.; Lu, J. COVID-19: Asymptomatic carrier transmission is an underestimated problem. Epidemiol. Infect. 2020, 148, e116. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, R.; Campbell, P.; Wu, Y.; Tong, S.; McVernon, J.; Geard, N. Implications of asymptomatic carriers for infectious disease transmission and control. R. Soc. Open Sci. 2018, 5, 172341. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Brook, C.E.; Northrup, G.R.; Ehrenberg, A.J.; Doudna, J.A.; Boots, M.; IGI SARS-CoV-2 Testing Consortium. Optimizing COVID-19 control with asymptomatic surveillance testing in a university environment. Epidemics 2021, 37, 100527. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.; Fyles, M.; Pi, L.; Panovska-Griffiths, J.; House, T.; Jay, C.; Fearon, E. The role of regular asymptomatic testing in reducing the impact of a COVID-19 wave. Epidemics 2023, 44, 100699. [Google Scholar] [CrossRef]
- Arcede, J.P.; Caga-Anan, R.L.; Mentuda, C.Q.; Mammeri, Y. Accounting for symptomatic and asymptomatic in a SEIR-type model of COVID-19. Math. Model. Nat. Phenom. 2020, 15, 34. [Google Scholar] [CrossRef]
- Ahmed, I.; Modu, G.U.; Yusuf, A.; Kumam, P.; Yusuf, I. A mathematical model of Coronavirus Disease (COVID-19) containing asymptomatic and symptomatic classes. Results Phys. 2021, 21, 103776. [Google Scholar] [CrossRef]
- Serhani, M.; Labbardi, H. Mathematical modeling of COVID-19 spreading with asymptomatic infected and interacting peoples. J. Appl. Math. Comput. 2021, 66, 1–20. [Google Scholar] [CrossRef]
- Huo, X.; Chen, J.; Ruan, S. Estimating asymptomatic, undetected and total cases for the COVID-19 outbreak in Wuhan: A mathematical modeling study. BMC Infect. Dis. 2021, 21, 476. [Google Scholar] [CrossRef]
- Chowdhury, S.; Forkan, M.; Ahmed, S.F.; Agarwal, P.; Ali, A.S.; Muyeen, S. Modeling the SARS-CoV-2 parallel transmission dynamics: Asymptomatic and symptomatic pathways. Comput. Biol. Med. 2022, 143, 105264. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Z.; Huang, X.; Cheng, K.; Guo, S.; Wang, X.; Liu, M.; Liu, X. A study on the transmission dynamics of COVID-19 considering the impact of asymptomatic infection. J. Biol. Dyn. 2023, 17, 2244980. [Google Scholar] [CrossRef]
- Ripoll, J.; Font, J. A Discrete Model for the Evolution of Infection Prior to Symptom Onset. Mathematics 2023, 11, 1092. [Google Scholar] [CrossRef]
- Bai, Z.; Gong, Y.; Tian, X.; Cao, Y.; Liu, W.; Li, J. The rapid assessment and early warning models for COVID-19. Virol. Sin. 2020, 35, 272–279. [Google Scholar] [CrossRef]
- Usuelli, M. The Lombardy region of Italy launches the first investigative COVID-19 commission. Lancet 2020, 396, e86–e87. [Google Scholar] [CrossRef]
- Goumenou, M.; Sarigiannis, D.; Tsatsakis, A.; Anesti, O.; Docea, A.O.; Petrakis, D.; Tsoukalas, D.; Kostoff, R.; Rakitskii, V.; Spandidos, D.A.; et al. COVID-19 in Northern Italy: An integrative overview of factors possibly influencing the sharp increase of the outbreak. Mol. Med. Rep. 2020, 22, 20–32. [Google Scholar] [CrossRef]
- Bernucci, C.; Brembilla, C.; Veiceschi, P. Effects of the COVID-19 outbreak in Northern Italy: Perspectives from the Bergamo Neurosurgery Department. World Neurosurg. 2020, 137, 465. [Google Scholar] [CrossRef] [PubMed]
- Palladino, R.; Bollon, J.; Ragazzoni, L.; Barone-Adesi, F. Excess Deaths and Hospital Admissions for COVID-19 Due to a Late Implementation of the Lockdown in Italy. Int. J. Environ. Res. Public Health 2020, 17, 5644. [Google Scholar] [CrossRef]
- Perico, N.; Fagiuoli, S.; Di Marco, F.; Laghi, A.; Cosentini, R.; Rizzi, M.; Gianatti, A.; Rambaldi, A.; Ruggenenti, P.; La Vecchia, C.; et al. Bergamo and COVID-19: How the dark can turn to light. Front. Med. 2021, 8, 609440. [Google Scholar] [CrossRef]
- Gaspari, M. The impact of test positivity on surveillance with asymptomatic carriers. Epidemiol. Methods 2023, 11, 20220125. [Google Scholar] [CrossRef]
- Fasina, C.; Salami, M.; Fasina, M.; Otekunrin, O.A.; Hoogesteijn, A.; Hittner, J.B. Test positivity–evaluation of a new metric to assess epidemic dispersal mediated by non-symptomatic cases. Methods 2021, 195, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y.; Ko, Y.K.; Ninomiya, K.; Suzuki, M.; Oshitani, H. Relationship of test positivity rates with COVID-19 epidemic dynamics. Int. J. Environ. Res. Public Health 2021, 18, 4655. [Google Scholar] [CrossRef]
- Al Dallal, A.; AlDallal, U.; Al Dallal, J. Positivity rate: An indicator for the spread of COVID-19. Curr. Med. Res. Opin. 2021, 37, 2067–2076. [Google Scholar] [CrossRef]
- Fenga, L.; Gaspari, M. Predictive capacity of COVID-19 test positivity rate. Sensors 2021, 21, 2435. [Google Scholar] [CrossRef]
- Vong, S.; Kakkar, M. Monitoring COVID-19 where capacity for testing is limited: Use of a three-step analysis based on test positivity ratio. WHO South-East Asia J. Public Health 2020, 9, 141–146. [Google Scholar] [CrossRef]
- Boschi, T.; Di Iorio, J.; Testa, L.; Cremona, M.A.; Chiaromonte, F. Functional data analysis characterizes the shapes of the first COVID-19 epidemic wave in Italy. Sci. Rep. 2021, 11, 17054. [Google Scholar] [CrossRef]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Jansen, L.; Tegomoh, B.; Lange, K.; Showalter, K.; Figliomeni, J.; Abdalhamid, B.; Iwen, P.C.; Fauver, J.; Buss, B.; Donahue, M. Investigation of a SARS-CoV-2 B. 1.1. 529 (Omicron) variant cluster—Nebraska, November–December 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1782. [Google Scholar] [CrossRef]
- Brandal, L.T.; MacDonald, E.; Veneti, L.; Ravlo, T.; Lange, H.; Naseer, U.; Feruglio, S.; Bragstad, K.; Hungnes, O.; Ødeskaug, L.E.; et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Eurosurveillance 2021, 26, 2101147. [Google Scholar] [CrossRef]
- World Health Organization. Considerations for Implementing and Adjusting Public Health and Social Measures in the Context of COVID-19: Interim Guidance, 4 November 2020; Technical Report; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Indicators for Monitoring COVID-19 Community Levels and Making Public Health Recommendations. In CDC COVID-19 Science Briefs; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. [Google Scholar]
- Faes, C.; Abrams, S.; Van Beckhoven, D.; Meyfroidt, G.; Vlieghe, E.; Hens, N.; Belgian Collaborative Group on COVID-19 Hospital Surveillance. Time between symptom onset, hospitalisation and recovery or death: Statistical analysis of Belgian COVID-19 patients. Int. J. Environ. Res. Public Health 2020, 17, 7560. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Z.; Lan, Y.; Wang, J.; Ma, J.; Jin, L.; Sun, Q.; Lv, W.; Lai, S.; Liao, Y.; et al. A nationwide web-based automated system for outbreak early detection and rapid response in China. West. Pac. Surveill. Response J. 2011, 2, 10. [Google Scholar]
- Presidenza del Consiglio dei Ministri; Dipartimento di Protezione Civile. Dati COVID-19 Italia. GitHub Repository. 2020. Available online: https://github.com/pcm-dpc/COVID-19 (accessed on 23 December 2023).
- Roccetti, M. Excess mortality and COVID-19 deaths in Italy: A peak comparison study. Math. Biosci. Eng. 2023, 20, 7042–7055. [Google Scholar] [CrossRef]
- Roccetti, M. Forecasting by Analogy: A Parallel between the Trend of Confirmed COVID-19 Deaths in the Winters of 2022/2023 and 2023/2024 in Italy. Qeios 2023. [Google Scholar] [CrossRef]
- Kulldorff, M.; Nagarwalla, N. Spatial disease clusters: Detection and inference. Stat. Med. 1995, 14, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M. A spatial scan statistic. Commun. Stat. Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Widdowson, M.A.; Bosman, A.; van Straten, E.; Tinga, M.; Chaves, S.; van Eerden, L.; van Pelt, W. Automated, laboratory-based system using the Internet for disease outbreak detection, the Netherlands. Emerg. Infect. Dis. 2003, 9, 1046. [Google Scholar] [CrossRef]
- Riccardo, F.; Ajelli, M.; Andrianou, X.D.; Bella, A.; Del Manso, M.; Fabiani, M.; Bellino, S.; Boros, S.; Urdiales, A.M.; Marziano, V.; et al. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Eurosurveillance 2020, 25, 2000790. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Blair, C.; Brown, A.; Gabos, S.; Honish, L.; Hughes, T.; Jaipaul, J.; Johnson, M.; Lo, E.; Lubchenko, A.; et al. A multi-function public health surveillance system and the lessons learned in its development: The Alberta Real Time Syndromic Surveillance Net. Can. J. Public Health 2010, 101, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Coelho, C.A.; Barcellos, C.; Carvalho, M.S.; Catao, R.D.C.; Coelho, G.E.; Ramalho, W.M.; Bailey, T.C.; Stephenson, D.B.; Rodo, X. Evaluating probabilistic dengue risk forecasts from a prototype early warning system for Brazil. eLife 2016, 5, e11285. [Google Scholar] [CrossRef] [PubMed]
- Nuvey, F.S.; Edu-Quansah, E.P.; Kuma, G.K.; Eleeza, J.; Kenu, E.; Sackey, S.; Ameme, D.; Abakar, M.F.; Kreppel, K.; Ngandolo, R.B.; et al. Evaluation of the sentinel surveillance system for influenza-like illnesses in the Greater Accra region, Ghana, 2018. PLoS ONE 2019, 14, e0213627. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Lai, S.; Clements, A.C.; Wang, L.; Yin, W.; Zhou, H.; Yu, H.; Hu, W.; Yang, W. Evaluation of the performance of a dengue outbreak detection tool for China. PLoS ONE 2014, 9, e106144. [Google Scholar] [CrossRef]
- Baghdadi, Y.; Gallay, A.; Caserio-Schönemann, C.; Fouillet, A. Evaluation of the French reactive mortality surveillance system supporting decision making. Eur. J. Public Health 2019, 29, 601–607. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.A.; Clements, C.F. Early warning signal reliability varies with COVID-19 waves. Biol. Lett. 2021, 17, 20210487. [Google Scholar] [CrossRef]

| Region | Mortality | Case Fatality | Alarm | Rule | Date |
|---|---|---|---|---|---|
| Lombardia | 166.141 | 0.177 | 1 | 1 | 1 March 2020 |
| Valle d’Aosta | 116.751 | 0.122 | 1 | 2 | 20 March 2020 |
| Liguria | 101.525 | 0.156 | 1 | 1 | 12 March 2020 |
| Emilia-Romagna | 98.652 | 0.155 | 1 | 1 | 2 March 2020 |
| Piemonte | 94.766 | 0.13 | 1 | 2 | 5 March 2020 |
| Trentino | 75.723 | 0.09 | 1 | 1 | 12 March 2020 |
| Marche | 65.275 | 0.146 | 1 | 1 | 1 March 2020 |
| ITA | 58.172 | 0.145 | 1 | 1 | 1 March 2020 |
| Alto Adige | 55.787 | 0.111 | 1 | 2 | 15 March 2020 |
| Veneto | 41.293 | 0.104 | 1 | 1 | 2 March 2020 |
| Abruzzo | 35.505 | 0.141 | 0 | - | - |
| Toscana | 29.779 | 0.108 | 0 | - | - |
| Friuli Venezia Giulia | 28.647 | 0.104 | 0 | - | - |
| Lazio | 14.324 | 0.103 | 0 | - | - |
| Puglia | 13.566 | 0.12 | 0 | - | - |
| Umbria | 9.109 | 0.056 | 0 | - | - |
| Sardegna | 8.143 | 0.096 | 0 | - | - |
| Molise | 7.597 | 0.052 | 0 | - | - |
| Campania | 7.456 | 0.088 | 0 | - | - |
| Sicilia | 5.664 | 0.081 | 0 | - | - |
| Calabria | 5.013 | 0.081 | 0 | - | - |
| Basilicata | 4.826 | 0.064 | 0 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspari, M. A Low-Cost Early Warning Method for Infectious Diseases with Asymptomatic Carriers. Healthcare 2024, 12, 469. https://doi.org/10.3390/healthcare12040469
Gaspari M. A Low-Cost Early Warning Method for Infectious Diseases with Asymptomatic Carriers. Healthcare. 2024; 12(4):469. https://doi.org/10.3390/healthcare12040469
Chicago/Turabian StyleGaspari, Mauro. 2024. "A Low-Cost Early Warning Method for Infectious Diseases with Asymptomatic Carriers" Healthcare 12, no. 4: 469. https://doi.org/10.3390/healthcare12040469
APA StyleGaspari, M. (2024). A Low-Cost Early Warning Method for Infectious Diseases with Asymptomatic Carriers. Healthcare, 12(4), 469. https://doi.org/10.3390/healthcare12040469






