Inverse Association between Exercising Blood Pressure Response and Left Ventricular Chamber Size and Mass in Women Who Habitually Resistance Train
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Screening Visit
2.3. Echocardiograph and Exercise Visit
2.4. Statistical Analysis
3. Results
3.1. Participant and Protocol Characteristics
3.2. Exercise Systolic Blood Pressure Response and Left Ventricular Mass
3.3. Independent Effects on Left Ventricular Mass
3.4. Independent Effects on Exercise Systolic Blood Pressure Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callaghan, K.N.; Hosick, P.A.; Brian, M.S.; Matthews, E.L. Impact of a Family History of Hypertension and Physical Activity on Left Ventricular Mass. J. Sports Med. Phys. Fit. 2023, 63, 949–956. [Google Scholar] [CrossRef]
- Devereux, R.B.; Koren, M.J.; de Simone, G.; Roman, M.J.; Laragh, J.H. Left Ventricular Mass as a Measure of Preclinical Hypertensive Disease. Am. J. Hypertens. 1992, 5, 175S–181S. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B. Prognostic Significance of Left Ventricular Mass Change During Treatment of Hypertension. JAMA 2004, 292, 2350. [Google Scholar] [CrossRef]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; de Simone, G.; Koren, M.J.; Roman, M.J.; Laragh, J.H. Left Ventricular Mass as a Predictor of Development of Hypertension. Am. J. Hypertens. 1991, 4, 603S–607S. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, L.T.; Schieken, R.M.; Clarke, W.R.; Lauer, R.M. Left Ventricular Mass and Exercise Responses Predict Future Blood Pressure. The Muscatine Study. Hypertension 1988, 12, 206–213. [Google Scholar] [CrossRef]
- Haufe, S.; Utz, W.; Engeli, S.; Kast, P.; Böhnke, J.; Pofahl, M.; Traber, J.; Haas, V.; Hermsdorf, M.; Mähler, A.; et al. Left Ventricular Mass and Function with Reduced-Fat or Reduced-Carbohydrate Hypocaloric Diets in Overweight and Obese Subjects. Hypertension 2012, 59, 70–75. [Google Scholar] [CrossRef]
- Musso, N.; Gatto, F.; Nista, F.; Dotto, A.; Shen, Z.; Ferone, D. Left Ventricular Mass Reduction by a Low-Sodium Diet in Treated Hypertensive Patients. Nutrients 2020, 12, 3714. [Google Scholar] [CrossRef]
- Hegde, S.M.; Gonçalves, A.; Claggett, B.; Evenson, K.R.; Cheng, S.; Shah, A.M.; Folsom, A.R.; Solomon, S.D. Cardiac Structure and Function and Leisure-Time Physical Activity in the Elderly: The Atherosclerosis Risk in Communities Study. Eur. Heart J. 2016, 37, 2544–2551. [Google Scholar] [CrossRef]
- Kang, E.; Lee, J.; Kim, H.J.; Han, M.; Kim, S.W.; Lee, K.-B.; Sung, S.; Yoo, T.-H.; Chung, W.; Ahn, C.; et al. The Association between Socioeconomic Disparities and Left Ventricular Hypertrophy in Chronic Kidney Disease: Results from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). BMC Nephrol. 2018, 19, 203. [Google Scholar] [CrossRef]
- Hosick, P.A.; Willett, J.M.; Matthews, E.L. Cardiovascular Strain and Metabolic Rate Are Higher Following 15 Minutes of Standing versus Seated Computer Work. Eur. J. Prev. Cardiol. 2020, 27, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Kop, W.J.; Gottdiener, J.S.; Patterson, S.M.; Krantz, D.S. Relationship between Left Ventricular Mass and Hemodynamic Responses to Physical and Mental Stress. J. Psychosom. Res. 2000, 48, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T. Physical Fitness and Activity as Separate Heart Disease Risk Factors: A Meta-Analysis. Med. Sci. Sports Exerc. 2001, 33, 754–761. [Google Scholar] [CrossRef]
- Hinderliter, A.; Sherwood, A.; Gullette, E.C.D.; Babyak, M.; Waugh, R.; Georgiades, A.; Blumenthal, J.A. Reduction of Left Ventricular Hypertrophy After Exercise and Weight Loss in Overweight Patients With Mild Hypertension. Arch. Intern. Med. 2002, 162, 1333. [Google Scholar] [CrossRef]
- Japanese Society of Hypertension Chapter 4. Lifestyle Modifications. Hypertens. Res. 2009, 32, 29–32. [CrossRef]
- Fagard, R.; Staessen, J.; Thijs, L.; Amery, A. Relation of Left Ventricular Mass and Filling to Exercise Blood Pressure and Rest Blood Pressure. Am. J. Cardiol. 1995, 75, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Morganroth, J.; Maron, B.J.; Henry, W.L.; Epstein, S.E. Comparative Left Ventricular Dimensions in Trained Athletes. Ann. Intern. Med. 1975, 82, 521–524. [Google Scholar] [CrossRef]
- Utomi, V.; Oxborough, D.; Whyte, G.P.; Somauroo, J.; Sharma, S.; Shave, R.; Atkinson, G.; George, K. Systematic Review and Meta-Analysis of Training Mode, Imaging Modality and Body Size Influences on the Morphology and Function of the Male Athlete’s Heart. Heart 2013, 99, 1727–1733. [Google Scholar] [CrossRef]
- Pluim, B.M.; Zwinderman, A.H.; van der Laarse, A.; van der Wall, E.E. The Athlete’s Heart. A Meta-Analysis of Cardiac Structure and Function. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef]
- Whyte, G.P.; George, K.; Nevill, A.; Shave, R.; Sharma, S.; McKenna, W.J. Left Ventricular Morphology and Function in Female Athletes: A Meta-Analysis. Int. J. Sports Med. 2004, 25, 380–383. [Google Scholar] [CrossRef]
- Beaumont, A.; Grace, F.; Richards, J.; Hough, J.; Oxborough, D.; Sculthorpe, N. Left Ventricular Speckle Tracking-Derived Cardiac Strain and Cardiac Twist Mechanics in Athletes: A Systematic Review and Meta-Analysis of Controlled Studies. Sports Med. 2017, 47, 1145–1170. [Google Scholar] [CrossRef]
- Caselli, S.; Di Paolo, F.M.; Pisicchio, C.; Pandian, N.G.; Pelliccia, A. Patterns of Left Ventricular Diastolic Function in Olympic Athletes. J. Am. Soc. Echocardiogr. 2015, 28, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Stelzer, K.; Ostad, M.A.; Post, F. Impact of Exaggerated Blood Pressure Response in Normotensive Individuals on Future Hypertension and Prognosis: Systematic Review According to PRISMA Guideline. Adv. Med. Sci. 2017, 62, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.L.; Greaney, J.L.; Wenner, M.M. Rapid Onset Pressor Response to Exercise in Young Women with a Family History of Hypertension. Exp. Physiol. 2017, 102, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, K.M.; Michelsen, S.; Otterstad, J.E.; Frøland, G.; Wasenius, A.; Stugaard, M. Correlations between Left Ventricular Mass and Systolic Blood Pressure at Rest and during Maximal Exercise in Moderately Hypertensive Men. J. Hum. Hypertens. 1991, 5, 149–154. [Google Scholar] [PubMed]
- Lauer, M.S.; Levy, D.; Anderson, K.M.; Plehn, J.F. Is There a Relationship between Exercise Systolic Blood Pressure Response and Left Ventricular Mass? The Framingham Heart Study. Ann. Intern. Med. 1992, 116, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Markovitz, J.H.; Raczynski, J.M.; Lewis, C.E.; Flack, J.; Chesney, M.; Chettur, V.; Hardin, J.M.; Johnson, E. Lack of Independent Relationships between Left Ventricular Mass and Cardiovascular Reactivity to Physical and Psychological Stress in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am. J. Hypertens. 1996, 9, 915–923. [Google Scholar] [CrossRef]
- Karjalainen, J.; Mäntysaari, M.; Viitasalo, M.; Kujala, U. Left Ventricular Mass, Geometry, and Filling in Endurance Athletes: Association with Exercise Blood Pressure. J. Appl. Physiol. 1997, 82, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Majahalme, S.; Turjanmaa, V.; Tuomisto, M.; Kautiainen, H.; Uusitalo, A. Intra-Arterial Blood Pressure during Exercise and Left Ventricular Indices in Normotension and Borderline and Mild Hypertension. Blood Press. 1997, 6, 5–12. [Google Scholar] [CrossRef]
- Molina, L.; Elosua, R.; Marrugat, J.; Pons, S. Relation of Maximum Blood Pressure during Exercise and Regular Physical Activity in Normotensive Men with Left Ventricular Mass and Hypertrophy. MARATHOM Investigators. Medida de La Actividad Fisica y Su Relación Ambiental Con Todos Los Lípidos En El HOMb. Am. J. Cardiol. 1999, 84, 890–893. [Google Scholar] [CrossRef]
- Lim, P.O.; Donnan, P.T.; MacDonald, T.M. Blood Pressure Determinants of Left Ventricular Wall Thickness and Mass Index in Hypertension: Comparing Office, Ambulatory and Exercise Blood Pressures. J. Hum. Hypertens. 2001, 15, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Ouyang, P.; Silber, H.A.; Bacher, A.C.; Turner, K.L.; DeRegis, J.R.; Hees, P.S.; Shapiro, E.P.; Stewart, K.J. Exercise Blood Pressure Response Is Related to Left Ventricular Mass. J. Hum. Hypertens. 2003, 17, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Raina, A.; Saif, H.; Swillens, A.; Gupta, A.K.; Townsend, R.; Emmi, A.G.; Kirkpatrick, J.N.; Keane, M.G.; et al. Arterial Pulsatile Hemodynamic Load Induced by Isometric Exercise Strongly Predicts Left Ventricular Mass in Hypertension. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H320–H330. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, E.A.; Balogun, M.O.; Akintomide, O.A.; Adebayo, R.A.; Ajayi, O.E.; Ikem, R.T.; Ogunyemi, S.A.; Oyedeji, A.T. Blood Pressure Response to an Exercise Treadmill Test, and Echocardiographic Left Ventricular Geometry in Nigerian Normotensive Diabetics. Cardiovasc. J. Afr. 2010, 21, 93–96. [Google Scholar] [PubMed]
- Kamada, Y.; Masuda, T.; Tanaka, S.; Akiyama, A.; Nakamura, T.; Hamazaki, N.; Okubo, M.; Kobayashi, N.; Ako, J. Muscle Weakness Is Associated With an Increase of Left Ventricular Mass Through Excessive Blood Pressure Elevation During Exercise in Patients With Hypertension. Int. Heart J. 2017, 58, 551–556. [Google Scholar] [CrossRef]
- Oh, M.S.; Cho, S.J.; Sung, J.; Hong, K.P. Higher Blood Pressure during Light Exercise Is Associated with Increased Left Ventricular Mass Index in Normotensive Subjects. Hypertens. Res. 2018, 41, 382–387. [Google Scholar] [CrossRef]
- Tanaka, S.; Masuda, T.; Kamada, Y.; Hamazaki, N.; Kamiya, K.; Ogura, M.N.; Maekawa, E.; Noda, C.; Yamaoka-Tojo, M.; Ako, J. Excessive SBP Elevation during Moderate Exercise Discriminates Patients at High Risk of Developing Left Ventricular Hypertrophy from Hypertensive Patients. J. Hypertens. 2018, 36, 1291–1298. [Google Scholar] [CrossRef]
- Iglesias Cubero, G.; Batalla, A.; Rodriguez Reguero, J.J.; Barriales, R.; González, V.; de la Iglesia, J.L.; Terrados, N. Left Ventricular Mass Index and Sports: The Influence of Different Sports Activities and Arterial Blood Pressure. Int. J. Cardiol. 2000, 75, 261–265. [Google Scholar] [CrossRef]
- Olga, V.; Lucio, M.; Giuseppe, G.; Stefano, M.; Paolo, P. Blood Pressure Response to Stress Tests Does Not Reflect Blood Pressure Variability and Degree of Cardiovascular Involvement in Young Hypertensives. Int. J. Cardiol. 1995, 48, 303–310. [Google Scholar] [CrossRef]
- Cardillo, C.; De Felice, F.; Campia, U.; Musumeci, V.; Folli, G. Relation of Stress Testing and Ambulatory Blood Pressure to Hypertensive Cardiac Damage. Am. J. Hypertens. 1996, 9, 162–170. [Google Scholar] [CrossRef]
- Hunter, S.K.; Angadi, S.S.; Bhargava, A.; Harper, J.; Hirschberg, A.L.; Levine, B.D.; Moreau, K.L.; Nokoff, N.J.; Stachenfeld, N.S.; Bermon, S. The Biological Basis of Sex Differences in Athletic Performance: Consensus Statement for the American College of Sports Medicine. Transl. J. Am. Coll. Sports Med. 2023, 8, 1–33. [Google Scholar] [CrossRef]
- Hurley, K.S.; Flippin, K.J.; Blom, L.C.; Bolin, J.E.; Hoover, D.L.; Judge, L.W. Practices, Perceived Benefits, and Barriers to Resistance Training Among Women Enrolled in College. Int. J. Exerc. Sci. 2018, 11, 226–238. [Google Scholar]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial Blood Pressure Response to Heavy Resistance Exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Tsai, K.-Z.; Han, C.-L.; Lee, J.-T.; Lin, G.-M. Athlete’s Heart Assessed by Sit-Up Strength Exercises in Military Men and Women: The CHIEF Heart Study. Front. Cardiovasc. Med. 2022, 8, 737607. [Google Scholar] [CrossRef]
- Robinson, A.T.; Wenner, M.M.; Bunsawat, K.; Watso, J.C.; Giersch, G.E.W.; Charkoudian, N. When It’s Time for the Sex Talk, Words Matter. Am. J. Physiol.-Heart Circ. Physiol. 2022, 322, H66–H70. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013; ISBN 9780203771587. [Google Scholar]
- Haff, G.G.; Triplett, N.T. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Du Bois, D. Clinical Calorimetry. Arch. Intern. Med. 1916, XVII, 863–871. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Otto, C.M.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A. Recommendations for Quantification of Doppler Echocardiography: A Report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Imholz, B. Fifteen Years Experience with Finger Arterial Pressure Monitoring: Assessment of the Technology. Cardiovasc. Res. 1998, 38, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.P.M.; Wessling, K.H.; Pott, F.; Jenstrup, M.; VAN Goudever, J.; Secher, N.H.; Van Lieshout, J.J. Continuous Stroke Volume Monitoring by Modelling Flow from Non-Invasive Measurement of Arterial Pressure in Humans under Orthostatic Stress. Clin. Sci. 1999, 97, 291–301. [Google Scholar] [CrossRef]
- American College of Sports Medicine American College of Sports Medicine Position Stand. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine American College of Sports Medicine Position Stand. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Tharpe, M.A.; Linder, B.A.; Babcock, M.C.; Watso, J.C.; Pollin, K.U.; Hutchison, Z.J.; Barnett, A.M.; Culver, M.N.; Kavazis, A.N.; Brian, M.S.; et al. Adjusting for Muscle Strength and Body Size Attenuates Sex Differences in the Exercise Pressor Reflex in Young Adults. Am. J. Physiol.-Heart Circ. Physiol. 2023, 325, H1418–H1429. [Google Scholar] [CrossRef]
- Bech-Hanssen, O.; Wallentin, I.; Houltz, E.; Beckman Suurküla, M.; Larsson, S.; Caidahl, K. Gender Differences in Patients with Severe Aortic Stenosis: Impact on Preoperative Left Ventricular Geometry and Function, as Well as Early Postoperative Morbidity and Mortality. Eur. J. Cardio-Thorac. Surg. 1999, 15, 24–30. [Google Scholar] [CrossRef]
- Gaasch, W.H.; Zile, M.R. Left Ventricular Structural Remodeling in Health and Disease. J. Am. Coll. Cardiol. 2011, 58, 1733–1740. [Google Scholar] [CrossRef]
- Basford, J.R. The Law of Laplace and Its Relevance to Contemporary Medicine and Rehabilitation. Arch. Phys. Med. Rehabil. 2002, 83, 1165–1170. [Google Scholar] [CrossRef]
- Scheuer, J.; Malhotra, A.; Schaible, T.F.; Capasso, J. Effects of Gonadectomy and Hormonal Replacement on Rat Hearts. Circ. Res. 1987, 61, 12–19. [Google Scholar] [CrossRef]
- Koenig, H.; Goldstone, A.; Lu, C.Y. Testosterone-Mediated Sexual Dimorphism of the Rodent Heart. Ventricular Lysosomes, Mitochondria, and Cell Growth Are Modulated by Androgens. Circ. Res. 1982, 50, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Szadkowska, I.; Guligowska, A.; Jegier, A.; Pawlikowski, M.; Pisarek, H.; Winczyk, K.; Kostka, T. Serum Testosterone Level Correlates with Left Ventricular Hypertrophy in Older Women. Front. Endocrinol. 2022, 13, 1079043. [Google Scholar] [CrossRef]
- La Gerche, A.; Brosnan, M.J. Cardiovascular Effects of Performance-Enhancing Drugs. Circulation 2017, 135, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, O.; Howlett, S.E. Testosterone Modulates Cardiac Contraction and Calcium Homeostasis: Cellular and Molecular Mechanisms. Biol. Sex. Differ. 2015, 6, 9. [Google Scholar] [CrossRef]
- Piro, M.; Della Bona, R.; Abbate, A.; Biasucci, L.M.; Crea, F. Sex-Related Differences in Myocardial Remodeling. J. Am. Coll. Cardiol. 2010, 55, 1057–1065. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Maron, B.A.; Wang, R.-S.; Carnethon, M.R.; Rowin, E.J.; Loscalzo, J.; Maron, B.J.; Maron, M.S. What Causes Hypertrophic Cardiomyopathy? Am. J. Cardiol. 2022, 179, 74–82. [Google Scholar] [CrossRef]
- Paliakaite, B.; Charlton, P.H.; Rapalis, A.; Plusciauskaite, V.; Piartli, P.; Kaniusas, E.; Marozas, V. Blood Pressure Estimation Based on Photoplethysmography: Finger Versus Wrist. In Proceedings of the 2021 Computing in Cardiology (CinC), Brno, Czech Republic, 13–15 September 2021; IEEE: New York, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Omenås, I.N.; Tronstad, C.; Rosseland, L.A. Accuracy and Trending Abilities of Finger Plethysmographic Blood Pressure and Cardiac Output Compared to Invasive Measurements during Caesarean Delivery in Healthy Women: An Observational Study. BMC Anesthesiol. 2020, 20, 157. [Google Scholar] [CrossRef]
- Bos, W.J.W.; van Goudoever, J.; van Montfrans, G.A.; van den Meiracker, A.H.; Wesseling, K.H. Reconstruction of Brachial Artery Pressure from Noninvasive Finger Pressure Measurements. Circulation 1996, 94, 1870–1875. [Google Scholar] [CrossRef]
| LVM/BSA | eSBP | ||||
|---|---|---|---|---|---|
| Variable | Mean ± SD | r | p | r | p |
| Age (years) | 23 ± 3 | 0.181 | 0.330 | 0.280 | 0.126 |
| Height (cm) | 164 ± 7 | - | - | 0.072 | 0.702 |
| Body Mass (kg) | 63.7 ± 10.3 | - | - | 0.151 | 0.417 |
| BSA (m2) | 1.69 ± 0.15 | - | - | 0.117 | 0.340 |
| Body Fat (%) | 25.5 ± 6.5 | 0.067 | 0.719 | 0.258 | 0.160 |
| Resting SBP (mmHg) | 110 ± 8 | 0.355 | 0.049 | 0.074 | 0.692 |
| Resting DBP (mmHg) | 74 ± 8 | 0.058 | 0.756 | 0.016 | 0.930 |
| Resting HR (beats/min) | 73 ± 10 | −0.054 | 0.775 | 0.039 | 0.835 |
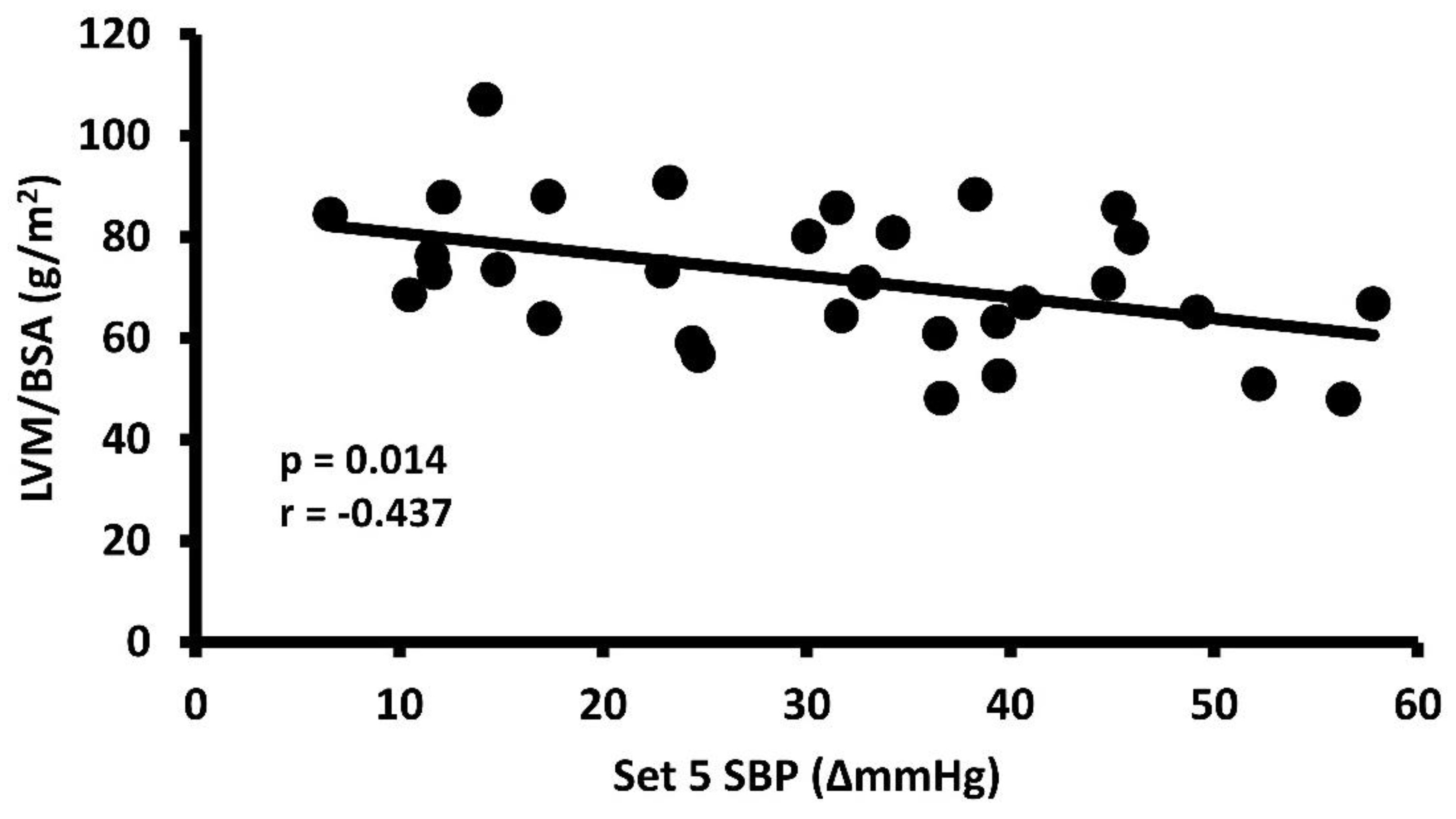
| eSBP (∆mmHg) | 30.8 ± 14.6 | −0.437 | 0.014 | - | - |
| eDBP (∆mmHg) | 16.6 ± 8.8 | −0.393 | 0.028 | 0.736 | <0.001 |
| eHR (∆beats/min) | 35.3 ± 12.9 | −0.178 | 0.338 | 0.467 | 0.008 |
| 1RM (kg) | 68 ± 24 | −0.153 | 0.412 | 0.370 | 0.040 |
| Exercising Weight (kg) | 49 ± 17 | −0.169 | 0.364 | 0.381 | 0.034 |
| R Ex (Days/week) | 3.7 ± 1.6 | −0.276 | 0.132 | 0.189 | 0.306 |
| A Ex (Days/week) | 2.6 ± 1.9 | 0.200 | 0.283 | −0.311 | 0.088 |
| Sedentary Time (min/day) | 457 ± 205 | −0.016 | 0.932 | −0.209 | 0.266 |
| MF Resting SV (mL/beat) | 36.0 ± 7.1 | 0.097 | 0.603 | −0.261 | 0.155 |
| MF eSV (mL/beat) | 34.0 ± 7.8 | 0.312 | 0.087 | −0.426 | 0.017 |
| MF Resting TPR (mmHg/mL/s) | 2.23 ± 0.70 | −0.109 | 0.558 | 0.234 | 0.205 |
| MF eTPR (mmHg/mL/s) | 1.99 ± 0.68 | −0.307 | 0.092 | 0.396 | 0.027 |
| Biplane Resting EDV (mL) | 70.6 ± 14.0 | 0.157 | 0.408 | −0.233 | 0.214 |
| Biplane Resting ESV (mL) | 28.1 ± 7.5 | 0.094 | 0.621 | −0.218 | 0.248 |
| Biplane Resting SV (mL/beat) | 42.5 ± 9.1 | 0.165 | 0.383 | −0.183 | 0.333 |
| Biplane Ejection Fraction (%) | 60.3 ± 6.0 | 0.015 | 0.938 | 0.122 | 0.520 |
| LVM/BSA (g/m2) | 72.0 ± 28.4 | - | - | −0.437 | 0.014 |
| IVSd (cm) | 0.88 ± 0.12 | - | - | −0.137 | 0.463 |
| LVIDd (cm) | 4.25 ± 0.33 | - | - | −0.411 | 0.021 |
| LVIDs (cm) | 2.86 ± 0.26 | 0.304 | 0.095 | −0.177 | 0.340 |
| PWTd (cm) | 0.91 ± 0.15 | - | - | −0.084 | 0.654 |
| RWT (Unitless) | 0.43 ± 0.08 | 0.227 | 0.219 | 0.103 | 0.582 |
| FS (%) | 32.8 ± 4.5 | 0.411 | 0.021 | −0.246 | 0.182 |
| ANOVA | R | R2 | df | p |
|---|---|---|---|---|
| LVM/BSA | 0.585 | 0.342 | 2 | 0.003 * |
| Coefficients | Unstandardized | Standard Error | Standardized | p |
| Intercept | 14.391 | 28.317 | - | 0.615 |
| Resting SBP (mmHg) | 0.650 | 0.257 | 0.389 | 0.017 * |
| eSBP (∆mmHg) | −0.449 | 0.148 | −0.466 | 0.005 * |
| ANOVA | R | R2 | df | p |
|---|---|---|---|---|
| eSBP | 0.537 | 0.289 | 2 | 0.009 * |
| Coefficients | Unstandardized | Standard Error | Standardized | p |
| Intercept | 46.166 | 14.987 | - | 0.005 * |
| LVM/BSA (g/m2) | −0.398 | 0.168 | −0.384 | 0.025 * |
| Exercising Weight (kg) | 0.271 | 0.139 | 0.317 | 0.060 |
| ANOVA | R | R2 | df | p |
|---|---|---|---|---|
| eSBP | 0.740 | 0.548 | 2 | <0.001 * |
| Coefficients | Unstandardized | Standard Error | Standardized | p |
| Intercept | 8.338 | 5.468 | - | 0.139 |
| eDBP (∆mmHg) | 1.133 | 0.251 | 0.686 | <0.001 * |
| eHR (∆beats/min) | 0.104 | 0.172 | 0.091 | 0.552 |
| ANOVA | R | R2 | df | p |
|---|---|---|---|---|
| eSBP | 0.432 | 0.187 | 2 | 0.056 |
| Coefficients | Unstandardized | Standard Error | Standardized | p |
| Intercept | 45.159 | 31.745 | - | 0.166 |
| eSV (mL) | −0.588 | 0.581 | −0.315 | 0.320 |
| eTPR (mmHg/mL/s) | 2.829 | 6.657 | 0.132 | 0.674 |
| ANOVA | R | R2 | df | p |
|---|---|---|---|---|
| eSBP | 0.426 | 0.181 | 3 | 0.139 |
| Coefficients | Unstandardized | Standard Error | Standardized | p |
| Intercept | 116.829 | 36.627 | - | 0.004 * |
| IVSd (cm) | 7.216 | 28.135 | 0.057 | 0.800 |
| LVIDd (cm) | −18.919 | 8.178 | −0.433 | 0.029 * |
| PWTd (cm) | −13.138 | 21.011 | −0.135 | 0.537 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, E.L.; Guers, J.J.; Ramick, M.G.; Hosick, P.A. Inverse Association between Exercising Blood Pressure Response and Left Ventricular Chamber Size and Mass in Women Who Habitually Resistance Train. Healthcare 2024, 12, 353. https://doi.org/10.3390/healthcare12030353
Matthews EL, Guers JJ, Ramick MG, Hosick PA. Inverse Association between Exercising Blood Pressure Response and Left Ventricular Chamber Size and Mass in Women Who Habitually Resistance Train. Healthcare. 2024; 12(3):353. https://doi.org/10.3390/healthcare12030353
Chicago/Turabian StyleMatthews, Evan L., John J. Guers, Meghan G. Ramick, and Peter A. Hosick. 2024. "Inverse Association between Exercising Blood Pressure Response and Left Ventricular Chamber Size and Mass in Women Who Habitually Resistance Train" Healthcare 12, no. 3: 353. https://doi.org/10.3390/healthcare12030353
APA StyleMatthews, E. L., Guers, J. J., Ramick, M. G., & Hosick, P. A. (2024). Inverse Association between Exercising Blood Pressure Response and Left Ventricular Chamber Size and Mass in Women Who Habitually Resistance Train. Healthcare, 12(3), 353. https://doi.org/10.3390/healthcare12030353









