Is the Combination of Robot-Assisted Therapy and Transcranial Direct Current Stimulation Useful for Upper Limb Motor Recovery? A Systematic Review with Meta-Analysis
Abstract
1. Introduction
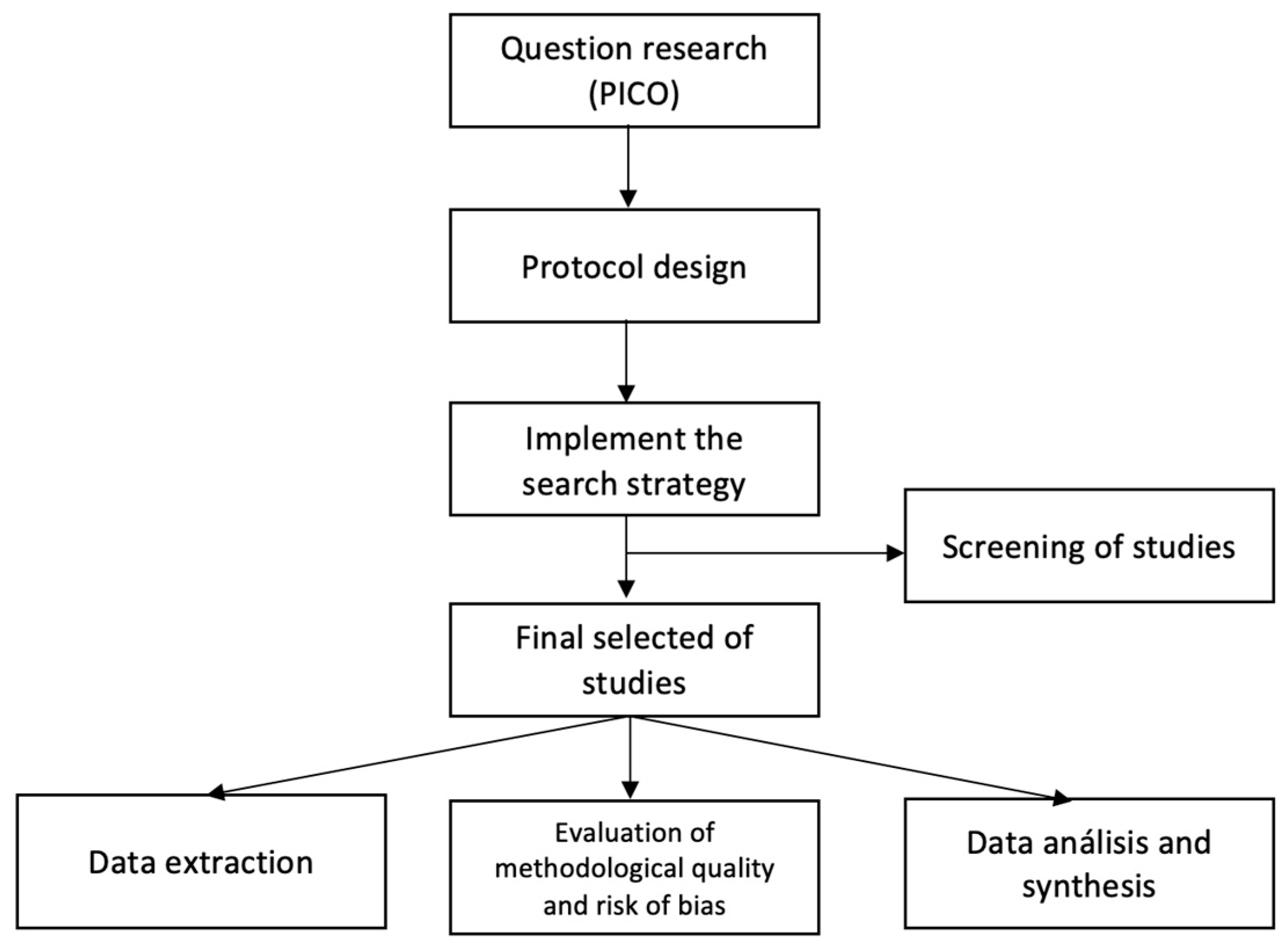
2. Materials and Methods
2.1. Selection Criteria, Identification, and Selection of Studies
2.2. Quality Evaluation of Involved Studies
2.3. Outcome Measures
2.4. Data Extraction and Analysis
3. Results
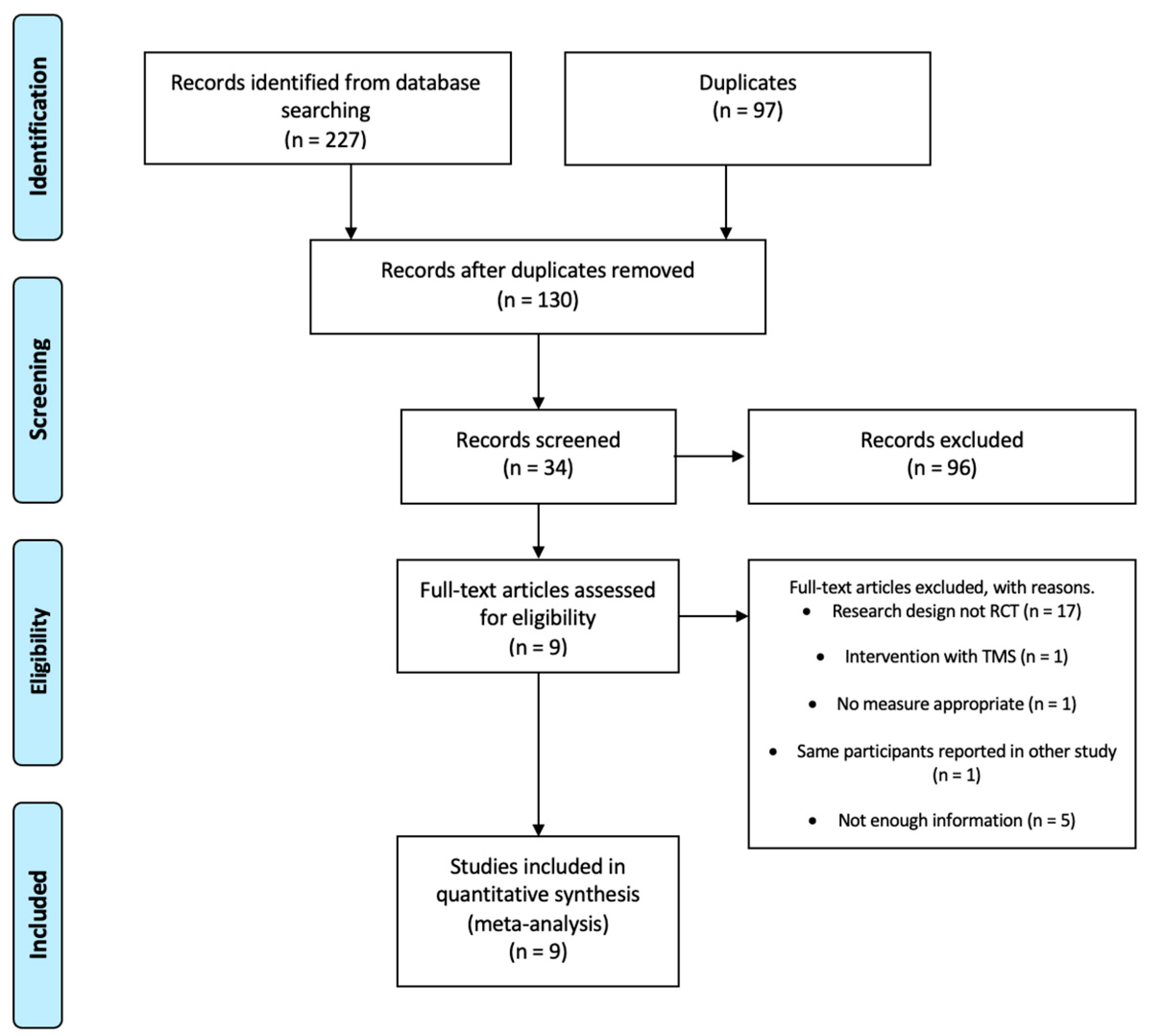
3.1. Flow of Studies through the Review
3.2. Characteristics of Included Studies
3.3. Quality
Outcome Measures
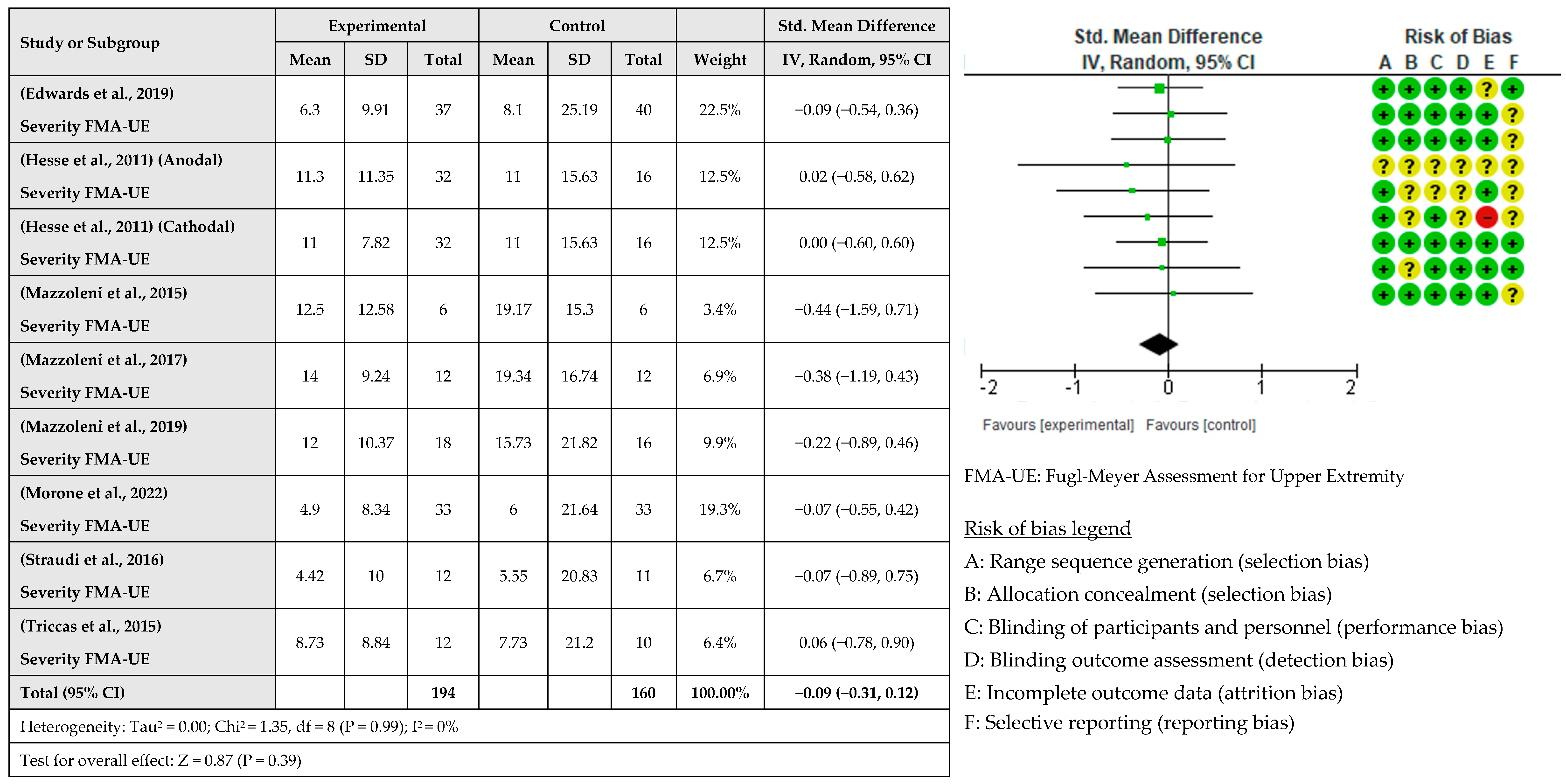
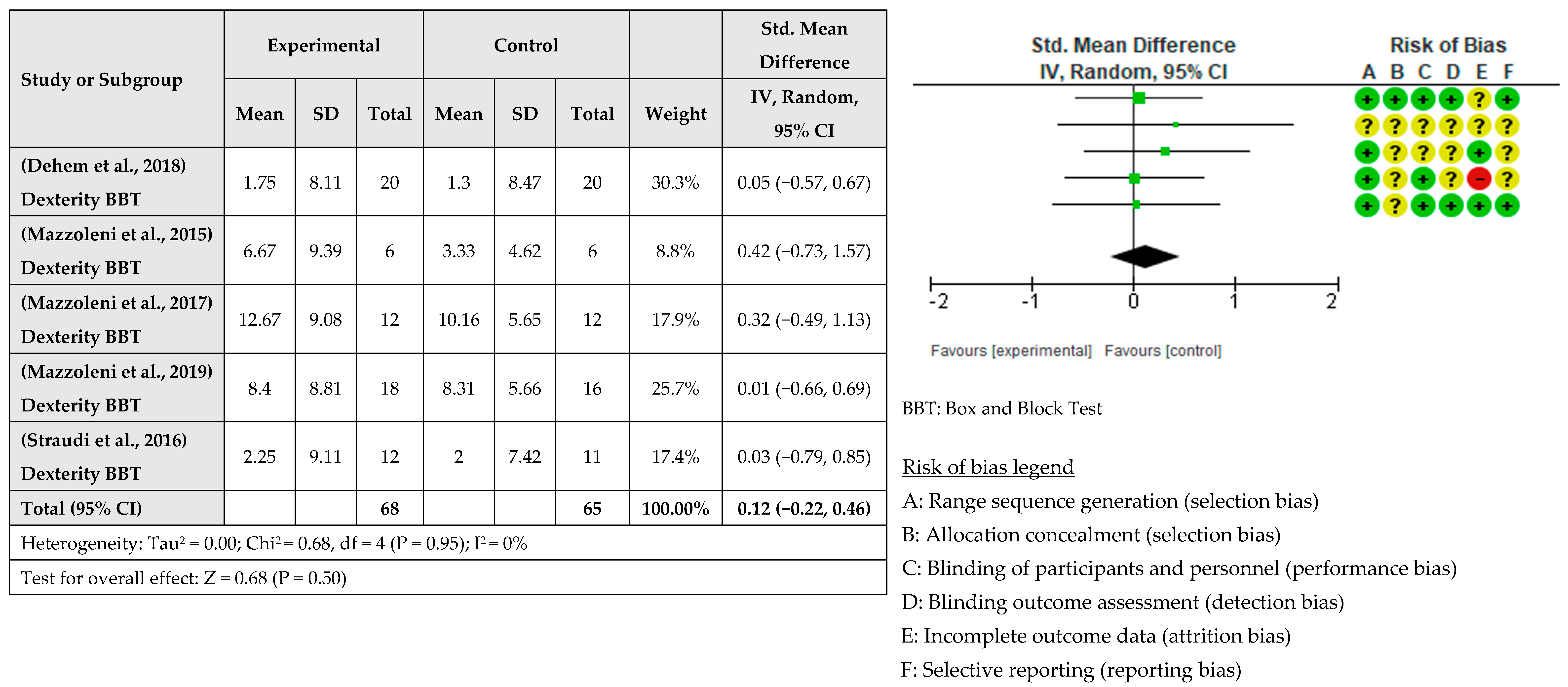
3.4. Intervention Effects
4. Discussion
- The presence of MEP: Most studies suggest that patients with the presence of MEP, those who preserve the corticospinal pathway, benefit more from the effects of tDCS and RAT [32,33,34,40,58], although there are other studies that point in the opposite direction [30]. It is not clear which patients benefit the most from NIBS, and this may be an important variable in establishing this criterion, so further research is required.
- The severity of deficits: Most studies indicate that subjects with moderate deficits, measured with FMA-EU, benefit most from the combination of both techniques [31,34,38]. Baseline scores are predictors of the evolution of the subjects, so this information can help us decide whether or not a patient will benefit from the intervention [71].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyu, H.H.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- Blomgren, C.; Jood, K.; Jern, C.; Holmegaard, L.; Redfors, P.; Blomstrand, C.; Claesson, L. Long-Term Performance of Instrumental Activities of Daily Living (IADL) in Young and Middle-Aged Stroke Survivors: Results from SAHLSIS Outcome. Scand. J. Occup. Ther. 2018, 25, 119–126. [Google Scholar] [CrossRef]
- Fekadu, G.; Chelkeba, L.; Kebede, A. Risk Factors, Clinical Presentations and Predictors of Stroke among Adult Patients Admitted to Stroke Unit of Jimma University Medical Center, South West Ethiopia: Prospective Observational Study. BMC Neurol. 2019, 19, 187. [Google Scholar] [CrossRef]
- Schinwelski, M.J.; Sitek, E.J.; Wąż, P.; Sławek, J.W. Prevalence and Predictors of Post-Stroke Spasticity and Its Impact on Daily Living and Quality of Life. Neurol. Neurochir. Pol. 2019, 53, 449–457. [Google Scholar] [CrossRef]
- Huang, J.; Ji, J.-R.; Liang, C.; Zhang, Y.-Z.; Sun, H.-C.; Yan, Y.-H.; Xing, X.-B. Effects of Physical Therapy-Based Rehabilitation on Recovery of Upper Limb Motor Function after Stroke in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Palliat. Med. 2022, 11, 521–531. [Google Scholar] [CrossRef]
- Chien, W.; Chong, Y.; Tse, M.; Chien, C.; Cheng, H. Robot-assisted Therapy for Upper-limb Rehabilitation in Subacute Stroke Patients: A Systematic Review and Meta-analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Song, W.-K. Effects of Two Different Robot-Assisted Arm Training on Upper Limb Motor Function and Kinematics in Chronic Stroke Survivors: A Randomized Controlled Trial. Top. Stroke Rehabil. 2021, 28, 241–250. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.T.; Bertolucci, F.; Torrealba-Acosta, G.; Huerta, R.; Fregni, F.; Thibaut, A. Non-invasive Brain Stimulation for Fine Motor Improvement after Stroke: A Meta-analysis. Eur. J. Neurol. 2018, 25, 1017–1026. [Google Scholar] [CrossRef]
- Elsner, B.; Kugler, J.; Pohl, M.; Mehrholz, J. Transcranial Direct Current Stimulation (tDCS) for Improving Activities of Daily Living, and Physical and Cognitive Functioning, in People after Stroke. Cochrane Database Syst. Rev. 2020, 11, CD009645. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; van Wegen, E.; van Peppen, R.; van der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What Is the Evidence for Physical Therapy Poststroke? A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Dorsch, S.; Weeks, K.; King, L.; Polman, E. In Inpatient Rehabilitation, Large Amounts of Practice Can Occur Safely without Direct Therapist Supervision: An Observational Study. J. Physiother. 2019, 65, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Pellicciari, M.C.; Bonnì, S.; Spanò, B.; Ponzo, V.; Salsano, I.; Giulietti, G.; Martino Cinnera, A.; Maiella, M.; Borghi, I.; et al. Evidence for Interhemispheric Imbalance in Stroke Patients as Revealed by Combining Transcranial Magnetic Stimulation and Electroencephalography. Hum. Brain Mapp. 2021, 42, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Heo, P.; Gu, G.M.; Lee, S.; Rhee, K.; Kim, J. Current Hand Exoskeleton Technologies for Rehabilitation and Assistive Engineering. Int. J. Precis. Eng. Manuf. 2012, 13, 807–824. [Google Scholar] [CrossRef]
- Niestanak, V.D.; Moshaii, A.A.; Moghaddam, M.M. A New Underactuated Mechanism of Hand Tendon Injury Rehabilitation. In Proceedings of the 2017 5th RSI International Conference on Robotics and Mechatronics (ICRoM), Tehran, Iran, 25–27 October 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 400–405. [Google Scholar]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and Robot-Assisted Arm Training for Improving Activities of Daily Living, Arm Function, and Arm Muscle Strength after Stroke. Cochrane Database Syst. Rev. 2018, 2018. [Google Scholar] [CrossRef]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of Brain Plasticity in Stroke: A Novel Model for Neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Di Pino, G.; Di Lazzaro, V. The Balance Recovery Bimodal Model in Stroke Patients between Evidence and Speculation: Do Recent Studies Support It? Clin. Neurophysiol. 2020, 131, 2488–2490. [Google Scholar] [CrossRef]
- Fuentes Calderón, M.A.; Miralles, A.N.; Pimienta, M.J.; Estella, J.M.G.; Ledesma, M.J.S. Analysis of the Factors Related to the Effectiveness of Transcranial Current Stimulation in Upper Limb Motor Function Recovery after Stroke: A Systematic Review. J. Med. Syst. 2019, 43, 69. [Google Scholar] [CrossRef]
- Muller, C.O.; Muthalib, M.; Mottet, D.; Perrey, S.; Dray, G.; Delorme, M.; Duflos, C.; Froger, J.; Xu, B.; Faity, G.; et al. Recovering Arm Function in Chronic Stroke Patients Using Combined Anodal HD-tDCS and Virtual Reality Therapy (ReArm): A Study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 747. [Google Scholar] [CrossRef]
- Liao, W.; Chiang, W.; Lin, K.; Wu, C.; Liu, C.; Hsieh, Y.; Lin, Y.; Chen, C. Timing-Dependent Effects of Transcranial Direct Current Stimulation with Mirror Therapy on Daily Function and Motor Control in Chronic Stroke: A Randomized Controlled Pilot Study. J. NeuroEngineering Rehabil. 2020, 17, 101. [Google Scholar] [CrossRef]
- Wang, T.; Dong, L.; Cong, X.; Luo, H.; Li, W.; Meng, P.; Wang, Q. Comparative Efficacy of Non-Invasive Neurostimulation Therapies for Poststroke Dysphagia: A Systematic Review and Meta-Analysis. Neurophysiol. Clin. 2021, 51, 493–506. [Google Scholar] [CrossRef]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; et al. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 256–313. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, D.; Zollo, L.; Milighetti, S.; Miccinilli, S.; Bravi, M.; Ranieri, F.; Magrone, G.; Guglielmelli, E.; Di Lazzaro, V.; Sterzi, S. Literature Review on the Effects of tDCS Coupled with Robotic Therapy in Post Stroke Upper Limb Rehabilitation. Front. Hum. Neurosci. 2017, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Comino-Suárez, N.; Moreno, J.C.; Gómez-Soriano, J.; Megía-García, Á.; Serrano-Muñoz, D.; Taylor, J.; Alcobendas-Maestro, M.; Gil-Agudo, Á.; del-Ama, A.J.; Avendaño-Coy, J. Transcranial Direct Current Stimulation Combined with Robotic Therapy for Upper and Lower Limb Function after Stroke: A Systematic Review and Meta-Analysis of Randomized Control Trials. J. NeuroEng. Rehabil. 2021, 18, 148. [Google Scholar] [CrossRef]
- Reis, S.B.; Bernardo, W.M.; Oshiro, C.A.; Krebs, H.I.; Conforto, A.B. Effects of Robotic Therapy Associated with Noninvasive Brain Stimulation on Upper-Limb Rehabilitation after Stroke: Systematic Review and Meta-Analysis of Randomized Clinical Trials. Neurorehabil. Neural Rep. 2021, 35, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Fonte, C.; Varalta, V.; Rocco, A.; Munari, D.; Filippetti, M.; Evangelista, E.; Modenese, A.; Smania, N.; Picelli, A. Combined Transcranial Direct Current Stimulation and Robot-Assisted Arm Training in Patients with Stroke: A Systematic Review. RNN 2021, 39, 435–446. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. The PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Veritas Health Innovation Covidence Systematic Review Software. Melbourne, Australia. Available online: www.covidence.org (accessed on 16 April 2023).
- Morone, G.; Capone, F.; Iosa, M.; Cruciani, A.; Paolucci, M.; Martino Cinnera, A.; Musumeci, G.; Brunelli, N.; Costa, C.; Paolucci, S.; et al. May Dual Transcranial Direct Current Stimulation Enhance the Efficacy of Robot-Assisted Therapy for Promoting Upper Limb Recovery in Chronic Stroke? Neurorehabil. Neural Repair. 2022, 36, 800–809. [Google Scholar] [CrossRef]
- Triccas, L.T.; Burridge, J.H.; Hughes, A.; Verheyden, G.; Desikan, M.; Rothwell, J. A Double-Blinded Randomised Controlled Trial Exploring the Effect of Anodal Transcranial Direct Current Stimulation and Uni-Lateral Robot Therapy for the Impaired Upper Limb in Sub-Acute and Chronic Stroke. NRE 2015, 37, 181–191. [Google Scholar] [CrossRef]
- Edwards, D.J.; Cortes, M.; Rykman-Peltz, A.; Chang, J.; Elder, J.; Thickbroom, G.; Mariman, J.J.; Gerber, L.M.; Oromendia, C.; Krebs, H.I.; et al. Clinical Improvement with Intensive Robot-Assisted Arm Training in Chronic Stroke Is Unchanged by Supplementary tDCS. RNN 2019, 37, 167–180. [Google Scholar] [CrossRef]
- Straudi, S.; Fregni, F.; Martinuzzi, C.; Pavarelli, C.; Salvioli, S.; Basaglia, N. tDCS and Robotics on Upper Limb Stroke Rehabilitation: Effect Modification by Stroke Duration and Type of Stroke. BioMed Res. Int. 2016, 2016, 5068127. [Google Scholar] [CrossRef]
- Hesse, S.; Waldner, A.; Mehrholz, J.; Tomelleri, C.; Pohl, M.; Werner, C. Combined Transcranial Direct Current Stimulation and Robot-Assisted Arm Training in Subacute Stroke Patients: An Exploratory, Randomized Multicenter Trial. Neurorehabil. Neural Repair. 2011, 25, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Dehem, S.; Gilliaux, M.; Lejeune, T.; Delaunois, E.; Mbonda, P.; Vandermeeren, Y.; Detrembleur, C.; Stoquart, G. Effectiveness of a Single Session of Dual-Transcranial Direct Current Stimulation in Combination with Upper Limb Robotic-Assisted Rehabilitation in Chronic Stroke Patients: A Randomized, Double-Blind, Cross-over Study. Int. J. Rehabil. Res. 2018, 41, 138–145. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Dario, P.; Posteraro, F.; Iardella, L. Effects of Combined Transcranial Direct Current Stimulation and Wrist Robot-Assisted Therapy in Subacute Stroke Patients: Preliminary Results. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), Singapore, 11–14 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 217–222. [Google Scholar]
- Mazzoleni, S.; Tran, V.D.; Iardella, L.; Dario, P.; Posteraro, F. Randomized, Sham-Controlled Trial Based on Transcranial Direct Current Stimulation and Wrist Robot-Assisted Integrated Treatment on Subacute Stroke Patients: Intermediate Results. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 555–560. [Google Scholar]
- Mazzoleni, S.; Tran, V.-D.; Dario, P.; Posteraro, F. Effects of Transcranial Direct Current Stimulation (tDCS) Combined with Wrist Robot-Assisted Rehabilitation on Motor Recovery in Subacute Stroke Patients: A Randomized Controlled Trial. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Calabrò, R.S. Improving Upper Limb and Gait Rehabilitation Outcomes in Post-Stroke Patients: A Scoping Review on the Additional Effects of Non-Invasive Brain Stimulation When Combined with Robot-Aided Rehabilitation. Brain Sci. 2022, 12, 1511. [Google Scholar] [CrossRef]
- Picelli, A.; Chemello, E.; Castellazzi, P.; Roncari, L.; Waldner, A.; Saltuari, L.; Smania, N. Combined Effects of Transcranial Direct Current Stimulation (tDCS) and Transcutaneous Spinal Direct Current Stimulation (tsDCS) on Robot-Assisted Gait Training in Patients with Chronic Stroke: A Pilot, Double Blind, Randomized Controlled Trial. RNN 2015, 33, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, G.; Wang, A.; Cheng, L.J.; Lau, Y. Robot-Assisted Distal Training Improves Upper Limb Dexterity and Function after Stroke: A Systematic Review and Meta-Regression. Neurol. Sci. 2022, 43, 1641–1657. [Google Scholar] [CrossRef]
- Lee, H.-C.; Kuo, F.-L.; Lin, Y.-N.; Liou, T.-H.; Lin, J.-C.; Huang, S.-W. Effects of Robot-Assisted Rehabilitation on Hand Function of People with Stroke: A Randomized, Crossover-Controlled, Assessor-Blinded Study. Am. J. Occup. Ther. 2021, 75, 7501205020p1–7501205020p11. [Google Scholar] [CrossRef]
- Cha, T.-H.; Hwang, H.-S. Rehabilitation Interventions Combined with Noninvasive Brain Stimulation on Upper Limb Motor Function in Stroke Patients. Brain Sci. 2022, 12, 994. [Google Scholar] [CrossRef]
- Bornheim, S.; Croisier, J.-L.; Maquet, P.; Kaux, J.-F. Transcranial Direct Current Stimulation Associated with Physical-Therapy in Acute Stroke Patients—A Randomized, Triple Blind, Sham-Controlled Study. Brain Stimul. 2020, 13, 329–336. [Google Scholar] [CrossRef]
- Fusco, A.; Assenza, F.; Iosa, M.; Izzo, S.; Altavilla, R.; Paolucci, S.; Vernieri, F. The Ineffective Role of Cathodal tDCS in Enhancing the Functional Motor Outcomes in Early Phase of Stroke Rehabilitation: An Experimental Trial. BioMed Res. Int. 2014, 2014, 1547290. [Google Scholar] [CrossRef]
- Koganemaru, S.; Fukuyama, H.; Mima, T. Two Is More Than One: How to Combine Brain Stimulation Rehabilitative Training for Functional Recovery? Front. Syst. Neurosci. 2015, 9, 154. [Google Scholar] [CrossRef]
- Capone, F.; Miccinilli, S.; Pellegrino, G.; Zollo, L.; Simonetti, D.; Bressi, F.; Florio, L.; Ranieri, F.; Falato, E.; Di Santo, A.; et al. Transcutaneous Vagus Nerve Stimulation Combined with Robotic Rehabilitation Improves Upper Limb Function after Stroke. Neural Plast. 2017, 2017, 7876507. [Google Scholar] [CrossRef]
- Hyakutake, K.; Morishita, T.; Saita, K.; Fukuda, H.; Abe, H.; Ogata, T.; Kamada, S.; Inoue, T. Effect of Robot-Assisted Rehabilitation to Botulinum Toxin A Injection for Upper Limb Disability in Patients with Chronic Stroke: A Case Series and Systematic Review. Neurol. Med. Chir. 2022, 62, 35–44. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Q.; Qu, Q.; Ding, L.; Chen, Z.; Huang, F.; Hu, S.; Deng, W.; Guo, F.; Wang, C.; et al. Comparative Effectiveness of Robot-Assisted Training Versus Enhanced Upper Extremity Therapy on Upper and Lower Extremity for Stroke Survivors: A Multicentre Randomized Controlled Trial. JRM 2022, 54, jrm00314. [Google Scholar] [CrossRef]
- Molteni, F.; Gasperini, G.; Cannaviello, G.; Guanziroli, E. Exoskeleton and End-Effector Robots for Upper and Lower Limbs Rehabilitation: Narrative Review. PMR 2018, 10, S174–S188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, G.; Cho, D.Y.; Kim, H.Y.; Lee, J.-Y.; Kim, S.; Park, S.-B.; Shin, J.-H. Comparisons between End-Effector and Exoskeleton Rehabilitation Robots Regarding Upper Extremity Function among Chronic Stroke Patients with Moderate-to-Severe Upper Limb Impairment. Sci. Rep. 2020, 10, 1806. [Google Scholar] [CrossRef]
- Krebs, H.I.; Mernoff, S.; Fasoli, S.E.; Hughes, R.; Stein, J.; Hogan, N. A Comparison of Functional and Impairment-Based Robotic Training in Severe to Moderate Chronic Stroke: A Pilot Study. NeuroRehabilitation 2008, 23, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.I.; Saitoh, E.; Hogan, N. Robotic Therapy and the Paradox of the Diminishing Number of Degrees of Freedom. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Schweighofer, N.; Wang, C.; Mottet, D.; Laffont, I.; Bakhti, K.; Reinkensmeyer, D.J.; Rémy-Néris, O. Dissociating Motor Learning from Recovery in Exoskeleton Training Post-Stroke. J. NeuroEng. Rehabil. 2018, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; MacDonald, J.R.; Reisman, D.S.; Boyd, L.; Jacobson Kimberley, T.; Schindler-Ivens, S.M.; Hornby, T.G.; Ross, S.A.; Scheets, P.L. Observation of Amounts of Movement Practice Provided During Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2009, 90, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.E.; Lohse, K.R.; Birkenmeier, R.L. Dose and Timing in Neurorehabilitation: Prescribing Motor Therapy after Stroke. Curr. Opin. Neurol. 2015, 28, 549–555. [Google Scholar] [CrossRef]
- Hayward, K.S.; Kramer, S.F.; Dalton, E.J.; Hughes, G.R.; Brodtmann, A.; Churilov, L.; Cloud, G.; Corbett, D.; Jolliffe, L.; Kaffenberger, T.; et al. Timing and Dose of Upper Limb Motor Intervention after Stroke: A Systematic Review. Stroke 2021, 52, 3706–3717. [Google Scholar] [CrossRef]
- Edwards, D.J.; Krebs, H.I.; Rykman, A.; Zipse, J.; Thickbroom, G.W.; Mastaglia, F.L.; Pascual-Leone, A.; Volpe, B.T. Raised Corticomotor Excitability of M1 Forearm Area Following Anodal tDCS Is Sustained during Robotic Wrist Therapy in Chronic Stroke. Restor. Neurol. Neurosci. 2009, 27, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Lungholt, B.K.S.; Nyborg, K.; Nielsen, J.B. Motor Skill Training Induces Changes in the Excitability of the Leg Cortical Area in Healthy Humans. Exp. Brain Res. 2004, 159, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Kaelin-Lang, A.; Sawaki, L.; Cohen, L.G. Role of Voluntary Drive in Encoding an Elementary Motor Memory. J. Neurophysiol. 2005, 93, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, G.; Ma, J.; Wang, S.; Cheng, L. Short and Long-Term Effects of Robot-Assisted Therapy on Upper Limb Motor Function and Activity of Daily Living in Patients Post-Stroke: A Meta-Analysis of Randomized Controlled Trials. J. NeuroEng. Rehabil. 2022, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pollock, A.; Pohl, M.; Kugler, J.; Elsner, B. Systematic Review with Network Meta-Analysis of Randomized Controlled Trials of Robotic-Assisted Arm Training for Improving Activities of Daily Living and Upper Limb Function after Stroke. J. NeuroEng. Rehabil. 2020, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Abbasimoshaei, A.; Chinnakkonda Ravi, A.K.; Kern, T.A. Development of a New Control System for a Rehabilitation Robot Using Electrical Impedance Tomography and Artificial Intelligence. Biomimetics 2023, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Bernhardi, L.E.; Eugenín, J. What Is Neural Plasticity? In The Plastic Brain; von Bernhardi, R., Eugenín, J., Muller, K.J., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Germany, 2017; Volume 1015, ISBN 978-3-319-62815-8. [Google Scholar]
- Chisari, C.; Fanciullacci, C.; Lamola, G. NIBS-Driven Brain Plasticity. Arch. Ital. Biol. 2015, 152, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.M.; Dundon, N.M.; Valyear, K.F. The Neural Basis of Hand Choice: An fMRI Investigation of the Posterior Parietal Interhemispheric Competition Model. NeuroImage 2019, 185, 208–221. [Google Scholar] [CrossRef]
- Finger, S. Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 95. [Google Scholar]
- Lindenberg, R.; Renga, V.; Zhu, L.L.; Nair, D.; Schlaug, G. Bihemispheric Brain Stimulation Facilitates Motor Recovery in Chronic Stroke Patients. Neurology 2010, 75, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Bradnam, L.V.; Stinear, C.M.; Barber, P.A.; Byblow, W.D. Contralesional Hemisphere Control of the Proximal Paretic Upper Limb Following Stroke. Cereb. Cortex 2012, 22, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- Iosa, M.; Morone, G.; Antonucci, G.; Paolucci, S. Prognostic Factors in Neurorehabilitation of Stroke: A Comparison among Regression, Neural Network, and Cluster Analyses. Brain Sci. 2021, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Vliet, R.; Selles, R.W.; Andrinopoulou, E.; Nijland, R.; Ribbers, G.M.; Frens, M.A.; Meskers, C.; Kwakkel, G. Predicting Upper Limb Motor Impairment Recovery after Stroke: A Mixture Model. Ann. Neurol. 2020, 87, 383–393. [Google Scholar] [CrossRef]
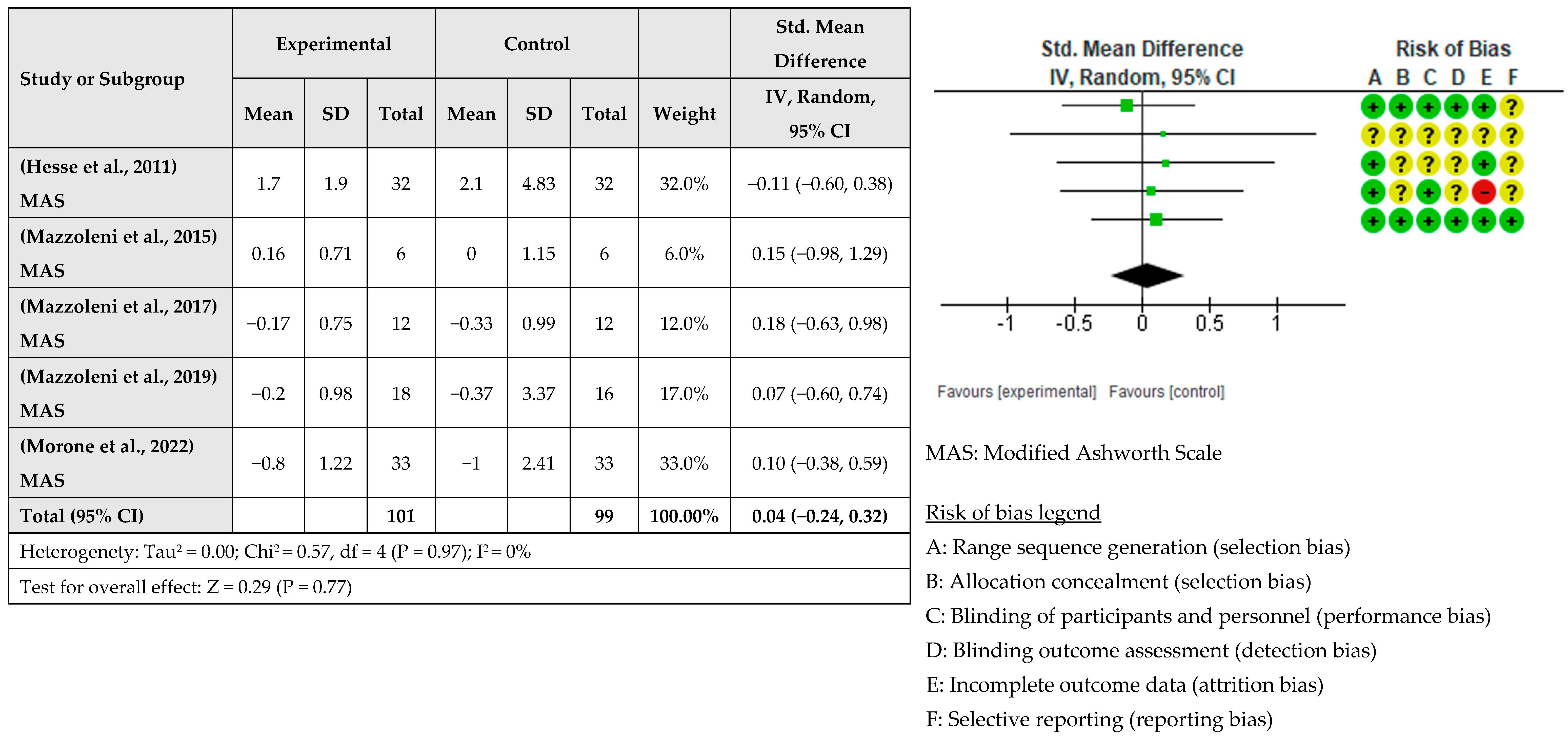
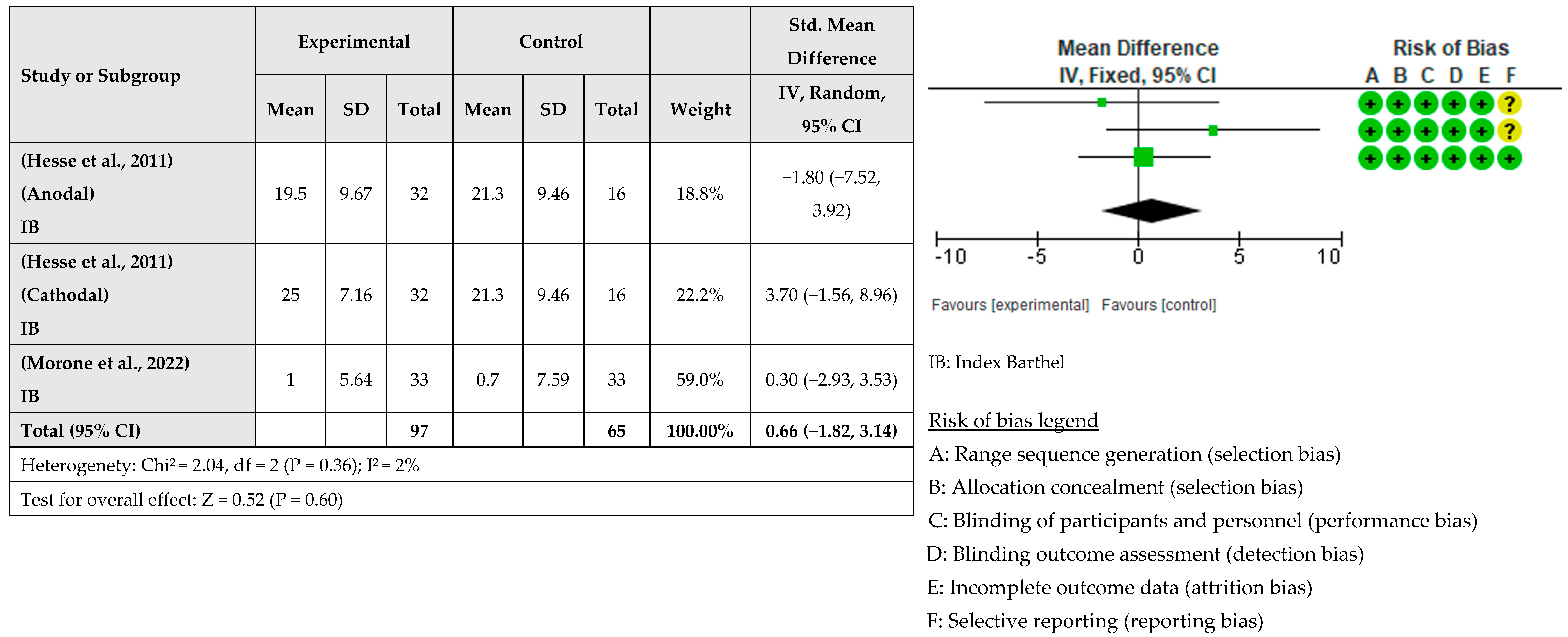
| Study | Study Design | Time Poststroke | No. of Sessions/Week | Groups | Number of Patients | Diagnosis (Hemisphere Affected) | Age (Mean ± SD) | Outcomes |
|---|---|---|---|---|---|---|---|---|
| (Morone et al., 2022) [30] | RCT | Chronic | 10 s/2 weeks | Group Ex: d-tDCS Group C: sham | 66 | 36 RH 30 LH | Group Ex: 59.7 ± 12.5 Group C: 60.2 ± 16.1 | FMA-UE; MAS; BBT; BI |
| (Triccas et al., 2015) [31] | RCT | Subacute and chronic | 18 s/8 weeks | Group Ex: a-tDCS Group C: sham | 23 | 11 LH 12 RH | Total Group: 63.4 ± 12 | FMA |
| (Edwards et al., 2019) [32] | RCT | Chronic | 36 s/12 weeks | Group Ex: a-tDCS Group C: sham | 82 | 82 RH | See footnote * | FMA-UE |
| (Straudi et al., 2016) [33] | RCT | Subacute and chronic | 10 s/2 weeks | Group Ex: d-tDCS Group C: sham | 23 | 15 LH 8 RH | Group Ex: 52.7 ± 16 Group C: 64.3 ± 9.7 | FMA; BBT |
| (Hesse et al., 2011) [34] | RCT | Subacute | 30 s/6 weeks | Group Ex1: a-tDCS Group Ex2: c-tDCS Group C: sham | 96 | 45 LH 51 RH | Group Ex1: 63.9 ± 10.5 Group Ex2: 65.4 ± 8.6 Group C: 65.6 ± 10.3 | FMA-UE; MAS; BBT; BI |
| (Dehem et al., 2018) [35] | RCT; crossover | Chronic | 2 s/1 weeks | Group Ex1: d-tDCS + ses sham Group Ex2: ses sham + d-tDCS | 21 | 11 LH 10 RH | Group Ex: 62.73 ± 8 Group C: 58.1 ± 10.8 | BBT |
| (Mazzoleni et al., 2015) [36] | RCT | Subacute | 30 s/6 weeks | Group Ex: a-tDCS Group C: sham | 12 | 4 RH 8 LH | Total Group: 75.9 ± 7 | FMA-UE; MAS; BBT |
| (Mazzoleni et al., 2017) [37] | RCT | Subacute | 30 s/6 weeks | Group Ex: a-tDCS Group C: sham | 24 | 12 RH 12 LH | Group Ex: 70.0 ± 12.8 Group C: 75.25 ± 8.01 | FMA-UE; MAS; BBT |
| (Mazzoleni et al., 2019) [38] | RCT | Subacute | 30 s/6 weeks | Group Ex: a-tDCS Group C: sham | 39 | 17 RH 22 LH | Group Ex: 67.5 ± 16.3 Group C: 68.74 ± 15.83 | FMA-UE; MAS; BBT |
| Study | Stimulated Area | Timing | tDCS Time | Characteristics | Intensity |
|---|---|---|---|---|---|
| (Morone et al., 2022) [30] | D-tDCS M1 | Online | 20 min | Electrodes 35 cm2. Saline | 2 mA |
| (Triccas et al., 2015) [31] | A-tDCS C3/C4 (M1) affected hemispheric | Online | 20 min | Electrodes 35 cm2. Saline | 1 mA |
| (Edwards et al., 2019) [32] | A-tDCS M1 | Offline pre | 20 min | Electrodes 35 cm2. Saline | 2 mA |
| (Straudi et al., 2016) [33] | D-tDCS M1 | Online | 30 min | Electrodes 35 cm2. Saline | 1 mA |
| (Hesse et al., 2011) [34] | A-tDCS: C3 C-tDCS: C3 | Online | 20 min | Electrodes 35 cm2. Saline | 2 mA |
| (Dehem et al., 2018) [35] | D-tDCS | Online | 20 min | Electrodes 35 cm2. Saline | 1 mA |
| (Mazzoleni et al., 2015) [36] | A-tDCS M1 | Online | 20 min | Electrodes 35 cm2. Saline | 2 mA |
| (Mazzoleni et al., 2017) [37] | A-tDCS M1 | Online | 20 min | Electrodes 35 cm2. Saline | 2 mA |
| (Mazzoleni et al., 2019) [38] | A-tDCS M1 | Online | 20 min | Electrodes 35 cm2. Saline | 2 mA |
| Study | Robot Device | Robot Device (End-Effector vs. Exoskeleton) | Robot Device (Bimanual vs. Unimanual) | Robot Device (Distal vs. Proximal) | DOFs (Degrees of Freedom) | Robot-Assisted Training Time | Type of Task | No. of Repetitions |
|---|---|---|---|---|---|---|---|---|
| (Morone et al., 2022) [30] | Armeo Power II | Exoskeleton | Unimanual | Distal | 6: elbow F; forearm S; wrist F; shoulder | 40 min | Exergames | No data |
| (Triccas et al., 2015) [31] | Armeo Spring | Exoskeleton | Unimanual | Proximal | No data | 75 min | Exergames | No data |
| (Edwards et al., 2019) [32] | MIT-Manus | End-effector | Unimanual | Distal | No data | 60 min | Visuomotor task | 1024 passives and assisted |
| (Straudi et al., 2016) [33] | REO Therapy System | End-effector | Unimanual | Distal | No data | 30 min | Visuomotor task | No data |
| (Hesse et al., 2011) [34] | Bi-Manu Track | End-effector | Bimanual | Distal | 2 F/E; P/S | 20 min | No data | 200 passives + 200 auto passives 800 total |
| (Dehem et al., 2018) [35] | REAplan robot | End-effector | Unimanual | Distal | No data | 20 min | Exergames | No data |
| (Mazzoleni et al., 2015) [36] | InMotion | End-effector | Unimanual | Distal | 3: F/E; P/S; ABD/ADD | No data | Visuomotor task | 960 assisted + 16 passives 976 total |
| (Mazzoleni et al., 2017) [37] | InMotion | End-effector | Unimanual | Distal | 3: F/E; P/S; ABD/ADD | No data | Visuomotor task | 960 assisted + 16 passives 976 total |
| (Mazzoleni et al., 2019) [38] | InMotion | End-effector | Unimanual | Distal | 3: F/E; P/S; ABD/ADD | No data | Visuomotor task | 960 assisted + 16 passives 976 total |
| Study | FMA-UE (Mean ± SD) | BBT (Mean ± SD) | MAS (Mean ± SD) | BI (Mean ± SD) |
|---|---|---|---|---|
| (Morone et al., 2022) [30] | Group Ex: 25.8 ± 15.2 Group C: 30.7 ± 15.0 | No data | Group Ex: 4.4 ± 2.3 Group C: 4.1 ± 1.7 | Group Ex: 85.1 ± 11.0 Group C: 79.9 ± 14.0 |
| (Triccas et al., 2015) [31] | Group Ex: 24.91 ± 16.01 Group C: 37.09 ± 13.57 | No data | No data | No data |
| (Edwards et al., 2019) [32] | Group Ex: 25.7 ± 16.3 Group C: 25.3 ± 16.3 | No data | No data | No data |
| (Straudi et al., 2016) [33] | Group Ex: 24.08 ± 16.6 Group C: 21.45 ± 13.23 | Group Ex: 10.42 ± 15.47 Group C: 6.55 ± 11.67 | No data | No data |
| (Hesse et al., 2011) [34] | Group Ex1: 7.8 ± 3.8 Group Ex2: 7.9 ± 3.4 Group C: 8.2 ± 4.4 | Group Ex1: 0 Group Ex2: 0 Group C: 0 | Group Ex1: 1.6 ± 2.9 Group Ex2: 1.0 ± 1.8 Group C: 1.4 ± 2.7 | Group Ex1: 34.1 ± 6.4 Group Ex2: 34.2 ± 7.6 Group C: 35.0 ± 7.8 |
| (Dehem et al., 2018) [35] | No data | Group R-S: 18.73 ± 13.3 Group S-R: 13.6 ± 14.3 | No data | No data |
| (Mazzoleni et al., 2015) [36] | Group Ex: 28.00 ± 20.91 Group C: 41.83 ± 14.48 | Group Ex: 11.33 ± 12.74 Group C: 20.5 ± 8.41 | Group Ex: 0.67 ± 1.21 Group C: 0.33 ± 0.81 | No data |
| (Mazzoleni et al., 2017) [37] | Group Ex: 37.33 ± 17.53 Group C: 37.83 ± 15.62 | Group Ex: 15.00 ± 9.99 Group C: 15.42 ± 9.78 | Group Ex: 0.75 ± 1.36 Group C: 0.50 ± 0.80 | No data |
| (Mazzoleni et al., 2019) [38] | Group Ex: 34.20 ± 18.35 Group C: 34.11 ± 15.48 | Group Ex: 15.95 ± 12.10 Group C: 12.32 ± 10.41 | Group Ex: 1.1 ± 1.86 Group C: 1.58 ± 2.34 | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Jiménez, J.J.; Polonio-López, B.; Sanz-García, A.; Martín-Conty, J.L.; Lerín-Calvo, A.; Segura-Fragoso, A.; Martín-Rodríguez, F.; Cantero-Garlito, P.A.; Corregidor-Sánchez, A.-I.; Mordillo-Mateos, L. Is the Combination of Robot-Assisted Therapy and Transcranial Direct Current Stimulation Useful for Upper Limb Motor Recovery? A Systematic Review with Meta-Analysis. Healthcare 2024, 12, 337. https://doi.org/10.3390/healthcare12030337
Bernal-Jiménez JJ, Polonio-López B, Sanz-García A, Martín-Conty JL, Lerín-Calvo A, Segura-Fragoso A, Martín-Rodríguez F, Cantero-Garlito PA, Corregidor-Sánchez A-I, Mordillo-Mateos L. Is the Combination of Robot-Assisted Therapy and Transcranial Direct Current Stimulation Useful for Upper Limb Motor Recovery? A Systematic Review with Meta-Analysis. Healthcare. 2024; 12(3):337. https://doi.org/10.3390/healthcare12030337
Chicago/Turabian StyleBernal-Jiménez, Juan J., Begoña Polonio-López, Ancor Sanz-García, José L. Martín-Conty, Alfredo Lerín-Calvo, Antonio Segura-Fragoso, Francisco Martín-Rodríguez, Pablo A. Cantero-Garlito, Ana-Isabel Corregidor-Sánchez, and Laura Mordillo-Mateos. 2024. "Is the Combination of Robot-Assisted Therapy and Transcranial Direct Current Stimulation Useful for Upper Limb Motor Recovery? A Systematic Review with Meta-Analysis" Healthcare 12, no. 3: 337. https://doi.org/10.3390/healthcare12030337
APA StyleBernal-Jiménez, J. J., Polonio-López, B., Sanz-García, A., Martín-Conty, J. L., Lerín-Calvo, A., Segura-Fragoso, A., Martín-Rodríguez, F., Cantero-Garlito, P. A., Corregidor-Sánchez, A.-I., & Mordillo-Mateos, L. (2024). Is the Combination of Robot-Assisted Therapy and Transcranial Direct Current Stimulation Useful for Upper Limb Motor Recovery? A Systematic Review with Meta-Analysis. Healthcare, 12(3), 337. https://doi.org/10.3390/healthcare12030337










