Overuse of Computed Tomography Pulmonary Angiography and Low Utilization of Clinical Prediction Rules in Suspected Pulmonary Embolism Patients at a Regional Australian Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
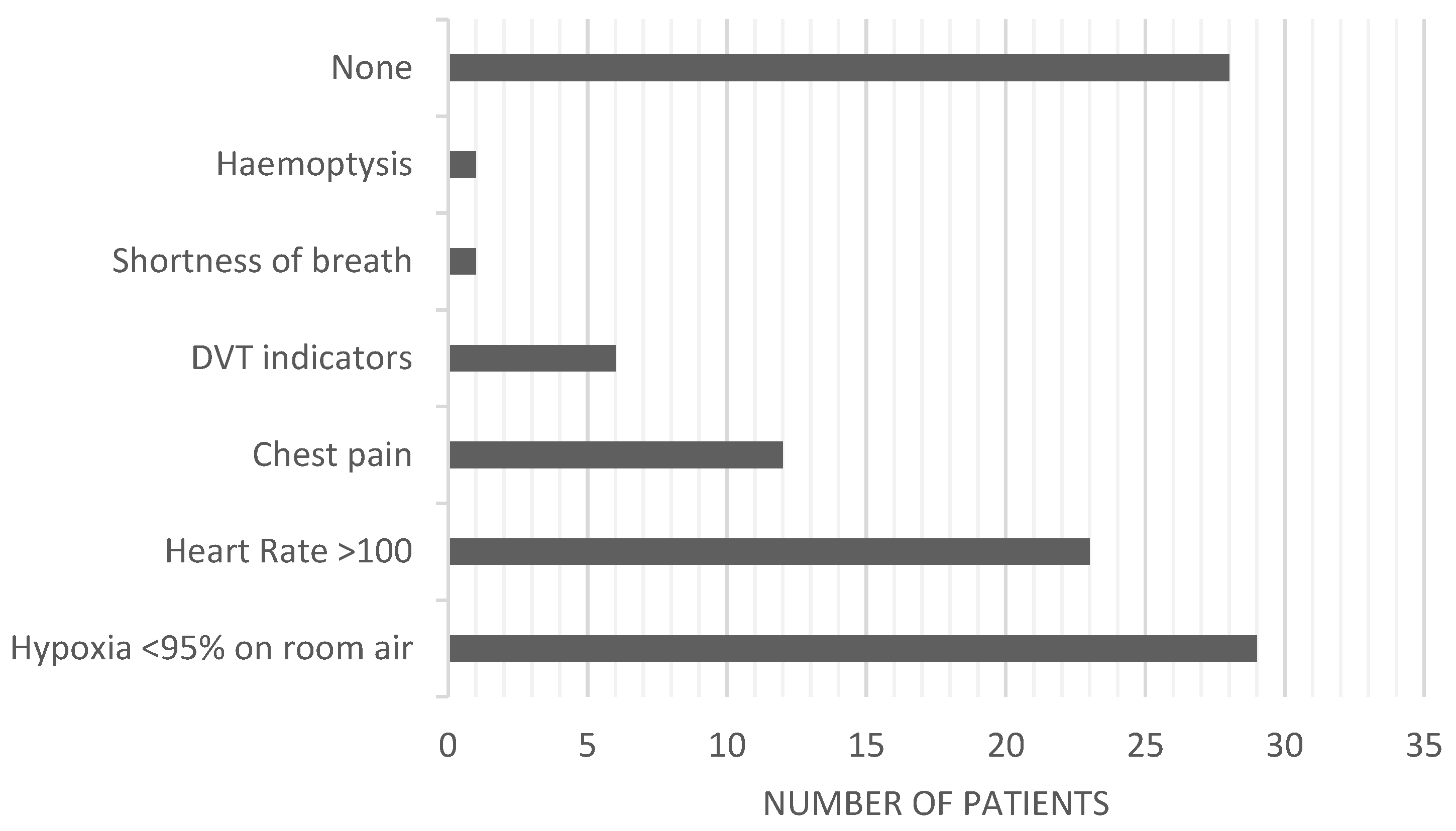
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turetz, M.; Sideris, A.T.; Friedman, O.A.; Triphathi, N.; Horowitz, J.M. Epidemiology, pathophysiology, and natural history of pulmonary embolism. Semin. Interv. Radiol. 2018, 35, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, M.; van Es, N.; Buller, H.R. Deep vein thrombosis and pulmonary embolism. Lancet 2016, 388, 3060–3073. [Google Scholar] [CrossRef] [PubMed]
- Righini, M.; Robert-Ebadi, H.; Le Gal, G. Diagnosis of acute pulmonary embolism. J. Thromb. Haemost. 2017, 15, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.S.; Anderson, D.R.; Rodger, M.; Stiell, I.; Dreyer, J.F.; Barnes, D.; Forgie, M.; Kovacs, G.; Ward, J.; Kovacs, M.J. Excluding pulmonary embolism at the bedside without diagnostic imaging: Management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Annals Inten. Med. 2001, 135, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Doherty, S. Pulmonary embolism: An update. Aust. Fam. Physician 2017, 46, 816–820. [Google Scholar] [PubMed]
- Duffett, L.; Castellucci, L.A.; Forgie, M.A. Pulmonary embolism: Update on management and controversies. BMJ 2020, 370, m2177. [Google Scholar] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Medson, K.; Yu, J.; Liwenborg, L.; Lindholm, P.; Westerlund, E. Comparing ‘clinical hunch’against clinical decision support systems (PERC rule, wells score, revised Geneva score and YEARS criteria) in the diagnosis of acute pulmonary embolism. BMC Pulm. Med. 2022, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.W.; Malipatil, V.; Lavercombe, M.; Teo, K.G.; Coughlin, P.B.; Leach, D.; Spanger, M.C.; Thien, F. Implementation of a clinical prediction tool for pulmonary embolism diagnosis in a tertiary teaching hospital reduces the number of computed tomography pulmonary angiograms performed. Intern. Med. J. 2013, 43, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Youens, D.; Doust, J.; Ha, N.T.; O’Leary, P.; Wright, C.; Parizel, P.M.; Moorin, R. Computed Tomography Angiography for Detection of Pulmonary Embolism in Western Australia Shows Increasing Use with Decreasing Diagnostic Yield. J. Clin. Med. 2023, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- OsmOsman, M.; Subedi, S.K.; Ahmed, A.; Khan, J.; Dawood, T.; Rios-Bedoya, C.F.; Bachuwa, G. Computed tomography pulmonary angiography is overused to diagnose pulmonary embolism in the emergency department of academic community hospital. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kline, J.A.; Garrett, J.S.; Sarmiento, E.J.; Strachan, C.C.; Courtney, D.M. Over-Testing for Suspected Pulmonary Embolism in American Emergency Departments: The Continuing Epidemic. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e005753. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.; Kourounis, G.; Trenear, R.; Messow, C.M.; Hrobar, P.; Mackay, A.; Isles, C. ECG in suspected pulmonary embolism. Postgrad. Med. J. 2019, 95, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Goldhaber, S.Z.; Henry, J.W.; Miller, A.C. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest 1996, 109, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Parsaik, A.K.; Agarwal, D.; Surana, A.; Mascarenhas, S.S.; Chandra, S. Diagnostic accuracy of pulmonary embolism rule-out criteria: A systematic review and meta-analysis. Ann. Emerg. Med. 2012, 59, 517–520.e4. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Tan, C.; Hilden, P.; Gesner, L.; Julius, B. Effectiveness of Clinical Decision Tools in Predicting Pulmonary Embolism. Pulm. Med. 2021, 2021, 8880893. [Google Scholar] [CrossRef] [PubMed]
- Sud, R.; Langfield, J.; Chu, G. Heightened clinical suspicion of pulmonary embolism and disregard of the D-dimer assay: A contemporary trend in an era of increased access to computed tomography pulmonary angiogram? Intern. Med. J. 2013, 43, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Goel, A. A study of modified Wells score for pulmonary embolism and age-adjusted D-dimer values in patients at risk for deep venous thrombosis. J. Family Med. Prim. Care 2023, 12, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.A.; Riordan, R.L.; Fraser, K.L.; Buchanan, J.D.; Goulston, K.J. The challenge of locum working arrangements in New South Wales public hospitals. Med. J. Aust. 2006, 185, 276–278. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Options |
|---|---|
| 1: Age | |
| 2: Gender |
|
| 3: Symptom Duration |
|
| 4: Signs and Symptoms |
|
| 5: Risk Factors |
|
| 6: Co-Morbidities |
|
| 7: D-Dimer (Age-Adjusted) |
|
| 8: Troponin |
|
| 9. Arterial Blood Gas (ABG) |
|
| 10: B-type Natriuretic Peptide (BNP) |
|
| 11. Electrocardiogram |
|
| 12. Chest X-ray |
|
| 13. PERC Rule |
|
| 14. Geneva Score |
|
| 15. Wells’ Score |
|
| 16. CTPA Findings | |
| 17. Initial Treatment for PE |
|
| Total (n = 100) | |
|---|---|
| Age, yrs, median (IQR) | 62 (46–72) |
| Female gender | 63 (63%) |
| Symptom duration | |
| 86 (86%) |
| 14 (14%) |
| Risk factors | |
| 20 (20%) |
| 8 (8%) |
| 2 (2%) |
| 13 (13%) |
| 57 (57%) |
| Co-morbidities | |
| 5 (5%) |
| 11 (11%) |
| 7 (7%) |
| 77 (77%) |
| Variable | No PE | PE Present | χ2 | p |
|---|---|---|---|---|
| D-Dimer | 0.38 | 0.83 | ||
| 36 | 3 | ||
| 2 | 0 | ||
| 53 | 6 | ||
| Troponin | 0.19 | 0.91 | ||
| 15 | 1 | ||
| 49 | 5 | ||
| 27 | 3 | ||
| Arterial Blood Gas | 3.97 | 0.265 | ||
| 39 | 6 | ||
| 3 | 1 | ||
| 4 | 0 | ||
| 45 | 2 | ||
| B-Type Natriuretic Peptide | 8.82 | 0.012 | ||
| 10 | 1 | ||
| 9 | 4 | ||
| 72 | 4 | ||
| Electrocardiogram | 6.36 | 0.384 | ||
| 37 | 2 | ||
| 2 | 0 | ||
| 17 | 2 | ||
| 4 | 0 | ||
| 2 | 1 | ||
| 7 | 0 | ||
| 11 | 0 | ||
| 11 | 4 | ||
| Chest X-ray | 3.57 | 0.312 | ||
| 19 | 1 | ||
| 2 | 0 | ||
| 8 | 3 | ||
| 14 | 2 | ||
| 48 | 3 | ||
| Initial Treatment for PE | 63.37 | <0.001 | ||
| 86 | 0 | ||
| 3 | 6 | ||
| 2 | 2 | ||
| 0 | 1 |
| Age | Signs and Symptoms | Risk Factors | Co-Morbidities | D-Dimer | Troponin | ABG | BNP | ECG | CXR | CPR | CTPA Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | Hypoxia < 95% on room air | None | None | Not ordered | Not elevated | VBG | Normal | Sinus tachycardia | No | Not calculated | Segmental and subsegmental PE |
| 42 | Heart rate > 100 | None | None | Not ordered | Not elevated | VBG | Normal | Sinus tachycardia | Normal | Not calculated | Segmental PE |
| 58 | Hypoxia < 95% on room air | None | None | Elevated | Not ordered | Not ordered | Normal | Normal | Pleural effusion | Not calculated | Segmental PE |
| 60 | Hypoxia < 95% on room air | None | Chronic lung disease | Not ordered | Not elevated | VBG | Not ordered | Right axis deviation | Normal | Not calculated | Linear filling defect involving the anterior subsegmental branches of the right upper lobe pulmonary artery, likely suggestive of pulmonary embolism |
| 62 | Heart rate > 100 | Immobilization/trauma | None | Not ordered | Not ordered | ABG with PCO2 normal | Not ordered | Normal | Pleural effusion | Not calculated | Two small bilateral non-occlusive segmental pulmonary emboli |
| 67 | Heart rate > 100 | Malignancy | None | Not ordered | Not ordered | VBG | Not ordered | Not ordered | Pleural effusion | Geneva and Well’s score | Positive for PE. Prominent pulmonary embolus at the origin of the pulmonary trunk. Further thromboembolic disease in right lower lobe |
| 84 | Hypoxia < 95% on room air | None | None | Elevated | Not elevated | VBG | Not ordered | Normal | Not ordered | Not calculated | Several segmental PEs |
| 85 | None | Immobilization/trauma | Chronic lung disease | Elevated | Elevated | VBG | Elevated | Not ordered | None of the above | Not calculated | Bilateral PE with evidence of right heart dysfunction |
| 86 | DVT signs | Immobilization/trauma | None of the above | Not ordered | Not elevated | Not ordered | Normal | Normal | Not ordered | Not calculated | Bilateral pulmonary emboli—w/saddle at R pulmonary trunk—R and L segmental and subsegmental involvement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chean, L.N.; Tan, C.; Hiskens, M.I.; Rattenbury, M.; Sundaram, P.; Perara, J.; Smith, K.; Kumar, P. Overuse of Computed Tomography Pulmonary Angiography and Low Utilization of Clinical Prediction Rules in Suspected Pulmonary Embolism Patients at a Regional Australian Hospital. Healthcare 2024, 12, 278. https://doi.org/10.3390/healthcare12020278
Chean LN, Tan C, Hiskens MI, Rattenbury M, Sundaram P, Perara J, Smith K, Kumar P. Overuse of Computed Tomography Pulmonary Angiography and Low Utilization of Clinical Prediction Rules in Suspected Pulmonary Embolism Patients at a Regional Australian Hospital. Healthcare. 2024; 12(2):278. https://doi.org/10.3390/healthcare12020278
Chicago/Turabian StyleChean, Li Ning, Clement Tan, Matthew I. Hiskens, Marie Rattenbury, Prahalath Sundaram, Jithmy Perara, Karen Smith, and Pranav Kumar. 2024. "Overuse of Computed Tomography Pulmonary Angiography and Low Utilization of Clinical Prediction Rules in Suspected Pulmonary Embolism Patients at a Regional Australian Hospital" Healthcare 12, no. 2: 278. https://doi.org/10.3390/healthcare12020278
APA StyleChean, L. N., Tan, C., Hiskens, M. I., Rattenbury, M., Sundaram, P., Perara, J., Smith, K., & Kumar, P. (2024). Overuse of Computed Tomography Pulmonary Angiography and Low Utilization of Clinical Prediction Rules in Suspected Pulmonary Embolism Patients at a Regional Australian Hospital. Healthcare, 12(2), 278. https://doi.org/10.3390/healthcare12020278






