Enhanced Recovery after Surgery (ERAS) Implementation and Barriers among Healthcare Providers in France: A Cross-Sectional Study
Abstract
1. Introduction
- What proportion of French healthcare providers actively practice ERAS for major surgery?
- What are the barriers to ERAS implementation among healthcare providers, both those who practice ERAS and those who do not?
- What disparities exist between participants who practice ERAS and those who do not?
2. Method
2.1. Study Design
2.2. Participants
2.3. Primary Outcome
2.4. Secondary Outcomes
2.5. Sample Size Calculation
2.6. Statistical Analysis
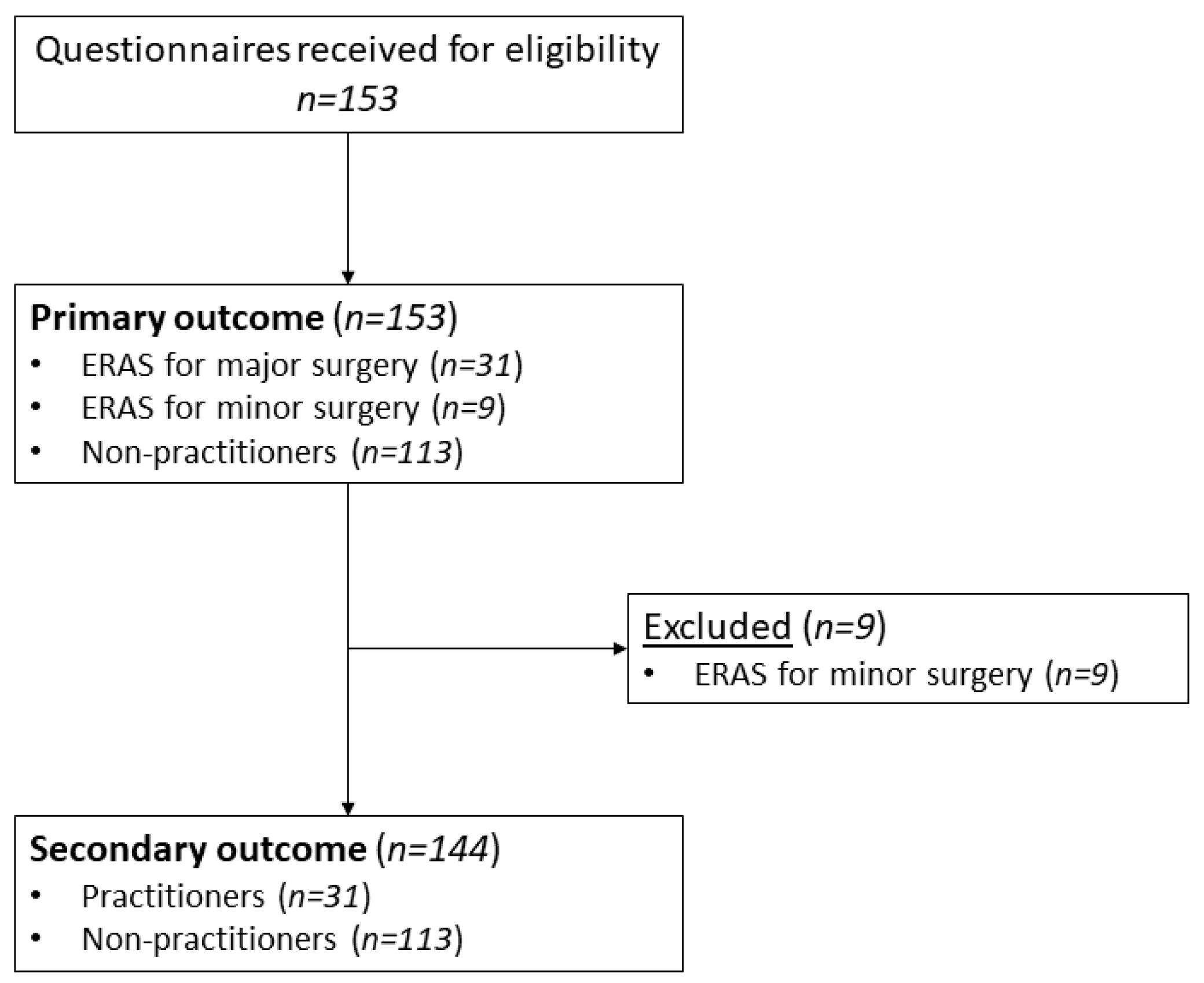
3. Results
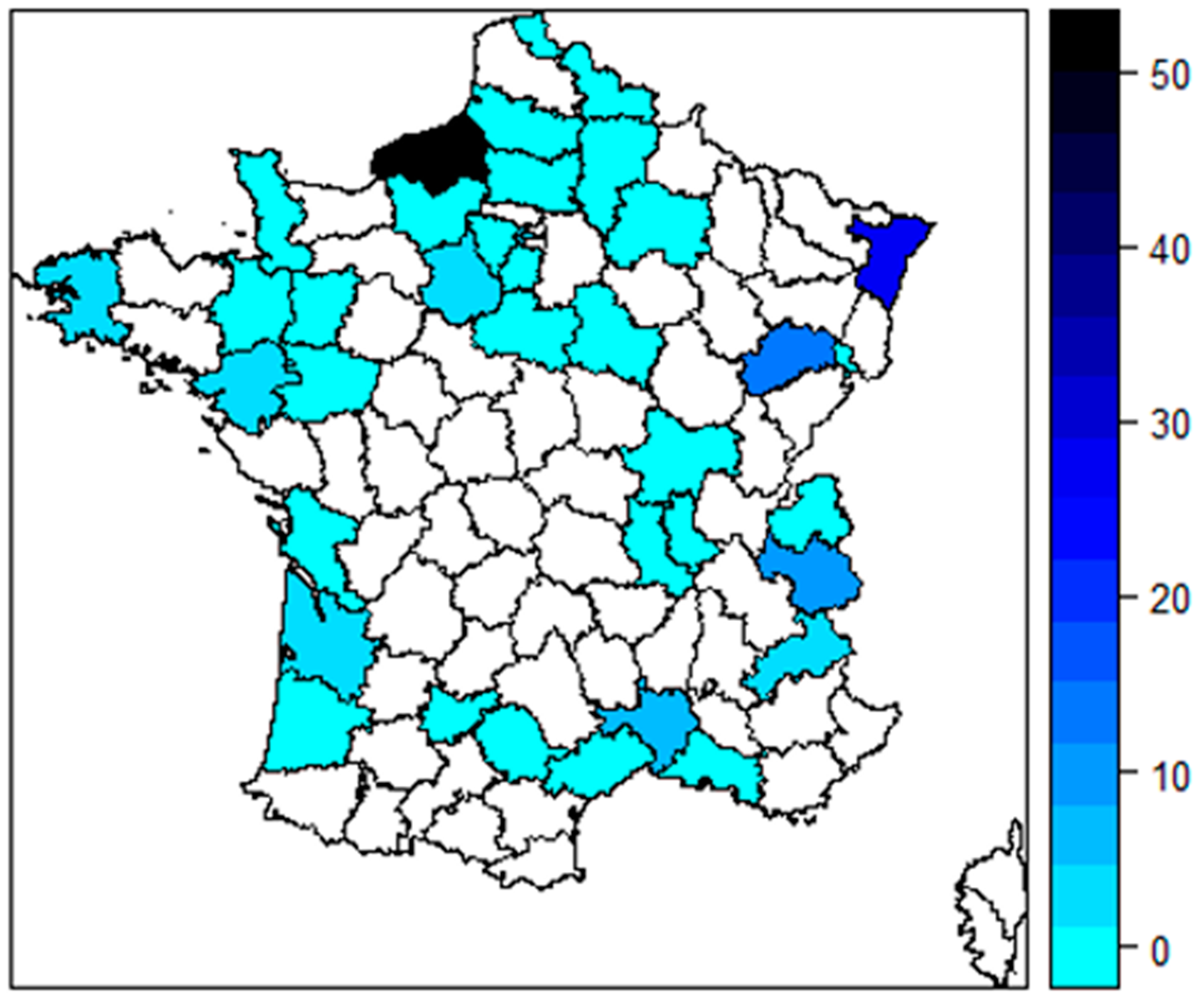
3.1. Participants
3.2. Primary Outcome: Proportion of Participants Who Practiced ERAS
3.3. Secondary Outcome
3.3.1. Barriers to Implementation
3.3.2. Differences
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- HAS. Rapport D’orientation-Programmes de Récupération Améliorée Après Chirurgie (RAAC): État des Lieux et Perspectives; HAS: Saint-Denis, France, 2016; 73p. [Google Scholar]
- Tapia Jurado, J. Challenges of surgery in the 21st century. Cirugía Cir. 2017, 85, 1–3. [Google Scholar] [CrossRef]
- Kehlet, H.; Dahl, J.B. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003, 362, 1921–1928. [Google Scholar] [CrossRef]
- GRACE. Grace Asso—Groupe Francophone de Réhabilitation Améliorée Après ChirurgiE. 2023. Available online: https://www.grace-asso.fr/ (accessed on 24 July 2020).
- Boden, I.; Skinner, E.H.; Browning, L.; Reeve, J.; Anderson, L.; Hill, C.; Robertson, I.K.; Story, D.; Denehy, L. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: Pragmatic, double blinded, multicentre randomised controlled trial. BMJ 2018, 360, j5916. [Google Scholar] [CrossRef]
- Gravier, F.-E.; Smondack, P.; Prieur, G.; Medrinal, C.; Combret, Y.; Muir, J.-F.; Baste, J.-M.; Cuvelier, A.; Boujibar, F.; Bonnevie, T. Effects of exercise training in people with non-small cell lung cancer before lung resection: A systematic review and meta-analysis. Thorax 2022, 77, 486–496. [Google Scholar] [CrossRef]
- ERAS. ERAS Society. ERAS® Society. 2023. Available online: https://erassociety.org/ (accessed on 24 July 2020).
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery after Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardio-Thorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Carmichael, J.C.; Keller, D.S.; Baldini, G.; Bordeianou, L.; Weiss, E.; Lee, L.; Boutros, M.; McClane, J.; Feldman, L.S.; Steele, S.R. Clinical Practice Guidelines for Enhanced Recovery after Colon and Rectal Surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis. Colon Rectum 2017, 60, 761–784. [Google Scholar] [CrossRef]
- Stowers, M.D.J.; Lemanu, D.P.; Hill, A.G. Health economics in Enhanced Recovery after Surgery programs. Can. J. Anesth. 2015, 62, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Noba, L.; Rodgers, S.; Chandler, C.; Balfour, A.; Hariharan, D.; Yip, V.S. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2020, 24, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Bisch, S.P.; Jago, C.A.; Kalogera, E.; Ganshorn, H.; Meyer, L.A.; Ramirez, P.T.; Dowdy, S.C.; Nelson, G. Outcomes of enhanced recovery after surgery (ERAS) in gynecologic oncology—A systematic review and meta-analysis. Gynecol. Oncol. 2021, 161, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Docherty, J.; Morgan-Bates, K.; Stather, P. A Systematic Review and Meta-Analysis of Enhanced Recovery for Open Abdominal Aortic Aneurysm Surgery. Vasc. Endovasc. Surg. 2022, 56, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, K.; Qu, C.; Qi, W.; Fang, T.; Yue, W.; Tian, H. The effect of the enhanced recovery after surgery program on lung cancer surgery: A systematic review and meta-analysis. J. Thorac. Dis. 2021, 13, 3566–3586. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Li, J.; Hong, D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: A meta-analysis of randomized controlled trials. Surg. Oncol. 2020, 32, 75–87. [Google Scholar] [CrossRef]
- Peerbocus, M.; Wang, Z.-J. Enhanced Recovery After Surgery and Radical Cystectomy: A Systematic Review and Meta-Analysis. Res. Rep. Urol. 2021, 13, 535–547. [Google Scholar] [CrossRef]
- Lode, L.; Oma, E.; Henriksen, N.A.; Jensen, K.K. Enhanced recovery after abdominal wall reconstruction: A systematic review and meta-analysis. Surg. Endosc. 2021, 35, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.A.; Kokorovic, A.; McGrath, J.S.; Kassouf, W.; Collins, J.W.; Black, P.C.; Douglas, J.; Djaladat, H.; Daneshmand, S.; Catto, J.W.F.; et al. Critical analysis of quality of life and cost-effectiveness of enhanced recovery after surgery (ERAS) for patient’s undergoing urologic oncology surgery: A systematic review. World J. Urol. 2022, 40, 1325–1342. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jensen, C. Patient Satisfaction and Quality of Life with Enhanced Recovery Protocols. Clin. Colon Rectal Surg. 2019, 32, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.P.; Vitous, C.A.; Stricklen, A.; Ross, R.; Ghaferi, A.A.; Finks, J.F. Implementation of an enhanced recovery after surgery protocol for bariatric surgery—A qualitative study. Am. J. Surg. 2022, 224, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Pearsall, E.A.; Meghji, Z.; Pitzul, K.B.; Aarts, M.-A.; McKenzie, M.; McLeod, R.S.; Okrainec, A. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann. Surg. 2015, 261, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Alawadi, Z.M.; Leal, I.; Phatak, U.R.; Flores-Gonzalez, J.R.; Holihan, J.L.; Karanjawala, B.E.; Millas, S.G.; Kao, L.S. Facilitators and barriers of implementing enhanced recovery in colorectal surgery at a safety net hospital: A provider and patient perspective. Surgery 2016, 159, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.; Solomon, M.J.; Harrison, J.D. A qualitative study assessing the barriers to implementation of enhanced recovery after surgery. World J. Surg. 2014, 38, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.E.; Doumouras, A.G.; Lethbridge, S.; Forbes, S.; Eskicioglu, C. A Provincial Assessment of the Barriers and Utilization of Enhanced Recovery after Colorectal Surgery. J. Surg. Res. 2019, 235, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Roulin, D.; Grass, F.; Addor, V.; Ljungqvist, O.; Demartines, N.; Hübner, M. A multicentre qualitative study assessing implementation of an Enhanced Recovery After Surgery program. Clin. Nutr. 2018, 37, 2172–2177. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Zhou, J.; Yang, J.; Chen, X.; Chang, C.; Liu, C.; Li, K.; Hu, J. Barriers to implementation of enhanced recovery after surgery (ERAS) by a multidisciplinary team in China: A multicentre qualitative study. BMJ Open 2022, 12, e053687. [Google Scholar] [CrossRef] [PubMed]
- Budacan, A.-M.; Mehdi, R.; Kerr, A.P.; Kadiri, S.B.; Batchelor, T.J.P.; Naidu, B. National survey of enhanced recovery after thoracic surgery practice in the United Kingdom and Ireland. J. Cardiothorac. Surg. 2020, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- von Meyenfeldt, E.M.; van Nassau, F.; de Betue, C.T.I.; Barberio, L.; Schreurs, W.H.; Marres, G.M.H.; Bonjer, H.J.; Anema, J. Implementing an enhanced recovery after thoracic surgery programme in the Netherlands: A qualitative study investigating facilitators and barriers for implementation. BMJ Open 2022, 12, e051513. [Google Scholar] [CrossRef] [PubMed]
- STROBE. Strengthening the Reporting of Observational Studies in Epidemiology. STROBE. 2023. Available online: https://www.strobe-statement.org/ (accessed on 19 April 2023).
- Ministère de la Santé et de la Prévention. Article R1121-1—Code de la Santé Publique—Légifrance. Santé Publique 1 July 2021. Available online: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000043723460 (accessed on 5 October 2023).
- Newsome, K.; McKenny, M.; Elkbuli, A. Major and minor surgery: Terms used for hundreds of years that have yet to be defined. Ann. Med. Surg. 2021, 66, 102409. [Google Scholar] [CrossRef] [PubMed]
- Earl, R. Definition of Major and Minor Surgery. Ann. Surg. 1917, 65, 799. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012, 7, 37. [Google Scholar] [CrossRef]
- Cox, N.S.; Oliveira, C.C.; Lahham, A.; Holland, A.E. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: A systematic review using the Theoretical Domains Framework. J. Physiother. 2017, 63, 84–93. [Google Scholar] [CrossRef]
- Redwood, J.N.; Matkin, A.E.; Temple-Oberle, C.F. Adoption of Enhanced Recovery after Surgery Protocols in Breast Reconstruction in Alberta Is High before a Formal Program Implementation. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2249. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Thompson, J.Y.; Menzies, J.C.; Manning, J.C.; McAnuff, J.; Brush, E.C.; Ryde, F.; Rapley, T.; Pathan, N.; Brett, S.; Moore, D.J.; et al. Early mobilisation and rehabilitation in the PICU: A UK survey. BMJ Paediatr. Open 2022, 6, e001300. [Google Scholar] [CrossRef]
- Aarons, G.A.; Sommerfeld, D.H.; Walrath-Greene, C.M. Evidence-based practice implementation: The impact of public versus private sector organization type on organizational support, provider attitudes, and adoption of evidence-based practice. Implement. Sci. 2009, 4, 83. [Google Scholar] [CrossRef]
- Li, X.; Krumholz, H.M.; Yip, W.; Cheng, K.K.; De Maeseneer, J.; Meng, Q.; Mossialos, E.; Li, C.; Lu, J.; Su, M.; et al. Quality of primary health care in China: Challenges and recommendations. Lancet 2020, 395, 1802–1812. [Google Scholar] [CrossRef]
- Watson, D.J. The role of the nurse coordinator in the enhanced recovery after surgery program. Nursing 2017, 47, 13. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Pelone, F.; Harrison, R.; Goldman, J.; Zwarenstein, M. Interprofessional Collaboration to Improve Professional Practice and Healthcare Outcomes. Cochrane Effective Practice and Organisation of Care Group, Editor. Cochrane Database of Systematic Reviews. 22 June 2017. Available online: http://doi.wiley.com/10.1002/14651858.CD000072.pub3 (accessed on 12 October 2023).
- Brown, B.B.; Patel, C.; McInnes, E.; Mays, N.; Young, J.; Haines, M. The effectiveness of clinical networks in improving quality of care and patient outcomes: A systematic review of quantitative and qualitative studies. BMC Health Serv. Res. 2016, 16, 360. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Reyes, J.-P.C.; Denham, Z.; Abdel-Rasoul, M.; Rasoul, E.; Humeidan, M.L. Survey of provider perceptions of enhanced recovery after surgery and perioperative surgical home protocols at a tertiary care hospital. Medicine 2021, 100, e26079. [Google Scholar] [CrossRef]
- Fraile Olivero, C.A.; Jarabo Sarceda, J.R.; Fernández Martín, E.; Santos Capa, P.; Arribas Manzanal, P.D.; Gómez Martínez, A.M.; Calatayud Gastardi, J.; Hernando Trancho, F. Implementation of a perioperative care App in elective thoracic surgery. Cir. Esp. 2022, 101, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Franssen, R.F.W.; Bongers, B.C.; Vogelaar, F.J.; Janssen-Heijnen, M.L.G. Feasibility of a tele-prehabilitation program in high-risk patients with colon or rectal cancer undergoing elective surgery: A feasibility study. Perioper. Med. 2022, 11, 28. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Shen, L.; Huang, Y. Level of ERAS understanding affects practitioners’ practice and perception of early postoperative resumption of oral intake: A nationwide survey. BMC Anesthesiol. 2021, 21, 279. [Google Scholar] [CrossRef]
- Lloyd, B.; Pfeiffer, D.; Dominish, J.; Heading, G.; Schmidt, D.; McCluskey, A. The New South Wales Allied Health Workplace Learning Study: Barriers and enablers to learning in the workplace. BMC Health Serv. Res. 2014, 14, 134. [Google Scholar] [CrossRef]
- Groves, R.M. (Ed.) Survey Methodology; Wiley Series in Survey Methodology; Wiley: Hoboken, NJ, USA, 2004; 424p. [Google Scholar]
- Vercruyssen, A. Are They Really Too Busy for Survey Participation? The Evolution of Busyness and Busyness Claims in Flanders. J. Off. Stat. 2011, 27, 619–632. [Google Scholar]
| Variable | Global, n = 153 |
|---|---|
| Age (Years), median (IQR) | 35 (28.5–47.5) |
| Gender n (%) | |
| Female | 116 (75.8%) |
| Profession, n (%) | |
| Physiotherapist | 48 (31.4%) |
| Nurse | 37 (24.2%) |
| Dietician | 22 (14.4%) |
| Surgeon | 12 (7.8%) |
| Occupational therapist | 11 (7.2%) |
| Psychologist | 7 (4.6%) |
| Anesthesiologist | 5 (3.3%) |
| Nurse anesthetist | 3 (2.0%) |
| Operating room nurse | 3 (2.0%) |
| General physician | 2 (1.3%) |
| Nursing assistant | 2 (1.3%) |
| Medical specialist | 1 (0.7%) |
| Private nurse | 0 (0.0%) |
| Country of studies n (%) | |
| France | 140 (91.5%) |
| Germany | 6 (3.9%) |
| Belgian | 5 (3.3%) |
| Spain | 2 (1.3%) |
| Time from graduation (Years), Median (IQR) | 12 (5.5–22.5) |
| Practice mode, n (%) | |
| Employee | 120 (78.4%) |
| Self-employed | 23 (15.0%) |
| Mixed | 10 (6.5%) |
| TDF * | Global, n = 144 | Global, % (95%CI) |
|---|---|---|
| Environmental context and resources | 83 | 57.6% (49.5–65.4) |
| Knowledge | 76 | 52.8% (44.7–60.8) |
| Social and professional role/identity | 30 | 20.8% (15.0–28.2) |
| Intentions | 19 | 13.2% (8.6–19.7) |
| Belief about consequences | 7 | 4.9% (2.4–9.7) |
| Variable | Participants Who Practiced ERAS, n = 31 | Participants Who Did Not Practice ERAS, n = 113 | p-Value |
|---|---|---|---|
| Age (Years), Median (IQR) | 44.0 (34–54) | 33.0 (27.0–43.5) | <0.001 * |
| Gender, n (%) | 0.096 | ||
| Female | 20 (65.0%) | 90 (80.0%) | |
| Profession, n (%) | <0.001 * | ||
| Physiotherapist | 9 (29.0%) | 35 (31.0%) | >0.999 |
| Nurse | 6 (19.4%) | 29 (25.7%) | 0.637 |
| Dietician | 3 (9.7%) | 19 (16.8%) | 0.410 |
| Occupational therapist | 11 (9.7%) | ||
| Surgeon | 7 (22.6%) | 3 (2.7%) | 0.001 * |
| Psychologist | 7 (6.2%) | ||
| Anesthesiologist | 3 (9.7%) | 1 (0.9%) | 0.031 * |
| Nurse anesthetist | 3 (2.7%) | ||
| Operating room nurse | 3 (9.7%) | ||
| General physician | 2 (1.8%) | ||
| Nursing assistant | 2 (1.8%) | ||
| Medical Specialist | 1 (0.9%) | ||
| Private nurse | |||
| Country of studies, n (%) | 0.846 | ||
| France | 28 (90.3%) | 105 (92.9%) | 0.703 |
| Germany | 2 (6.5%) | 4 (3.5%) | 0.610 |
| Belgian | 1 (3.2%) | 3 (2.7%) | >0.999 |
| Spain | 1 (0.9%) | ||
| Time from graduation, Median (IQR) | 15 (8–29) | 11 (4.5–21) | 0.052 |
| Practice mode, n (%) | 0.016 * | ||
| Employee | 30 (96.8%) | 82 (72.6%) | 0.003 * |
| Self-employed | 1 (3.2%) | 22 (19.5%) | 0.028 * |
| Mixed | 9 (8.0%) |
| TDF | Participants Who Practiced ERAS, n = 31 | Participants Who Did Not Practice ERAS, n = 113 | Relative Risk (95%CI) | p-Value |
|---|---|---|---|---|
| Environmental context and resources, % (95%CI) | 67.7% (50.1–81.4) | 54.9% (45.7–63.7) | 0.8 (0.6–1.1) | 0.224 |
| Knowledge, % (95%CI) | 19.4% (9.2–36.3) | 61.9% (52.7–70.4) | 3.2 (1.5–6.7) | <0.001 * |
| Social and professional role/identity, % (95%CI) | 22.6% (11.4–39.8) | 20.4% (14.0–28.7) | 0.9 (0.4–1.9) | 0.805 |
| Intentions, % (95%CI) | 61.3% (43.8–76.3) | 0.0% (0.0–0.0) | <0.001 * | |
| Belief about consequences, % (95%CI) | 16.1% (7.1–32.6) | 1.8% (0.5–6.2) | 0.1 (0.2–0.5) | 0.005 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clet, A.; Guy, M.; Muir, J.-F.; Cuvelier, A.; Gravier, F.-E.; Bonnevie, T. Enhanced Recovery after Surgery (ERAS) Implementation and Barriers among Healthcare Providers in France: A Cross-Sectional Study. Healthcare 2024, 12, 436. https://doi.org/10.3390/healthcare12040436
Clet A, Guy M, Muir J-F, Cuvelier A, Gravier F-E, Bonnevie T. Enhanced Recovery after Surgery (ERAS) Implementation and Barriers among Healthcare Providers in France: A Cross-Sectional Study. Healthcare. 2024; 12(4):436. https://doi.org/10.3390/healthcare12040436
Chicago/Turabian StyleClet, Augustin, Marin Guy, Jean-François Muir, Antoine Cuvelier, Francis-Edouard Gravier, and Tristan Bonnevie. 2024. "Enhanced Recovery after Surgery (ERAS) Implementation and Barriers among Healthcare Providers in France: A Cross-Sectional Study" Healthcare 12, no. 4: 436. https://doi.org/10.3390/healthcare12040436
APA StyleClet, A., Guy, M., Muir, J.-F., Cuvelier, A., Gravier, F.-E., & Bonnevie, T. (2024). Enhanced Recovery after Surgery (ERAS) Implementation and Barriers among Healthcare Providers in France: A Cross-Sectional Study. Healthcare, 12(4), 436. https://doi.org/10.3390/healthcare12040436





