Mobile Apps for COVID-19: A Systematic Review of Reviews
Abstract
1. Introduction
Objective
2. Materials and Methods
2.1. Systematic Literature Search
2.2. Data Extraction
2.3. Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
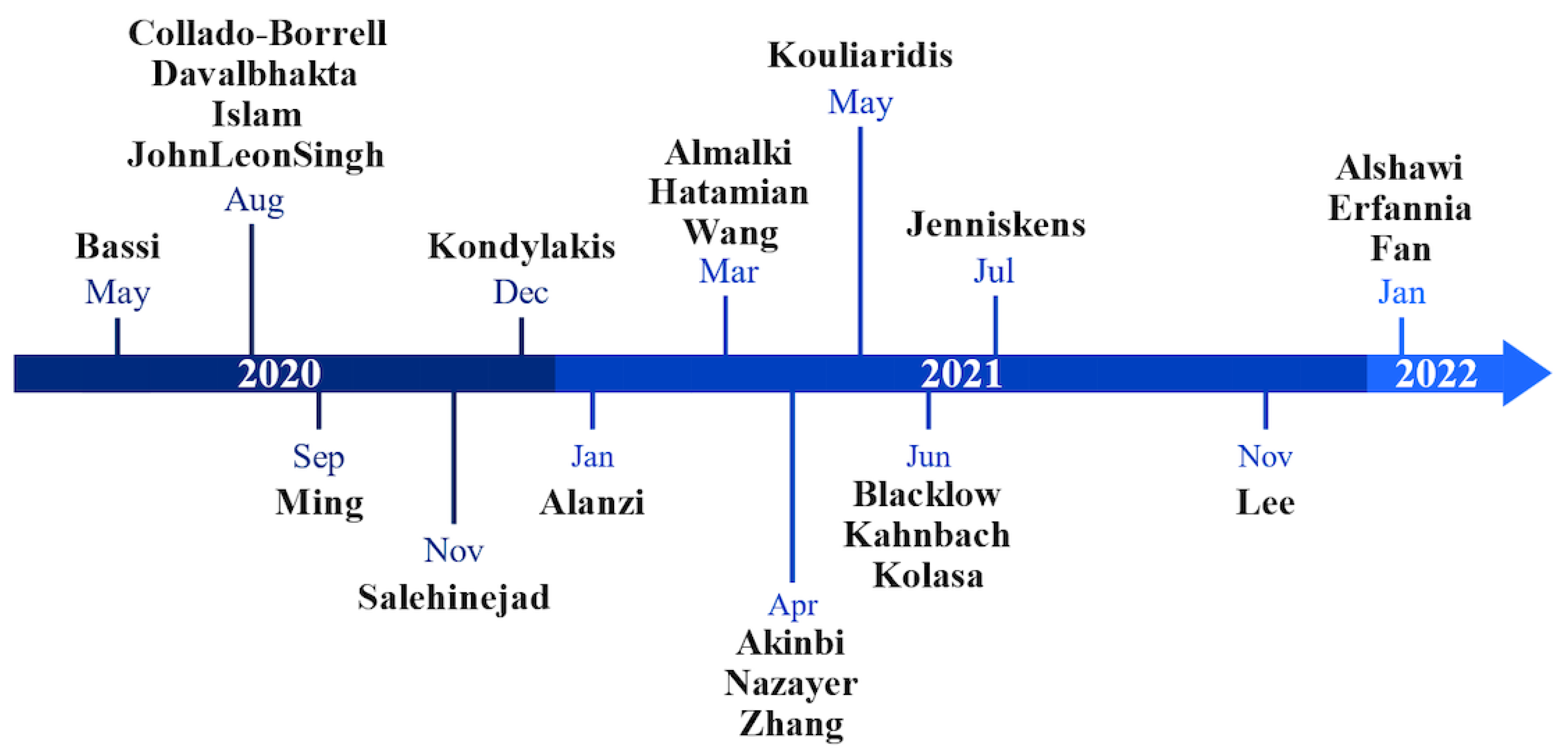
3.1. Systematic Literature Search
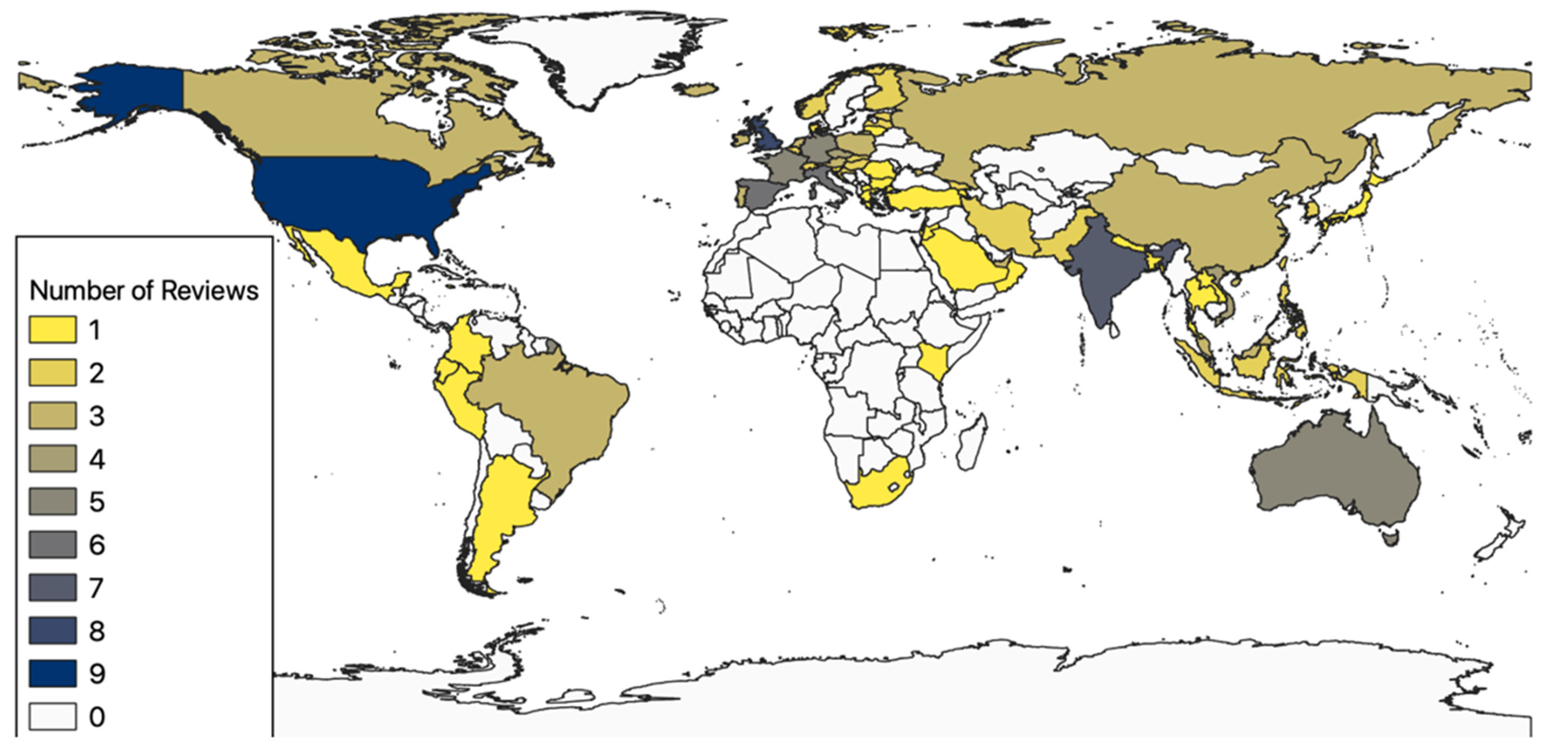
3.2. Spatiotemporal Analysis
3.3. Categorization
3.3.1. App Overview
3.3.2. Privacy and Security
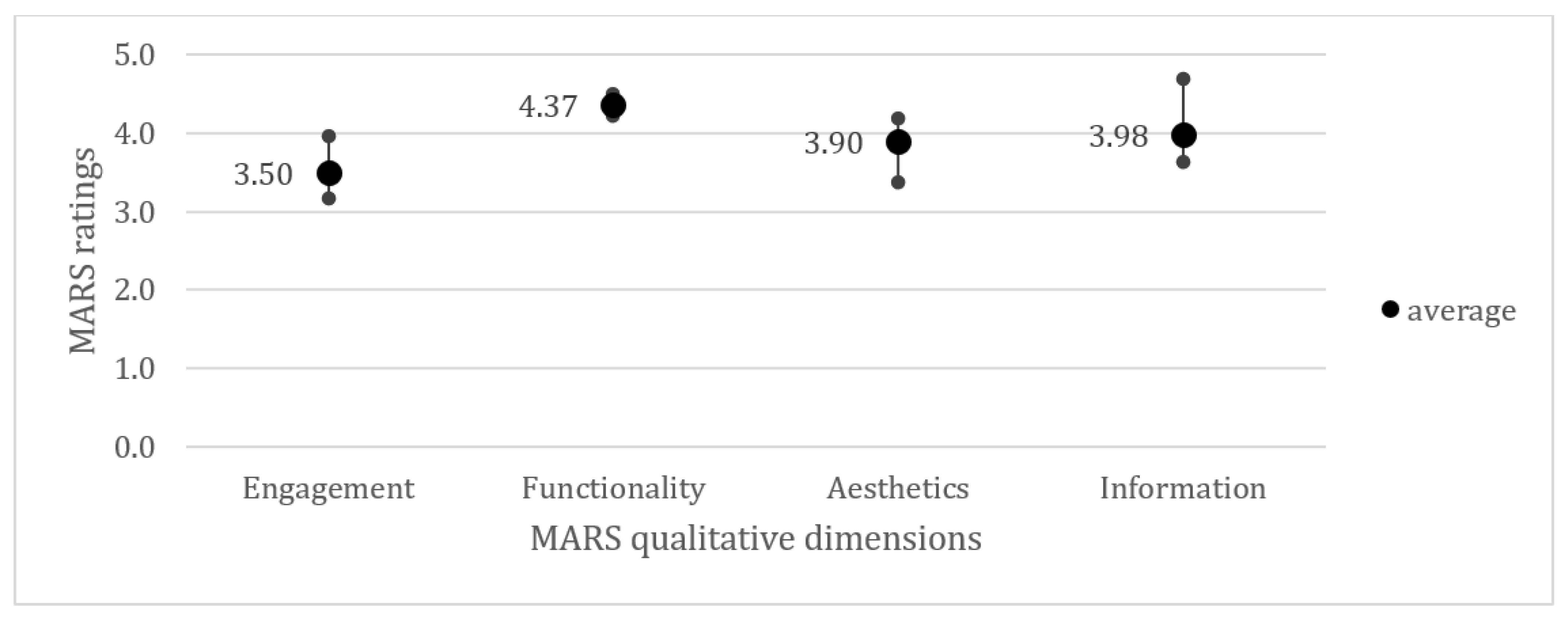
3.3.3. App Reviews Using the Mobile Application Rating Scale
3.3.4. Miscellaneous
3.4. Quality Assessment
4. Discussion
4.1. Principal Results
4.2. Limitations
4.3. Comparison with Prior Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Available online: https://coronavirus.jhu.edu/map.html (accessed on 21 August 2022).
- Ayouni, I.; Maatoug, J.; Dhouib, W.; Zammit, N.; Fredj, S.B.; Ghammam, R.; Ghannem, H. Effective public health measures to mitigate the spread of COVID-19: A systematic review. BMC Public Health 2021, 21, 1015. [Google Scholar] [CrossRef] [PubMed]
- Girum, T.; Lentiro, K.; Geremew, M.; Migora, B.; Shewamare, S. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: A systematic review. Trop. Med. Health 2020, 48, 91. [Google Scholar] [CrossRef]
- Hamine, S.; Gerth-Guyette, E.; Faulx, D.; Green, B.B.; Ginsburg, A.S. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: A systematic review. J. Med. Internet Res. 2015, 17, e52. [Google Scholar] [CrossRef]
- Marcolino, M.S.; Oliveira, J.A.Q.; D’Agostino, M.; Ribeiro, A.L.; Alkmim, M.B.M.; Novillo-Ortiz, D. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth 2018, 6, e23. [Google Scholar] [CrossRef]
- Baniasadi, T.; Niakan Kalhori, S.R.; Ayyoubzadeh, S.M.; Zakerabasali, S.; Pourmohamadkhan, M. Study of challenges to utilise mobile-based health care monitoring systems: A descriptive literature review. J. Telemed. Telecare 2018, 24, 661–668. [Google Scholar] [CrossRef]
- Brinkel, J.; Krämer, A.; Krumkamp, R.; May, J.; Fobil, J. Mobile phone-based mHealth approaches for public health surveillance in sub-Saharan Africa: A systematic review. Int. J. Environ. Res. Public Health 2014, 11, 11559–11582. [Google Scholar] [CrossRef]
- Rajput, Z.A.; Mbugua, S.; Amadi, D.; Chepngeno, V.; Saleem, J.J.; Anokwa, Y.; Hartung, C.; Borriello, G.; Mamlin, B.W.; Ndege, S.K.; et al. Evaluation of an Android-based mHealth system for population surveillance in developing countries. J. Am. Med. Inform. Assoc. 2012, 19, 655–659. [Google Scholar] [CrossRef]
- Li, J.; Moore, N.; Akter, S.; Bleisten, S.; Ray, P. mHealth for Influenza Pandemic Surveillance in Developing Countries. In Proceedings of the 2010 43rd Hawaii International Conference on System Sciences, Honolulu, HI, USA, 5–8 January 2010; pp. 1–9. [Google Scholar]
- Tom-Aba, D.; Nguku, P.M.; Arinze, C.C.; Krause, G. Assessing the Concepts and Designs of 58 Mobile Apps for the Management of the 2014–2015 West Africa Ebola Outbreak: Systematic Review. JMIR Public Health Surveill. 2018, 4, e68. [Google Scholar] [CrossRef] [PubMed]
- Wymant, C.; Ferretti, L.; Tsallis, D.; Charalambides, M.; Abeler-Dorner, L.; Bonsall, D.; Hinch, R.; Kendall, M.; Milsom, L.; Ayres, M.; et al. The epidemiological impact of the NHS COVID-19 app. Nature 2021, 594, 408–412. [Google Scholar] [CrossRef]
- Abbas, R.; Michael, K. COVID-19 Contact Trace App Deployments: Learnings From Australia and Singapore. IEEE Consum. Electron. Mag. 2020, 9, 65–70. [Google Scholar] [CrossRef]
- Zhou, S.L.; Jia, X.; Skinner, S.P.; Yang, W.; Claude, I. Lessons on mobile apps for COVID-19 from China. J. Saf. Sci. Resil. 2021, 2, 40–49. [Google Scholar] [CrossRef]
- Alanzi, T. A Review of Mobile Applications Available in the App and Google Play Stores Used During the COVID-19 Outbreak. J. Multidiscip. Healthc. 2021, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.J.L.; Couch, D.; Yap, K. Mobile Health Apps That Help With COVID-19 Management: Scoping Review. JMIR Nurs. 2020, 3, e20596. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Islam, I.; Munim, K.M.; Islam, A. A Review on the Mobile Applications Developed for COVID-19: An Exploratory Analysis. IEEE Access 2020, 8, 145601–145610. [Google Scholar] [CrossRef]
- Almalki, M.; Giannicchi, A. Health Apps for Combating COVID-19: Descriptive Review and Taxonomy. JMIR Mhealth Uhealth 2021, 9, e24322. [Google Scholar] [CrossRef]
- Kondylakis, H.; Katehakis, D.G.; Kouroubali, A.; Logothetidis, F.; Triantafyllidis, A.; Kalamaras, I.; Votis, K.; Tzovaras, D. COVID-19 Mobile Apps: A Systematic Review of the Literature. J. Med. Internet Res. 2020, 22, e23170. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Radua, J. Ten simple rules for conducting umbrella reviews. Evid.-Based Ment. Health 2018, 21, 95. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef] [PubMed]
- Medicine, N.L.o. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 22 November 2022).
- Golder, S.; Wright, K. Searching Evidence. Umbrella Rev. 2016, 95–106. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software. Available online: www.covidence.org (accessed on 28 November 2022).
- Coenen, M.; Schuetz, G.M.; Dewey, M. Evaluation of methodological quality of systematic reviews and meta-analyses: AMSTAR (A Measurement Tool for the Assessment of Multiple Systematic Reviews). Rofo 2013, 184, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps. JMIR mHealth uHealth 2015, 3, e27. [Google Scholar] [CrossRef]
- Holl, F.; Kircher, J.; Swoboda, W.J.; Schobel, J. Methods Used to Evaluate mHealth Applications for Cardiovascular Disease: A Quasi-Systematic Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 12315. [Google Scholar] [CrossRef]
- Meidani, Z.; Omidvar, A.; Asghari, F.; Akbari, H.; Khajouei, R.; Nabovati, E.; Zare, S.; Holl, F. Evaluating the Quality of a Clinical Mobile App for Physicians’ CT Scan Ordering Using the MARS Rating Scale; IOS Press: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Bassi, A.; Arfin, S.; John, O.; Jha, V. An overview of mobile applications (apps) to support the coronavirus disease 2019 response in India. Indian J. Med. Res. 2020, 151, 468–473. [Google Scholar] [CrossRef]
- Collado-Borrell, R.; Escudero-Vilaplana, V.; Villanueva-Bueno, C.; Herranz-Alonso, A.; Sanjurjo-Saez, M. Features and Functionalities of Smartphone Apps Related to COVID-19: Systematic Search in App Stores and Content Analysis. J. Med. Internet Res. 2020, 22, e20334. [Google Scholar] [CrossRef]
- Ming, L.C.; Untong, N.; Aliudin, N.A.; Osili, N.; Kifli, N.; Tan, C.S.; Goh, K.W.; Ng, P.W.; Al-Worafi, Y.M.; Lee, K.S.; et al. Mobile Health Apps on COVID-19 Launched in the Early Days of the Pandemic: Content Analysis and Review. JMIR Mhealth Uhealth 2020, 8, e19796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.W.B.; Chow, A.; Ho, R.C.M.; Smith, H.E. An Overview of Commercially Available Apps in the Initial Months of the COVID-19 Pandemic. Front. Psychiatry 2021, 12, 557299. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Ibrahim, S.A.; Zhang, T. Mobile Apps Leveraged in the COVID-19 Pandemic in East and South-East Asia: Review and Content Analysis. JMIR Mhealth Uhealth 2021, 9, e32093. [Google Scholar] [CrossRef] [PubMed]
- Erfannia, L.; Amraei, M.; Arji, G.; Yazdani, A.; Sabzehgar, M.; Yaghoobi, L. Reviewing and Content Analysis of Persian Language Mobile Health Apps for COVID-19 Management. Stud. Health Technol. Inform. 2022, 289, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Hatamian, M.; Wairimu, S.; Momen, N.; Fritsch, L. A privacy and security analysis of early-deployed COVID-19 contact tracing Android apps. Empir. Softw. Eng. 2021, 26, 36. [Google Scholar] [CrossRef]
- Nazayer, M.; Madanian, S.; Mirza, F. Contact-tracing applications: A review of technologies. BMJ Innov. 2021, 7, 368–378. [Google Scholar] [CrossRef]
- Kouliaridis, V.; Kambourakis, G.; Chatzoglou, E.; Geneiatakis, D.; Wang, H. Dissecting contact tracing apps in the Android platform. PLoS ONE 2021, 16, e0251867. [Google Scholar] [CrossRef]
- Kolasa, K.; Mazzi, F.; Leszczuk-Czubkowska, E.; Zrubka, Z.; Péntek, M. State of the Art in Adoption of Contact Tracing Apps and Recommendations Regarding Privacy Protection and Public Health: Systematic Review. JMIR Mhealth Uhealth 2021, 9, e23250. [Google Scholar] [CrossRef]
- Alshawi, A.; Al-Razgan, M.; AlKallas, F.H.; Bin Suhaim, R.A.; Al-Tamimi, R.; Alharbi, N.; AlSaif, S.O. Data privacy during pandemics: A systematic literature review of COVID-19 smartphone applications. PeerJ Comput. Sci. 2022, 8, e826. [Google Scholar] [CrossRef]
- Davalbhakta, S.; Advani, S.; Kumar, S.; Agarwal, V.; Bhoyar, S.; Fedirko, E.; Misra, D.; Goel, A.; Gupta, L.; Agarwal, V. A systematic review of the smartphone applications available for coronavirus disease 2019 (COVID19) and their assessment using the mobile app rating scale (MARS). medRxiv 2020. [Google Scholar] [CrossRef]
- Salehinejad, S.; Niakan Kalhori, S.R.; Hajesmaeel Gohari, S.; Bahaadinbeigy, K.; Fatehi, F. A review and content analysis of national apps for COVID-19 management using Mobile Application Rating Scale (MARS). Inform. Health Soc. Care 2021, 46, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Markert, C.; Sasangohar, F. Investigating Popular Mental Health Mobile Application Downloads and Activity During the COVID-19 Pandemic. Hum. Factors 2021, 65, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Kahnbach, L.; Lehr, D.; Brandenburger, J.; Mallwitz, T.; Jent, S.; Hannibal, S.; Funk, B.; Janneck, M. Quality and Adoption of COVID-19 Tracing Apps and Recommendations for Development: Systematic Interdisciplinary Review of European Apps. J. Med. Internet Res. 2021, 23, e27989. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Z.; Deng, S.; Lv, H.; Wang, F. The function and quality of individual epidemic prevention and control apps during the COVID-19 pandemic: A systematic review of Chinese apps. Int. J. Med. Inform. 2022, 160, 104694. [Google Scholar] [CrossRef] [PubMed]
- Akinbi, A.; Forshaw, M.; Blinkhorn, V. Contact tracing apps for the COVID-19 pandemic: A systematic literature review of challenges and future directions for neo-liberal societies. Health Inf. Sci. Syst. 2021, 9, 18. [Google Scholar] [CrossRef]
- Blacklow, S.O.; Lisker, S.; Ng, M.Y.; Sarkar, U.; Lyles, C. Usability, inclusivity, and content evaluation of COVID-19 contact tracing apps in the United States. J. Am. Med. Inform. Assoc. 2021, 28, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Jenniskens, K.; Bootsma, M.C.J.; Damen, J.A.A.G.; Oerbekke, M.S.; Vernooij, R.W.M.; Spijker, R.; Moons, K.G.M.; Kretzschmar, M.E.E.; Hooft, L. Effectiveness of contact tracing apps for SARS-CoV-2: A rapid systematic review. BMJ Open 2021, 11, e050519. [Google Scholar] [CrossRef]
- Nouri, R.; Niakan Kalhori, S.R.; Ghazisaeedi, M.; Marchand, G.; Yasini, M. Criteria for assessing the quality of mHealth apps: A systematic review. J. Am. Med. Inform. Assoc. 2018, 25, 1089–1098. [Google Scholar] [CrossRef]
- Izahar, S.; Lean, Q.Y.; Hameed, M.A.; Murugiah, M.K.; Patel, R.P.; Al-Worafi, Y.M.; Wong, T.W.; Ming, L.C. Content Analysis of Mobile Health Applications on Diabetes Mellitus. Front. Endocrinol. 2017, 8, 318. [Google Scholar] [CrossRef]
- Hatamian, M. Engineering Privacy in Smartphone Apps: A Technical Guideline Catalog for App Developers. IEEE Access 2020, 8, 35429–35445. [Google Scholar] [CrossRef]
- Exit through the App Store? Available online: https://www.adalovelaceinstitute.org/wp-content/uploads/2020/04/Ada-Lovelace-Institute-Rapid-Evidence-Review-Exit-through-the-App-Store-April-2020-2.pdf (accessed on 12 November 2022).
- Privacy Code of Conduct on Mobile Health Apps. Available online: https://digital-strategy.ec.europa.eu/en/policies/privacy-mobile-health-apps (accessed on 2 December 2023).
- Guidelines 04/2020 on the Use of Location Data and Contact Tracing Tools in the Context of the COVID-19 Outbreak. Available online: https://edpb.europa.eu/sites/default/files/files/file1/edpb_guidelines_20200420_contact_tracing_covid_with_annex_en.pdf (accessed on 12 November 2022).
- Davalbhakta, S.; Advani, S.; Kumar, S.; Agarwal, V.; Bhoyar, S.; Fedirko, E.; Misra, D.P.; Goel, A.; Gupta, L.; Agarwal, V. A Systematic Review of Smartphone Applications Available for Corona Virus Disease 2019 (COVID19) and the Assessment of their Quality Using the Mobile Application Rating Scale (MARS). J. Med. Syst. 2020, 44, 164. [Google Scholar] [CrossRef] [PubMed]
- Carlo, A.D.; Hosseini Ghomi, R.; Renn, B.N.; Areán, P.A. By the numbers: Ratings and utilization of behavioral health mobile applications. NPJ Digit. Med. 2019, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Messner, E.-M.; Terhorst, Y.; Barke, A.; Baumeister, H.; Stoyanov, S.; Hides, L.; Kavanagh, D.; Pryss, R.; Sander, L.; Probst, T. The German Version of the Mobile App Rating Scale (MARS-G): Development and Validation Study. JMIR mHealth uHealth 2020, 8, e14479. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.H.; Ciliska, D.; Dobbins, M.; Micucci, S. A Process for Systematically Reviewing the Literature: Providing the Research Evidence for Public Health Nursing Interventions. Worldviews Evid.-Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chow, A.; Smith, H. COVID-19 Contact-Tracing Apps: Analysis of the Readability of Privacy Policies. J. Med. Internet Res. 2020, 22, e21572. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, B.; Zhang, S.; Huang, N.; Zhao, T.; Lu, Q.B.; Cui, F. Differences in incidence and fatality of COVID-19 by SARS-CoV-2 Omicron variant versus Delta variant in relation to vaccine coverage: A world-wide review. J. Med. Virol. 2023, 95, e28118. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Google. Requirements for Coronavirus Disease 2019 (COVID-19) Apps. Available online: https://support.google.com/googleplay/android-developer/answer/9889712?hl=en (accessed on 10 November 2022).
- Apple Inc. Ensuring the Credibility of Health & Safety Information. Available online: https://developer.apple.com/news/?id=03142020a (accessed on 10 November 2022).
- Rowe, F. Contact tracing apps and values dilemmas: A privacy paradox in a neo-liberal world. Int. J. Inf. Manag. 2020, 55, 102178. [Google Scholar] [CrossRef]
- Wangler, J.; Jansky, M. The use of health apps in primary care—Results from a survey amongst general practitioners in Germany. Wien. Med. Wochenschr. 2021, 171, 148–156. [Google Scholar] [CrossRef]
- Wicks, P.; Chiauzzi, E. ‘Trust but verify’—Five approaches to ensure safe medical apps. BMC Med. 2015, 13, 205. [Google Scholar] [CrossRef]
- Zhou, L.; Bao, J.; Watzlaf, V.; Parmanto, B. Barriers to and Facilitators of the Use of Mobile Health Apps From a Security Perspective: Mixed-Methods Study. JMIR mHealth uHealth 2019, 7, e11223. [Google Scholar] [CrossRef] [PubMed]
- Holl, F.; Flemisch, F.; Swoboda, W.; Schobel, J. Apps for COVID-19 in Germany: Assessment using the German Mobile App Rating Scale. JAMIA Open 2022, 5, ooac082. [Google Scholar] [CrossRef] [PubMed]
- Guazzini, A.; Fiorenza, M.; Panerai, G.; Duradoni, M.; Guazzini, A.; Fiorenza, M.; Panerai, G.; Duradoni, M. What Went Wrong? Predictors of Contact Tracing Adoption in Italy during COVID-19 Pandemic. Future Internet 2021, 13, 286. [Google Scholar] [CrossRef]


| Dimension | Description |
|---|---|
| Population | People at risk of being exposed to SARS-CoV-2 or who were diagnosed with COVID-19 |
| Intervention | Reviews of COVID-19-related apps |
| Control | With or without a comparator |
| Outcome | Metadata Review’s subject Methods used Results |
| Database | Search String |
|---|---|
| Medline | (“SARS-CoV-2”[Mesh] OR SARS-CoV-2[tw] OR COVID-19[tw] OR CORONA) AND (“Mobile Applications”[Mesh] OR Mobile Applications[tw] OR Smartphone[tw] OR Telemedicine [tw]) |
| Embase | (‘mobile application’/exp OR ‘mobile application’ OR ‘smartphone’/exp OR smartphone OR ‘mobile phone’/exp OR ‘mobile phone’ OR ‘telemedicine’/exp OR ‘telemedicine’) AND (‘coronavirus disease 2019’ OR ‘19’) |
| Study ID | Title | Objective | Category | AMSTAR Rating |
|---|---|---|---|---|
| Bassi 2020 [35] | An overview of mobile applications (apps) to support the coronavirus disease 2019 response in India | To identify COVID-19-related mobile apps and highlight gaps to inform the development of future mHealth initiatives. | Overview | Low |
| Islam 2020 [20] | A Review on the Mobile Applications Developed for COVID-19: An Exploratory Analysis | To explore the existing mobile applications developed for the COVID-19 pandemic. | Overview | Low |
| Collado-Borrell 2020 [36] | Features and Functionalities of Smartphone Apps Related to COVID-19: Systematic Search in App Stores and Content Analysis | To identify smartphone apps designed to address the COVID-19 pandemic and analyze their characteristics. | Overview | Low |
| Ming 2020 [37] | Mobile Health Apps on COVID-19 Launched in the Early Days of the Pandemic: Content Analysis and Review | To analyze and evaluate the contents and features of COVID-19 mobile apps. | Overview | Critically low |
| Alanzi 2021 [18] | A Review of Mobile Applications Available in the App and Google Play Stores Used During the COVID-19 Outbreak | To review the functionalities and effectiveness of mHealth apps during the COVID-19 outbreak. | Overview | Critically low |
| Almalki 2021 [21] | Health Apps for Combating COVID-19: Descriptive Review and Taxonomy | To categorize health apps related to COVID-19, explore their key technical features, and classify their purposes. | Overview | Low |
| Zhang 2021 [38] | An Overview of Commercially Available Apps in the Initial Months of the COVID-19 Pandemic | To identify the commercial applications that are currently available for COVID-19 and explore their functionalities. | Overview | Critically low |
| Lee 2021 [39] | Mobile Apps Leveraged in the COVID-19 Pandemic in East and South-East Asia: Review and Content Analysis | To examine free apps from East and Southeast Asian countries, highlight their key characteristics, and interpret the relation of apps’ release dates and commencement dates of other COVID-19 public health policies. | Overview | Low |
| Erfannia 2022 [40] | Reviewing and Content Analysis of Persian Language Mobile Health Apps for COVID-19 Management. | To carry out a content analysis of free Persian mobile health apps in the management of COVID-19 and determine the relationship between the popularity and quality of these apps. | Overview | Low |
| JohnLeonSingh 2020 [19] | Mobile Health Apps That Help With COVID-19 Management: Scoping Review | To scope the evidence base on apps that were developed in response to COVID-19. | Privacy and Security | Low |
| Hatamian 2021 [41] | A privacy and security analysis of early-deployed COVID-19 contact tracing Android apps. | To analyze the privacy and security performance of Android contact-tracing applications, including code privileges, promises, privacy policies, and static and dynamic performance. | Privacy and Security | Critically low |
| Nazayer 2021 [42] | Contact-tracing applications: A review of technologies | To examine design decisions related to COVID-19 contact-tracing applications and the implications of these decisions. | Privacy and Security | Critically low |
| Kouliaridis 2021 [43] | Dissecting contact tracing apps in the Android platform. | To analyze all the official Android contact-tracing apps deployed by European countries regarding privacy and security via static and dynamic code analysis. | Privacy and Security | Critically low |
| Kolasa 2021 [44] | State of the Art in Adoption of Contact Tracing Apps and Recommendations Regarding Privacy Protection and Public Health: Systematic Review | To analyze available COVID-19 contact-tracing apps and verify to what extent public health interests and data privacy standards can be fulfilled simultaneously in the process of the adoption of digital health technologies. | Privacy and Security | Low |
| Alshawi 2022 [45] | Data privacy during pandemics: a systematic literature review of COVID-19 smartphone applications. | To provide a better study of privacy concerns in the context of COVID-19 apps, examine and analyze existing studies on COVID-19 apps and privacy concerns and their findings, and provide summaries. | Privacy and Security | Critically low |
| Davalbhakta 2020 [46] | A Systematic Review of Smartphone Applications Available for Corona Virus Disease 2019 (COVID19) and the Assessment of their Quality Using the Mobile Application Rating Scale (MARS) | To assess mobile applications for COVID-19 using the Mobile Application Rating Scale. | MARS | Moderate |
| Salehinejad 2021 [47] | A review and content analysis of national apps for COVID-19 management using Mobile Application Rating Scale (MARS) | To develop a reliable measure and rate the quality of COVID-19 mobile health apps. | MARS | Low |
| Wang 2021 [48] | Investigating Popular Mental Health Mobile Application Downloads and Activity During the COVID-19 Pandemic. | To analyze downloads and the user activity of select popular mental health apps during COVID-19 | MARS | Low |
| Kahnbach 2021 [49] | Quality and Adoption of COVID-19 Tracing Apps and Recommendations for Development: Systematic Interdisciplinary Review of European Apps | To investigate the quality characteristics of national European COVID-19 contact-tracing apps, investigate associations between app quality and adoption, and identify app features contributing to higher app quality. | MARS | Low |
| Fan 2022 [50] | The function and quality of individual epidemic prevention and control apps during the COVID-19 pandemic: A systematic review of Chinese apps. | To investigate the functional characteristics of individual epidemic prevention and control apps in China and evaluate their quality. | MARS | Moderate |
| Kondylakis 2020 [22] | COVID-19 Mobile Apps: A Systematic Review of the Literature | To review studies that have used and evaluated mobile apps for COVID-19. | Miscellaneous | Low |
| Akinbi 2021 [51] | Contact tracing apps for the COVID-19 pandemic: a systematic literature review of challenges and future directions for neo-liberal societies | To encompass current challenges facing contact-tracing applications and recommendations that address such challenges in the fight against the COVID-19 pandemic in neo-liberal societies. | Miscellaneous | Critically low |
| Blacklow 2021 [52] | Usability, inclusivity, and content evaluation of COVID-19 contact tracing apps in the United States. | To evaluate COVID-19 contact-tracing apps via an evaluation framework with objective measures of usability that are presented in this work. | Miscellaneous | Low |
| Jenniskens 2021 [53] | Effectiveness of contact tracing apps for SARS-CoV-2: A rapid systematic review | To systematically review evidence on the effectiveness of contact-tracing apps (CTAs) for SARSCoV-2 on epidemiological and clinical outcomes. | Miscellaneous | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holl, F.; Schobel, J.; Swoboda, W.J. Mobile Apps for COVID-19: A Systematic Review of Reviews. Healthcare 2024, 12, 139. https://doi.org/10.3390/healthcare12020139
Holl F, Schobel J, Swoboda WJ. Mobile Apps for COVID-19: A Systematic Review of Reviews. Healthcare. 2024; 12(2):139. https://doi.org/10.3390/healthcare12020139
Chicago/Turabian StyleHoll, Felix, Johannes Schobel, and Walter J. Swoboda. 2024. "Mobile Apps for COVID-19: A Systematic Review of Reviews" Healthcare 12, no. 2: 139. https://doi.org/10.3390/healthcare12020139
APA StyleHoll, F., Schobel, J., & Swoboda, W. J. (2024). Mobile Apps for COVID-19: A Systematic Review of Reviews. Healthcare, 12(2), 139. https://doi.org/10.3390/healthcare12020139









