Effects of Barefoot Walking in Urban Forests on CRP, IFNγ, and Serotonin Levels
Abstract
1. Introduction
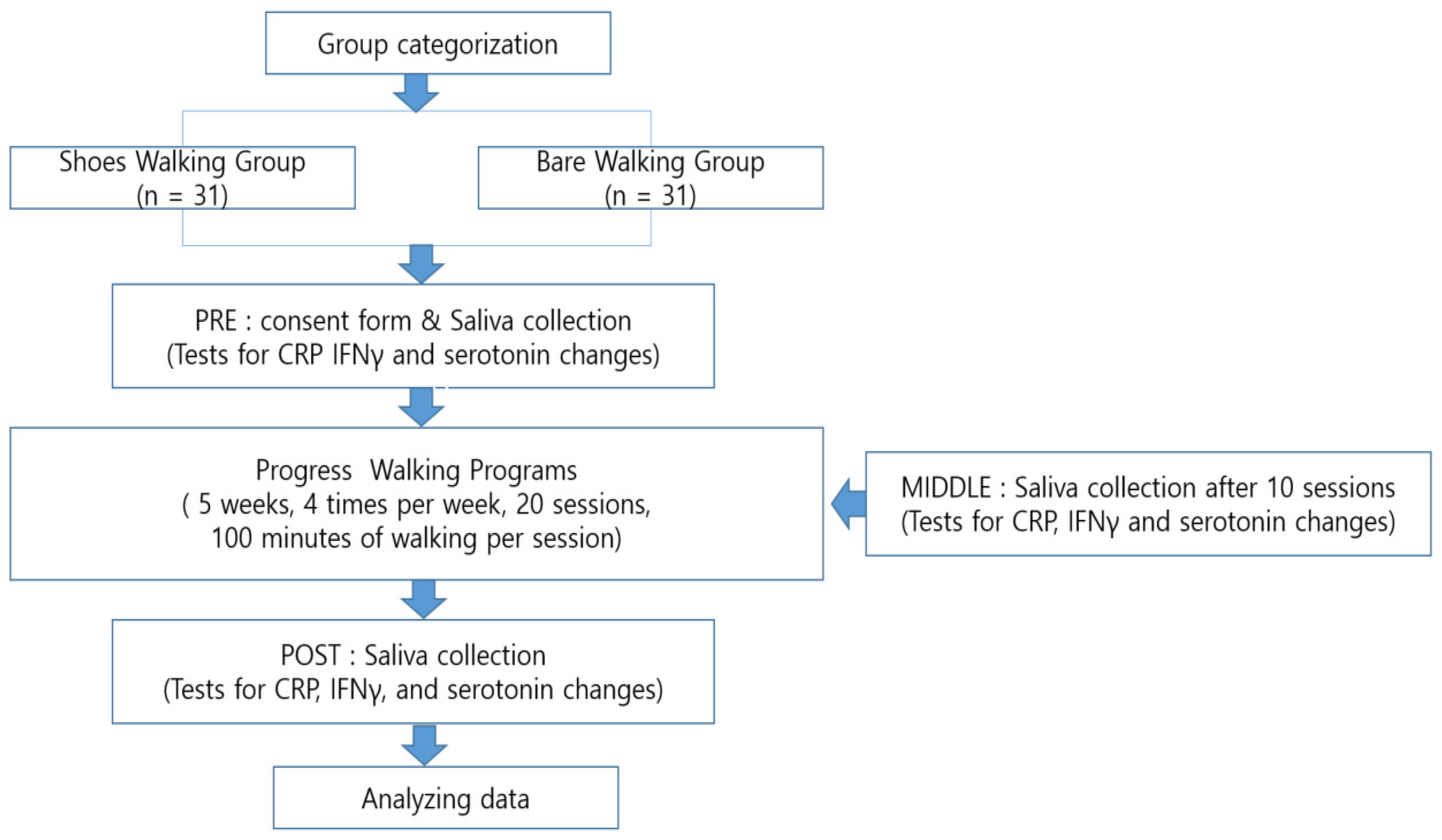
2. Materials and Methods
2.1. Participants
2.2. Site
2.3. Methodology
2.4. Walking Program
2.4.1. Program Configuration
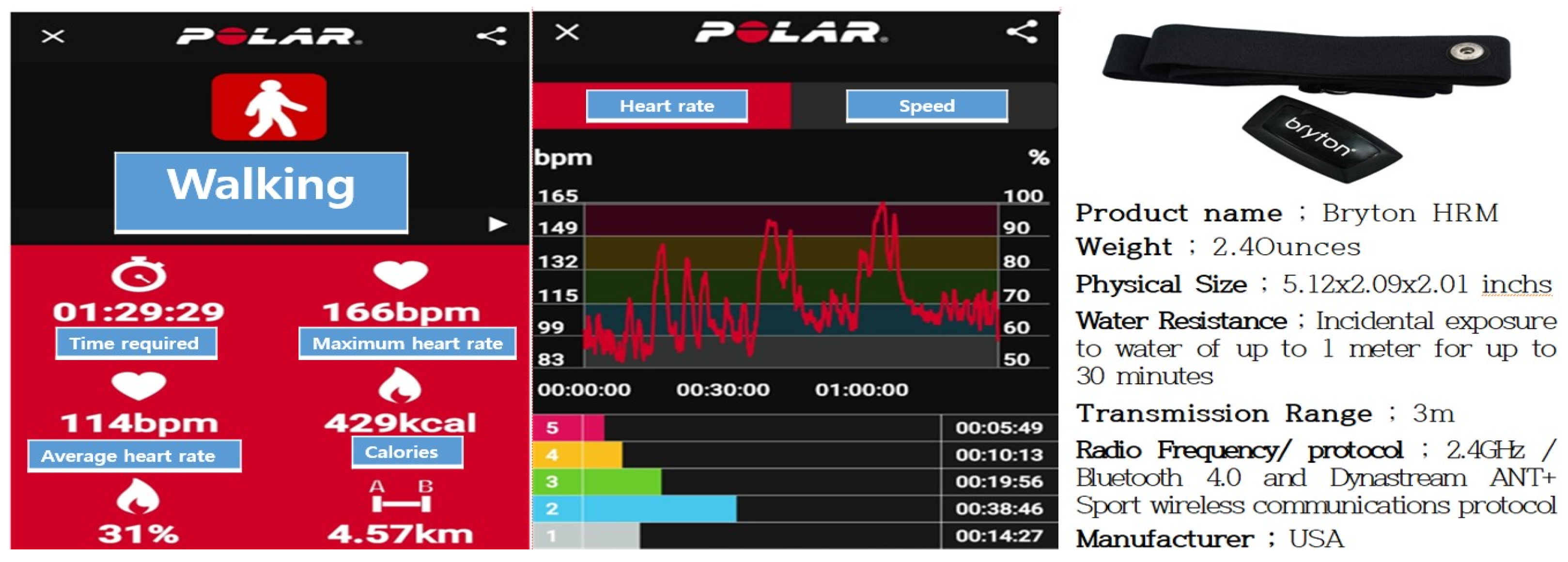
2.4.2. Exercise Intensity Measurement
2.5. Analyses
2.5.1. Saliva Analysis
2.5.2. Data Analysis
3. Results
3.1. General Characteristics
3.2. Preliminary Homogeneity Test
3.2.1. Physical Characteristics
3.2.2. Physiological Characteristics
3.2.3. Normality Tests
3.3. Physiological Change Measurement Results
3.3.1. CRP
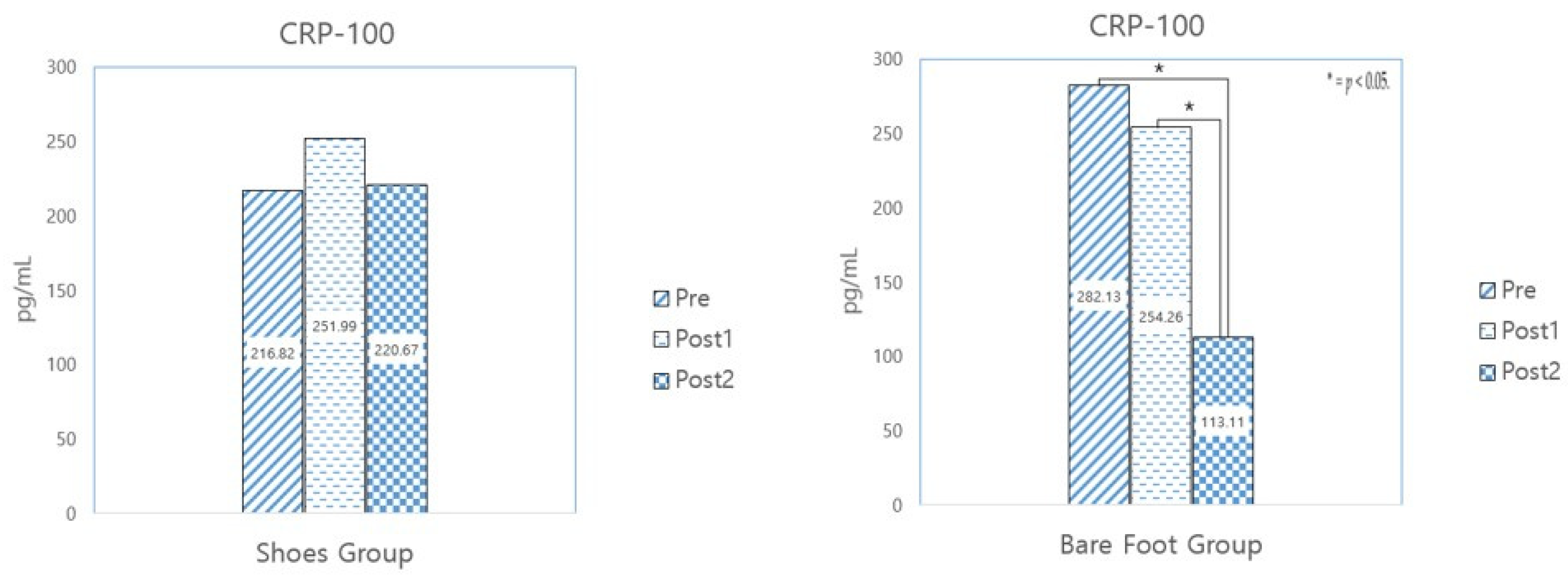
3.3.2. CRP-100
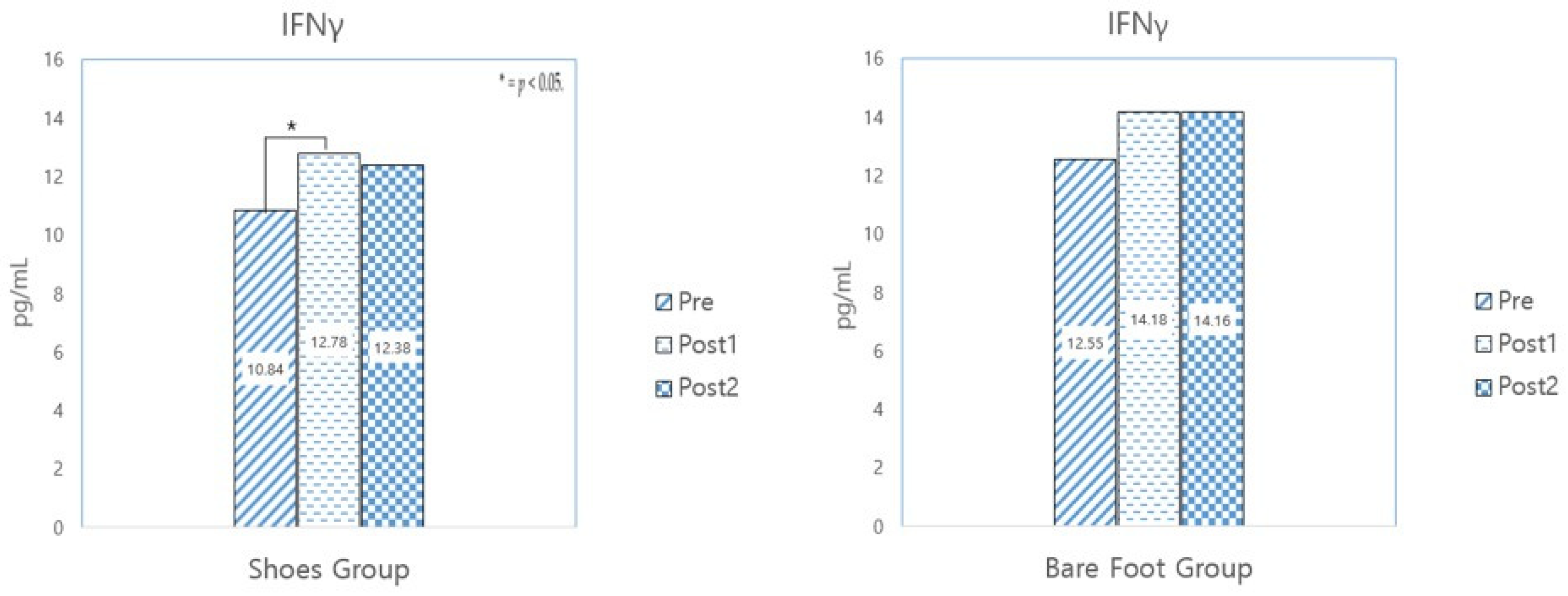
3.3.3. IFNγ
3.3.4. Serotonin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Ulrich, R.S. Aesthetic and Affective Response to Natural Environment. In Behavior and the Natural Environment; Springer: Berlin/Heidelberg, Germany, 1983; pp. 85–125. [Google Scholar] [CrossRef]
- Ulrich, R.S. Natural versus urban scenes: Some psychophysiological effects. Environ. Behav. 1981, 13, 523–556. [Google Scholar] [CrossRef]
- Bernstein, A. Wilderness as a therapeutic behavior setting. Ther. Recreat. J. Fourth Quart. 1972, 161–165. [Google Scholar]
- Orians, G.H. Habitat selection: General theory and applications to human behavior. In The Evolution of Human Social Behavior; Lockard, J.S., Ed.; Elsevier: New York, NY, USA, 1980; pp. 49–66. [Google Scholar]
- Orians, G.H. An ecological and evolutionary approach to landscape aesthetics. In Landscape Meanings and Values; Penning-Rowsell, E.C., Lowenthal, D., Eds.; Allen and Unwin: London, UK, 1986; pp. 4–25. [Google Scholar]
- Korea Ministry of Government Legislation. Forest Culture and Recreation Law. Available online: https://moleg.go.kr/ (accessed on 23 May 2024).
- Kim, M.J.; Lee, J.W.; Kim, K.W.; Lee, Y.H.; Choi, Y.H.; Park, B.J. An application of major facilities for forest therapy. J. Korean Inst. For. Rec. 2011, 2, 65–68. [Google Scholar]
- Kang, J.S. Effects of 10 Week Forest Trekking on Isokinetic Muscle Function, Dynamic Balance, Cardiorespiratory Endurance and Immune Function in Middle Aged Women. Doctoral Dissertation, Dankook University, Yongin-si, Republic of Korea, 2021. [Google Scholar]
- Shin, M.J.; Shin, C.S.; Lee, M.O.; Lee, J.S.; Choi, A.R.; Choi, J.H. Effects of exercise program with different forest slopes on BDNF and depression of women in 50s. J. Korean Inst. For. Rec. 2015, 19, 109–115. [Google Scholar]
- Shin, J.W. Effects of Single Session Forest Walking on Physiological and Psychological State of Myocardial Infarction Patients. Master’s Thesis, Chungbuk National University, Cheongju-si, Republic of Korea, 2016. [Google Scholar]
- Lee, J.S. The Effect of 12-Weeks Forest Walking on Functional Fitness, Self-Efficacy, and Stress in the Middle-Aged Women. Master’s Thesis, Chungbuk National University, Cheongju-si, Republic of Korea, 2016. [Google Scholar]
- Lee, M.O. The Effect of Forest Walking-Exercise on Immune Function and Antioxidant Hormone of Women in Their 50s. Doctoral Dissertation, Chungbuk National University, Cheongju-si, Republic of Korea, 2018. [Google Scholar]
- So, J.R. Foot Reflex Health Act; Truth Seeking Publisher: Seoul, Republic of Korea, 1998. [Google Scholar]
- Jones, J.; Thomson, P.; Lauder, W.; Howie, K.; Leslie, S.J. Reflexology has an acute (immediate) haemodynamic effect in healthy volunteers: A double-blind randomised controlled trial. Complement. Ther. Clin. Pract. 2012, 18, 204–211. [Google Scholar] [CrossRef]
- Park, S.H. Effects of foot-reflexology massage on body weight, lower extremity edema and serum lipids in postpartum women. Korean J. Women Health Nurs. 2007, 13, 105–114. [Google Scholar] [CrossRef]
- Lee, O.K.; Kim, E.J. The effect of foot reflex-massage on EEG variation & blood velocity. Korean J. Aesthet. Cosmet. Soc. 2009, 7, 131–144. [Google Scholar]
- Ober, C.; Sinatra, S.; Zucker, M. Earthing Contact with the Ground Heal; Hinausystems Publisher: Seoul, Republic of Korea, 2011. [Google Scholar]
- Oschman, J.L.; Chevalier, G.; Brown, R. The effects of grounding (earthing) on inflammation, the immune response, wound healing, and prevention and treatment of chronic inflammatory and autoimmune diseases. J. Inflamm. Res. 2015, 8, 83–96. [Google Scholar] [CrossRef]
- Park, J.P. The Effects of Bare Feet Walking Exercise on the Body Composition and Blood Lipid Profiles of Obese Fe-male-Students in the Middle School. Master’s Thesis, Changwon National University, Changwon-si, Republic of Korea, 2001. [Google Scholar]
- Kim, T.H. The effect of barefoot walking on waist to hip ratio and physiological variables among obese male middle school students. J. Hum. Soc. Sci. 2020, 11, 951–961. [Google Scholar]
- Choi, K.S. Effects of Barefoot Walking on the Health Related Physical Fitness and Physical Self-Efficacy in the Male High School Students’. Master’s Thesis, Korea Teachers’ University, Cheongju-si, Republic of Korea, 2020. [Google Scholar]
- Lee, G.I. The Effect of Barefoot Walking on the Sandy Beach on Pain, Motor Function, Sleep Satisfaction, and Quality of Life in the Elderly with Low Back Pain. Master’s Thesis, University of Dental Medicine and Dentistry, Seoul, Republic of Korea, 2019. [Google Scholar]
- Kwon, T.H. Qualitative research on the barefoot walking experience of elementary and middle school teachers. J. Hum. Soc. Sci. 2017, 8, 261–273. [Google Scholar]
- Lee, G.N. Collaborative Action Research on the Curriculum Operation for the Barefoot-Walking Experience Activity. Doctoral Dissertation, International Graduate School of Brain Education, Cheonan-si, Republic of Korea, 2020. [Google Scholar]
- Han, S.M. Phenomenological study on barefoot walking experiences of middle-aged women. J. Hum. Soc. Sci. 2020, 11, 767–782. [Google Scholar]
- Jung, J.S. Effects of 8 Weeks of Walking and Barefoot Walking on Blood Serotonin, Cortisol, Epinephrine, and Norepi-nephrine in Middle-Aged Women. Master’s Thesis, Daejeon National University, Daejeon, Republic of Korea, 2018. [Google Scholar]
- Ho, E.S. Effects of Barefoot Walking Exercise on Female Hormone, Brain Nerve Growth Factors and Immunoglobulins in Postmenopausal Women. Doctoral Dissertation, Korea Teachers University, Cheongju-si, Republic of Korea, 2021. [Google Scholar]
- Jung, H.J. Effects of Barefoot Walking on Forest Roads on Vascular Health, Stress and Well-being. Master’s Thesis, Chungbuk National University, Cheongju-si, Republic of Korea, 2023. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, be-havioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- In, J.Y. Considerations when calculating the sample size for an inequality test. Korean J. Anesthesiol. 2016, 69, 327–331. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 2013. Available online: https://books.google.co.in/books/about/ACSM_s_Guidelines_for_Exercise_Testing_a.html?id=TtiCAwAAQBAJ&redir_esc=y (accessed on 23 May 2024).
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Park, H.S. Effects of weight loss on immune function and inflammatory cytokines in judo athletes. J. Sport Leis. Stud. 2010, 42, 873–889. [Google Scholar] [CrossRef]
- Woods, J.A.; Vieira, V.J.; Keylock, K.T. Exercise, inflammation, and innate immunity. Immunol. Allergy Clin. N. Am. 2009, 29, 381–393. [Google Scholar] [CrossRef]
- Seo, Y.K.; Kwak, Y.S. Exercise and cancer protection: From ‘prevention’ to ‘therapeutics’? Korean J. Sport Sci. 2020, 31, 603–611. [Google Scholar] [CrossRef]
- Nimmo, M.A.; Leggate, M.; Viana, J.L.; King, J.A. The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology 2002, 13, 561–568. [Google Scholar]
- Karstoft, K.; Pedersen, B.K. Exercise and type 2 diabetes: Focus on metabolism and inflammation. Immunol. Cell Biol. 2016, 94, 146–150. [Google Scholar] [CrossRef]
- Tan, T.-T.; Coussens, L.M. Humoral immunity, inflammation and cancer. Curr. Opin. Immunol. 2007, 19, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Coventry, B.J.; Ashdown, M.L.; Quinn, M.A.; Markovic, S.N.; Yatomi-Clarke, S.L.; Robinson, A.P. CRP identifies homeo-static immune oscillations in cancer patients: A potential treatment targeting tool? J. Transl. Med. 2009, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Karin, M. The wolf in sheep’s clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 2008, 14, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Song, H.G. The Story of Inflammation and Immunity; BookLab Publishers: Jeonju-si, Republic of Korea, 2017. [Google Scholar]
- Woods, J.A.; Wilund, K.R.; Martin, S.A.; Kistler, B.M. Exercise, inflammation and aging. Aging Dis. 2012, 3, 130–140. [Google Scholar] [PubMed]
- Kim, H.J.; Ji, S.H. Insulin resistance and cancer. Korean J. Epidemiol. 2005, 27, 38–50. [Google Scholar]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Albert, M.A.; Glynn, R.J.; Ridker, P.M. Effects of physical activity on serum C-reactive protein. Am. J. Cardiol. 2004, 93, 221–225. [Google Scholar] [CrossRef]
- Whiteside, T.L. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin. Cancer Biol. 2006, 16, 3–15. [Google Scholar] [CrossRef]
- Yang, E.S.; Seo, S.H.; Jung, K.S.; Yoon, H.R.; Lee, D.H.; Hwang, B.G. The effects of short-term meditation and walking exercise in a forest on blood pressure, heart rate, NK-cell and POMS. Asian J. Kinesiol. 2011, 13, 31–40. [Google Scholar]
- Lim, J.G. The Effects of the Differences of Place Environment on Blood Pressure, Resting Heart Rate, Cortisol, Serotonin, NK-Cell, and Mood State During Meditation and Walking Exercise. Doctoral Dissertation, Hallim University, Chuncheon-si, Republic of Korea, 2019. [Google Scholar]
- Sapolsky, R.M. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 2000, 57, 925–935. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Dechelotte, P. The new link between gut–brain axis and neuropsychiatric disorders. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The microbiota-gut-brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Habib, K.E.; Weld, K.P.; Rice, K.C. Oral Administration of a Corticotropin-Releasing Hormone Receptor Antagonist Sig-nificantly Attenuates Behavioral, Neuroendocrine, and Autonomic Responses to Stress in Primates. Proc. Natl. Acad. Sci. USA 2020, 23, 6079–6084. [Google Scholar]
- Cho, J.H.; Ha, S.Y. Effects of exercise intensity on serotonin and cortisol levels in middle-aged women. Korean J. Sport 2016, 1, 383–392. [Google Scholar]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Lawrence, A.; Potempa, L.A. C-reactive protein and cancer—Diagnostic and therapeutic insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef]



| Separation | All (n = 62) | SWG (n = 31) | BWG (n = 31) | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age range | 40 s | 5 | 8.1 | 4 | 12.9 | 1 | 3.2 |
| 50 s | 26 | 41.9 | 12 | 38.7 | 14 | 45.2 | |
| 60 s | 27 | 43.5 | 12 | 38.7 | 15 | 48.4 | |
| 70 s | 4 | 6.5 | 3 | 9.7 | 1 | 3.2 | |
| Exercise | Duration | Frequency | Total Number | Distance | Workout Intensity | Activity Durations |
|---|---|---|---|---|---|---|
| Walking | 5 weeks | 4 times a week | 20 | 4.4 km | THR; 50–70% RPE; 10–13 | Warm-up: 5 min Main exercise: 90 min Cool-down: 5 min |
| Substance Category | Analysis Material |
|---|---|
| CRP | Salivary CRP ELISA kit (Generation II), (Cat. No. 1-2102), Salimetrics Ltd. (Carlsbad, CA, USA) |
| IFNγ | Human IFNγ high-sensitivity ELISA kit, (ab236895), Abcam Ltd. (Cambridge, UK) |
| Serotonin | Serotonin ELISA kit, (ADI-900-175), Enzo Ltd. (Farmingdale, NY, USA) |
| Demographic | Variable | SWG (N = 31) | BWG (N = 31) | p |
|---|---|---|---|---|
| M ± SE | M ± SE | |||
| Age (years) | - | 58.74 ± 4.46 | 59.58 ± 5.01 | 0.605 |
| Age range | 40 s | 4 (12.9%) | 1 (3.2%) | 0.349 |
| 50 s | 12 (38.7%) | 14 (45.2%) | ||
| 60 s | 12 (38.7%) | 15 (48.4%) | ||
| 70 s | 3 (9.7%) | 1 (3.2%) | ||
| Sex | Male | 6 (19.4%) | 6 (19.4%) | 1.00 |
| Female | 25 (80.6%) | 25 (80.6%) | ||
| Alcohol consumption | Yes | 7 (22.6%) | 12 (38.7%) | 0.168 |
| No | 24 (77.4%) | 19 (61.3%) | ||
| Smoking | Yes | 0 (0.0%) | 1 (1.6%) | 0.313 |
| No | 31 (100%) | 30 (98.4%) | ||
| Regular exercise | Yes | 24 (77.4%) | 26 (83.9%) | 0.520 |
| No | 7 (22.6%) | 5 (16.1%) | ||
| Exercise routine (Number of weekly exercise sessions) | None | 11 (35.5%) | 8 (25.8%) | 0.536 |
| Under 3 | 11 (35.5%) | 10 (32.3%) | ||
| 4 or more | 9 (29.0%) | 13 (41.9%) | ||
| Daily exercise duration (h) | 0 | 10 (32.3%) | 8 (25.8%) | 0.755 |
| Under 1 | 5 (16.1%) | 3 (9.7%) | ||
| Under 2 | 10 (32.3%) | 12 (38.7%) | ||
| 2 or more | 6 (19.3%) | 8 (25.8%) | ||
| Health condition | - | 3.26 ± 0.86 | 3.35 ± 0.61 | 0.231 |
| Regular eating habits | - | 3.45 ± 0.85 | 3.42 ± 0.62 | 0.060 |
| Favorite foods | Meat | 0 (0.0%) | 1 (3.2%) | 0.455 |
| Vegetables | 4 (12.9%) | 6 (19.4%) | ||
| Balanced | 27 (87.1%) | 24 (77.4%) |
| Variable | SWG (N = 31) | BWG (N = 31) | t | p |
|---|---|---|---|---|
| Age (years) | 58.74 ± 7.46 | 59.58 ± 5.01 | −0.520 | 0.605 |
| Height (cm) | 160.84 ± 5.49 | 161.08 ± 0.35 | −0.161 | 0.873 |
| Weight (kg) | 57.02 ± 7.40 | 59.55 ± 7.50 | −1.338 | 0.186 |
| BMI (kg/m2) | 21.99 ± 2.14 | 22.90 ± 2.00 | −1.720 | 0.091 |
| Variable | SWG (N = 31) | BWG (N = 31) | t | p |
|---|---|---|---|---|
| CRP (pg/mL) | 98.75 ± 107.50 | 144.80 ± 191.29 | −1.169 | 0.248 |
| IFNγ (pg/mL) | 10.84 ± 3.13 | 12.55 ± 4.25 | −1.810 | 0.075 |
| Serotonin (ng/mL) | 0.070 ± 0.04 | 0.053 ± 0.04 | 1.669 | 0.100 |
| Variable | Group | Skewness | Kurtosis | Shapiro–Wilk |
|---|---|---|---|---|
| Lg10_CRP (pg/mL) | SWG (N = 31) | −0.495 | 0.144 | 0.111 |
| BWG (N = 31) | −0.111 | −1.138 | 0.153 | |
| Lg10_IFNγ (pg/mL) | SWG (N = 31) | −0.824 | 0.193 | 0.049 |
| BWG (N = 31) | −0.996 | −0.027 | 0.002 | |
| Lg10_Serotonin (ng/mL) | SWG (N = 31) | −0.770 | 1.398 | 0.138 |
| BWG (N = 31) | −1.355 | 2.451 | 0.006 |
| Variable | Group | Pre | Post1 | Post2 | Pre-Post1 | Post1-Post2 | Pre-Post2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Program | After 10 Sessions | After 20 Sessions | t | p | t | p | t | p | ||
| CRP (pg/mL) | SWG (N = 31) | 98.74 ± 107.50 | 129.11 ± 174.31 | 185.15 ± 213.76 | −0.976 | 0.337 | −1.265 | 0.216 | −2.085 | 0.046 * |
| BWG (N = 31) | 144.80 ± 191.29 | 196.94 ± 232.65 | 123.67 ± 137.39 | −1.110 | 0.276 | 1.156 | 0.140 | 0.519 | 0.608 | |
| Variable | Group | Pre | Post1 | Post2 | Pre-Post1 | Post1-Post2 | Pre-Post2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Program | After 10 Sessions | After 20 Sessions | t | p | t | p | t | p | ||
| CRP-100 (pg/mL) | SWG (N = 9) | 216.82 ± 137.81 | 251.99 ± 265.98 | 220.67 ± 169.74 | −0.345 | 0.739 | 0.308 | 0.766 | −0.048 | 0.963 |
| BWG (N = 14) | 282.13 ± 215.26 | 254.26 ± 233.00 | 113.11 ± 93.28 | 0.354 | 0.729 | 2.445 | 0.030 | 3.027 | 0.010 * | |
| Variable | Group | Pre | Post1 | Post2 | Pre-Post1 | Post1-Post2 | Pre-Post2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Program | After 10 Sessions | After 20 Sessions | t | p | t | p | t | p | ||
| IFNγ (pg/mL) | SWG (N = 31) | 10.84 ± 3.13 | 12.78 ± 2.47 | 12.38 ± 3.23 | −3.095 | 0.004 * | 0.555 | 0.583 | −1.800 | 0.082 |
| BWG (N = 31) | 12.55 ± 4.25 | 14.18 ± 4.29 | 14.16 ± 6.31 | −1.624 | 0.115 | 0.017 | 0.986 | −1.416 | 0.167 | |
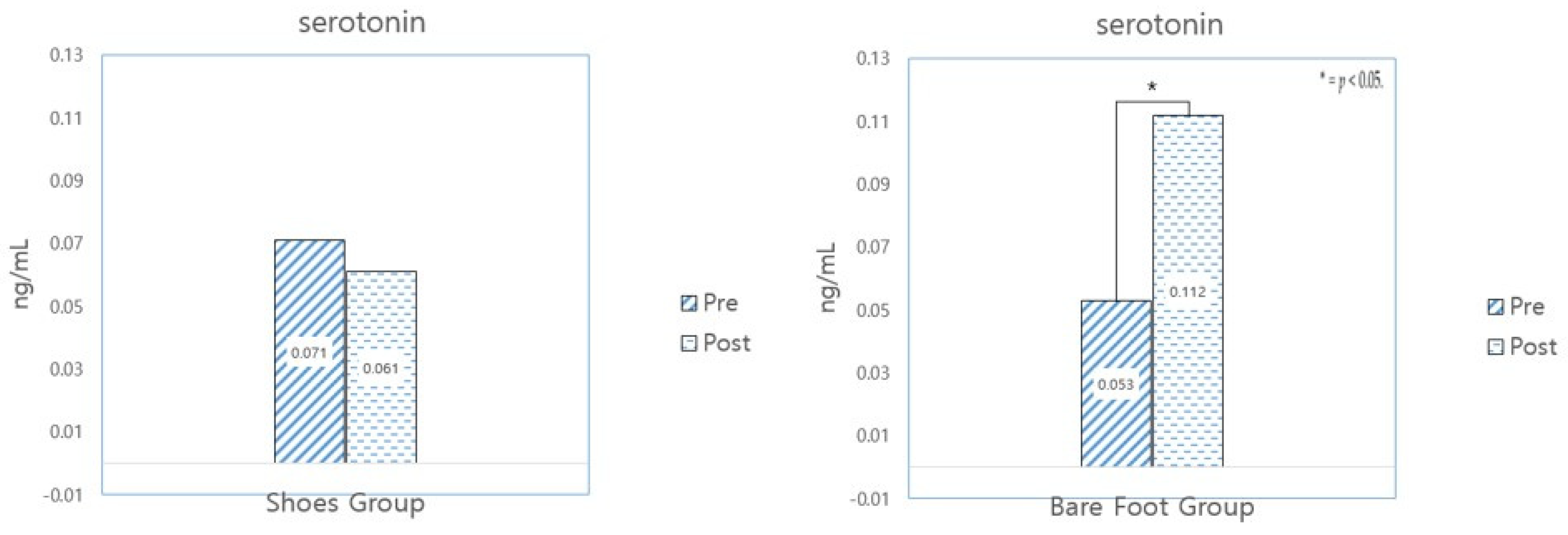
| Variable | Group | Pre | Post | t | p |
|---|---|---|---|---|---|
| Before Program | After 20 Sessions | ||||
| Serotonin (ng/mL) | SWG (N = 31) | 0.071 ± 0.44 | 0.061 ± 0.62 | 0.710 | 0.483 |
| BWG (N = 31) | 0.053 ± 0.040 | 0.112 ± 0.160 | −2.081 | 0.046 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.S.; Lee, M.M.; Kim, D.S.; Shin, C.S. Effects of Barefoot Walking in Urban Forests on CRP, IFNγ, and Serotonin Levels. Healthcare 2024, 12, 2372. https://doi.org/10.3390/healthcare12232372
Kim JS, Lee MM, Kim DS, Shin CS. Effects of Barefoot Walking in Urban Forests on CRP, IFNγ, and Serotonin Levels. Healthcare. 2024; 12(23):2372. https://doi.org/10.3390/healthcare12232372
Chicago/Turabian StyleKim, Jae Sun, Mi Mi Lee, Dong Soo Kim, and Chang Seob Shin. 2024. "Effects of Barefoot Walking in Urban Forests on CRP, IFNγ, and Serotonin Levels" Healthcare 12, no. 23: 2372. https://doi.org/10.3390/healthcare12232372
APA StyleKim, J. S., Lee, M. M., Kim, D. S., & Shin, C. S. (2024). Effects of Barefoot Walking in Urban Forests on CRP, IFNγ, and Serotonin Levels. Healthcare, 12(23), 2372. https://doi.org/10.3390/healthcare12232372





