Abstract
Introduction: A residual false lumen after treatment for Aortic Dissection type A (AD) has been associated with early complications, such as A malperfusion or rupture and mid-term or delayed complications, such as aneurysm formation or dissection expansion. Thoracic Endovascular Aortic Repair (TEVAR) is considered an effective solution by several surgical teams to prevent future complications. In this systematic review, all published data regarding the implementation of TEVAR after previous treatment for AD were collected in order to investigate indications, methods, clinical outcomes and aortic remodeling in these patients. Methods: The aim of this study was to investigate the indications, the methods and the efficacy of TEVAR usage after surgical treatment of AD. Data for this study were collected from four widely used medical databases (MEDLINE, SCIENCE DIRECT, GOOGLE SCHOLAR, OVID). All the results for each database were recorded and were analyzed with a systematic method. Techniques and clinical outcomes were investigated. Aortic remodeling was evaluated based on the following parameters in these studies: aortic diameter, true lumen diameter, false lumen diameter, false lumen thrombosis and false lumen patency. Results: The results obtained from the search among all databases comprised 1410 articles and of these articles 9 were included in the review. The majority of the studies were retrospective (seven out of nine studies), while no study was randomized. The total number of patients was 157 and 133 of them (84.7% of patients) were treated with TEVAR in zone 3 without extension below the diaphragm intraoperatively. Among 142 patients, the calculated mortality rate was 12.7% (18 of 142 patients), with 2.8% (4 of 142 patients) presenting with stroke. The percentage of patients with total or partial thrombosis combined was 65.9% (62 patients in a population of 92). The reintervention rate was 18.7%. Conclusions: TEVAR after AD surgery is an approach usually chosen in clinical practice, but the criteria of its usage are uncertain. This method is safe and enhances aortic remodeling with an acceptable reintervention rate. Definite guidelines in this field should be created in order to delineate whether TEVAR after AD surgery is beneficial as a preventive measure to aorta-related complications and to decide under which criteria this approach should be chosen.
1. Introduction
The surgical treatment of aortic dissection type A (AD) has sufficiently evolved during the last decade and the outcomes have improved significantly. The mortality rate decreased from 25% in 1995 to 18% in 2013 according to IRAD (International Registry of Acute Aortic Dissection) data [1]. Although surgery was effective in reducing mortality, aorta-related long-term complications continue to be a major cause of concern for patients surgically treated for AD. More specifically, recent evidence showed that almost half of the patients (46.1%) presented with major adverse events related to AD, while 17.3% of patients required reintervention [2]. Residual false lumen after treatment for AD has been associated with early complications, such as malperfusion or rupture and mid-term or delayed complications, such as aneurysm formation or dissection expansion [3]. Indeed, false lumen patency is a negative predictive factor for future aortic degeneration and reinterventions [3].
Despite the fact that the majority of surgeons still believe that the main goal in AD patients is in-hospital survival, mid-and long-term outcomes are undoubtedly critical. Thus, the modern treatment of AD takes into serious consideration possible future reinterventions [4] and aims to primarily address the long-term consequence of patent distal false lumen. In line with this, Thoracic Endovascular Aortic Repair (TEVAR) is considered an effective solution by several surgical teams to prevent future complications. In this systematic review, all published data regarding the implementation of TEVAR after previous treatment for AD were collected in order to investigate indications, methods, clinical outcomes and aortic remodeling in these patients.
2. Materials and Methods
Data for this study were collected from four widely used medical databases (MEDLINE, SCIENCE DIRECT, GOOGLE SCHOLAR, OVID). The key words used were aortic remodeling, type A dissection, residual dissection, TEVAR, and false lumen, and we included articles published in English up to 31 December 2023. All the results for each database were recorded and were analyzed by two researchers in the same method from 1 January 2024 to 15 January 2024. The search algorithm was: ((Aortic remodeling)) AND ((Type A aortic dissection) OR (DeBakey TYPE I aortic dissection)) AND ((Residual dissection) OR (False lumen)) AND ((TEVAR) OR (Endovascular aortic repair)). Firstly, abstracts of the articles were evaluated regarding relevance with the topic. Subsequently, relevant articles were fully reviewed and eligible articles were included in the review.
The inclusion criteria consisted of studies in which TEVAR was used for the treatment of residual false lumen after open surgical treatment for AS. Prospective and retrospective studies were included. In studies with various methods or comparative studies, only the patients receiving TEVAR were studied. The exclusion criteria for this review were: (1) studies in which no implementation of TEVAR was performed, (2) Frozen or Elephant Trunk procedure (3), and no follow-up among patients who had TEVAR after surgical repair for AD. Only data from patients who fulfilled the inclusion criteria for this review were included in the final analysis.
3. Results
A total of 1410 articles were obtained from the search among all databases. A detailed presentation of the number of publications from each database is presented in Table 1. After the initial screening, 59 articles were relevant to the topic of the review. After a review of the abstracts, we detected 32 articles with high relevance, which were fully reviewed. Among these, seven studies were finally identified as eligible for inclusion in the review, while two more studies were found through the author’s secondary search of the references of the included manuscripts (Table 1) [2,5,6,7,8,9,10,11,12]. Additionally, studies with non-randomized and comparative data were evaluated for their quality with the Newcastle—Ottawa Scale [13]. The outcomes of article evaluation are presented in Table 2 according to the aforementioned scale (Table 2). Out of five studies in total, two were categorized as medium-quality studies (four and six stars) and three as high-quality studies (seven stars for each study). Consequently, the number of publications included in the review was nine and the flow chart is depicted in Figure 1.

Table 1.
Table with the data of the article selection process.

Table 2.
Evaluation of the quality of comparative and non-randomized studies included in the systematic review. Studies between 4 and 6 stars are considered as medium-quality and studies with more than 7 stars as high-quality studies.
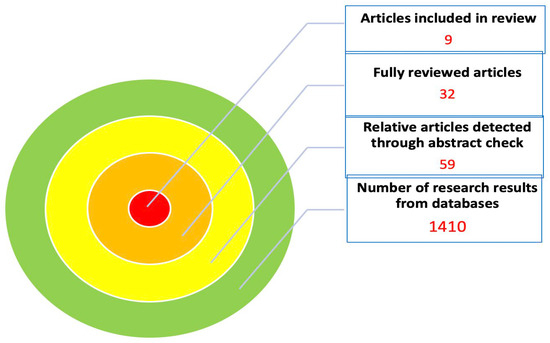
Figure 1.
Flow diagram of the article selection process.
All articles were published between 2008 and 2022, including data from 1994 to 2019. The duration of each study varied from 1 year to 10 years. The majority of the studies were retrospective (seven out of nine studies), while no study was randomized (Table 3). The total number of patients was 157 and 133 of them (84.7% of patients) were treated with TEVAR in zone 3 without extension below the diaphragm intraoperatively. The criteria for TEVAR vary a lot and the indications implemented in each study are presented in Table 3. Similarly, the techniques, the devices and the grafts used vary too.

Table 3.
All studies regarding TEVAR usage after surgically treated aortic dissection type A. Information regarding the type of study, study period, number of patients, time of intervention, criteria of TEVAR and technical parameters.
Studies also vary a lot in design and reporting evidence. As regards pre-TEVAR patients’ characteristics, CT findings and clinical status are poorly reported, and, as a result, this heterogeneity makes a comparison of the patient groups inappropriate. Clinical outcomes after TEVAR are reported in seven of the nine studies. In one study, mixed data with other methods are reported (Jakob et al.) [5] and in another study the clinical outcomes are not reported at all [2] (Table 4). Therefore, among 142 patients, the calculated mortality rate was 12.7% (18 of 142 patients), with 2.8% (4 of 142 patients) presenting stroke (Table 4, Figure 2).

Table 4.
Data regarding preoperative findings regarding pre-TEVAR false lumen characteristics and clinical status. Additionally, the clinical outcomes (mortality, stroke) after TEVAR are presented.
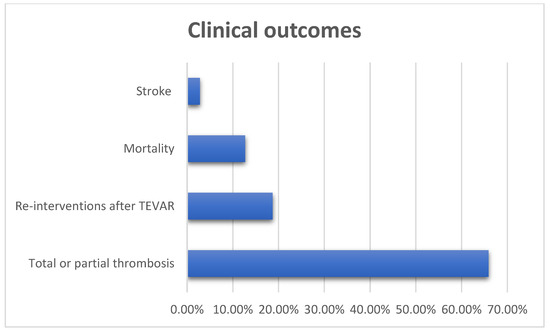
Figure 2.
Clinical outcomes after TEVAR for the treatment of residual false lumen.
The follow-up examination period ranged from 4.84 months to 8 years, while in almost all studies the aortic remodeling investigation was performed in 12 months after TEVAR. Aortic remodeling was evaluated based on the following parameters in these studies: aortic diameter, true lumen diameter, false lumen diameter, false lumen thrombosis and false lumen patency (Table 5).

Table 5.
Information regarding follow-up, aortic remodeling parameters and reinterventions.
Only three studies reported aortic diameter measurements in the follow-up examinations. Pochettino et al. [7] and Sultan et al. [9] reported very satisfying outcomes as regards aortic diameter in all cases, while 4 out of 11 patients presented aortic diameter increase in Di Tommaso et al.’s study [10]. Two studies included information about true lumen diameter, both indicating that TEVAR contributed to the true lumen diameter and area increase. Evidence about false lumen diameter were reported from three studies, showing that TEVAR was associated with a decrease in false lumen diameter. More specifically, Shimamura et al. [6] found the complete disappearance of false lumen in 30.3% and Pochettino et al. [7] in 17% of cases. The patency of the false lumen was reported as a parameter of aortic remodeling in three studies. A total of 22 cases out of 60 (percentage 36.6%) had a patent false lumen. Most researchers had chosen to include the degree of thrombosis in the results of their studies (seven of nine studies mention this information). The percentage of patients with total or partial thrombosis combined was 65.9% (62 patients in a population of 92) (Table 5).
Evidence on reinterventions after TEVAR was also included in the majority of the studies (five studies out of nine). The reintervention rate was 18.7% (14 cases out of 75) (Figure 2). It is worth mentioning that two patients were submitted to open surgery, one patient was treated with left subclavian artery occlusion with coils due to retrograde perfusion and the rest were treated with endovascular repair (Table 5).
4. Discussion
The deployment of a stent graft in the descending aorta to improve aortic remodeling and avoid aorta-related complications after surgical treatment for AD has been increased. This strategy is widely used in clinical practice but there are no definite guidelines and criteria for its implementation. A significant heterogeneity among the eligible studies regarding the indication for TEVAR was captured in our review. The indications vary a lot, but several predictors of negative aortic remodeling are commonly acceptable. Among these predictors, an aortic diameter > 35 mm, a false lumen diameter > 22 mm and an entry tear >1 mm were reported by the eligible studies [14]. Undoubtfully, the development of certain criteria for TEVAR usage after aortic dissection may offer the better management of such cases and improved documentation of the outcomes. We believe that the usage of TEVAR may be considered in patients with a total aortic diameter of more than 40 mm, a patent false lumen, the presence of an entry tear in zones 3 and/or 4 (based on Ishimaru zones) and a diameter of a false lumen more than 20 mm, but we have to highlight the fact that there is not enough evidence to support these recommendations at present.
In the majority of patients receiving TEVAR after surgery for AD, TEVAR was placed intraoperatively in approximately 84.7% of the patients, but preoperative evidence is poorly reported in almost all studies. Hence, it is not feasible to detect the profile of a patient receiving TEVAR intraoperatively. Another point that should be underlined is the follow-up after surgically treated AD patients. According to the reported evidence, most researchers did not stick to a certain follow-up protocol. It should be highlighted that close follow-up with repetitive Computed Tomography Angiography (CTA) is suggested in all patients surgically treated for AD, regardless of the extent of the false lumen. More specifically, CTA should be performed in the first month, 6 months and 1 year after surgery, and then annually for at least the first five years from the surgery, except in the cases where the findings suggest an even closer follow-up [15]. CTA is the preferred method of follow-up while Magnetic Resonance Angiography (MRA) is a reliable alternative.
Luminal communication between the true and false lumen is related to aortic diameter increase and consequently to reintervention rate. The 3-year freedom from reintervention is 96% when communication is absent and 47% in the presence of luminal communication [16]. Evidence shows that communication occlusion and the stenting of the descending aorta enhances aortic remodeling, as the partial or complete thrombosis of the false lumen reaches 88.9% [17]. In this review, partial or complete thrombosis was achieved in 65.9% of cases and the patency of false lumen was present in 34.1% (Table 5).
The reintervention rate was 18.7% and 2 out of 14 patients were submitted to open surgery. In a retrospective study including 534 patients surgically treated for AD, 37 of them required reintervention [18]. Of these patients, 30 were submitted to open surgery and 7 were in endovascular repair, while additional interventions were necessary in several patients [17]. These findings suggest that in cases of TEVAR implementation after surgery for AD, endovascular repair is the main approach for reintervention (2/14 cases), while in cases that no intervention is performed after AD surgery, open extended surgeries are required (30/37 cases). Other methods that aim to improve aortic remodeling such as the Frozen Elephant Trunk hybrid method are associated with a high rate of reintervention, both intentional or urgent. More specifically, the percentage of reinterventions reaches 33% while the usage of TEVAR after FET shows satisfactory outcomes regarding technical success and aorta remodeling [19,20]. Although indications and the extent of intervention are different between TEVAR after AD surgery and FET after AD, the findings show that the reintervention rate is lower in cases of TEVAR.
5. Limitations
As the studies were heterogenous, statistical analysis was not feasible. The statistical analysis would be biased and may lead to misleading conclusions. This fact is mainly due to (a) differences in the methods among studies, (b) differences in indications for TEVAR, (c) differences in the evidence presentation, (d) great variability in the follow-up period and in the choice of clinical or depicting outcomes presentation and (e) the deficit of randomized multicenter clinical trials on this field. Hence, in order to avoid weak or false conclusions, we present the evidence after meticulous investigation without statistical analysis.
6. Conclusions
TEVAR after AD surgery is an approach usually chosen in clinical practice, but the criteria of its usage are uncertain. This method is safe and enhances aortic remodeling with an acceptable reintervention rate. Definite guidelines on this field should be created in order to delineate whether TEVAR after AD surgery is beneficial as a preventive measure to aorta-related complications and to decide under which criteria this approach should be chosen.
Author Contributions
Conceptualization: N.S. and D.C.A.; Methodology: G.N.; Software: G.N. and I.S.; Validation: C.N.A. and D.C.A.; Investigation: N.S.; Resources: N.S., I.S. and G.N.; Data cuation: C.N.A. and I.S.; Writing—original draft preparation: N.S.; Writing review and editing: C.N.A. and D.C.A.; Visualization: G.N.; Supervision: D.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics approval was obtained from the local ethics committee. Patient consent was granted for all participants.
Informed Consent Statement
Informed consent was granted from all participants. The study is in accordance with the Bioethics regulations and the Greek lesgislation. The personal data are protected according to the law.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, M.; Porto, A.; Guivier-Curien, C.; Blanchard, A.; Bal, L.; Resseguier, N.; Omnes, V.; De Masi, M.; Ejargue, M.; Jacquier, A.; et al. Results of a prospective follow-up study after type A aortic dissection repair: A high rate of distal aneurysmal evolution and reinterventions. Eur. J. Cardio-Thorac. Surg. 2021, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Bozso, S.J.; Ouzounian, M.; Chu, M.W.A.; Moon, M.C.; Canadian Thoracic Aortic Collaborative. Acute type A aortic dis-section and the consequences of a patent false lumen. JTCVS Tech. 2021, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Kreibich, M.; Morlock, J.; Kondov, S.; Scheumann, J.; Schröfel, H.; Kari, F.A.; Berger, T.; Siepe, M.; Beyersdorf, F.; et al. Chronic type B “residual” after type A: What I would do? J. Vis. Surg. 2018, 4, 14. [Google Scholar] [CrossRef][Green Version]
- Jakob, H.; Tsagakis, K.; Tossios, P.; Massoudy, P.; Thielmann, M.; Buck, T.; Eggebrecht, H.; Kamler, M. Combining classic surgery with descending stent grafting for acute DeBakey type I dissection. Ann. Thorac. Surg. 2008, 86, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, K.; Kuratani, T.; Matsumiya, G.; Kato, M.; Shirakawa, Y.; Takano, H.; Ohta, N.; Sawa, Y. Long-term results of the open stent-grafting technique for extended aortic arch disease. J. Thorac. Cardiovasc. Surg. 2008, 135, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Pochettino, A.; Brinkman, W.T.; Moeller, P.; Szeto, W.Y.; Moser, W.; Cornelius, K.; Bowen, F.W.; Woo, Y.J.; Bavaria, J.E. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann. Thorac. Surg. 2009, 88, 482–489, discussion 489–490. [Google Scholar] [CrossRef] [PubMed]
- Preventza, O.; Cervera, R.; Cooley, D.A.; Bakaeen, F.G.; Mohamed, A.S.; Cheong, B.Y.; Cornwell, L.; Simpson, K.H.; Coselli, J.S. Acute type I aortic dissection: Traditional versus hybrid repair with antegrade stent delivery to the descending thoracic aorta. J. Thorac. Cardiovasc. Surg. 2014, 148, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Wallen, T.J.; Habertheuer, A.; Siki, M.; Arnaoutakis, G.J.; Bavaria, J.; Szeto, W.Y.; Milewski, R.; Vallabhajosyula, P. Concomitant antegrade stent grafting of the de-scending thoracic aorta during transverse hemiarch reconstruction for acute DeBakey I aortic dissection repair improves aortic remodeling. J. Card. Surg. 2017, 32, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.; Di Tommaso, E.; Di Palo, G.; Iannelli, G.; Di Tommaso, L. Treatment with transfemoral bare-metal stent of residual aortic arch dissection after surgical repair of acute type an aortic dissection. J. Thorac. Dis. 2018, 10, 6097–6106. [Google Scholar] [CrossRef] [PubMed]
- Morishita, K.; Baba, T.; Shingaki, M.; Shibata, T.; Narayama, K. 1-Year follow-up study of preemptive TEVAR for residually dissected aortas after proximal open repair of acute type a aortic dissection in high-risk patients for late re-intervention. Eur. J. Vasc. Endovasc. Surg. 2019, 58 (Suppl. S1), e30. [Google Scholar] [CrossRef]
- Li, J.; Stadlbauer, A.; Terrazas, A.; Floerchinger, B.; Pfister, K.; Creutzenberg, M.; Oikonomou, K.; Schmid, C.; Rupprecht, L. Mid-Term Outcomes of a Hybrid Approach Involving Open Surgery Plus TEVAR of the Descending Aorta in the Treatment of Complex Type A Dissection. Thorac. Cardiovasc. Surg. 2022, 70, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in me-ta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Rathore, K.S. Distal Aortic Remodeling after Type A Dissection Repair: An Ongoing Mirage. J. Chest Surg. 2021, 54, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hebert, A.M.; Smith-Washington, A.; Hoffstaetter, T.; Goldenberg, R.; Vemulapalli, S.; Del Río-Solá, D.; Arnaoutakis, G.J.; Mussa, F.; Ota, T.; et al. Knowledge gaps in surgical management for aortic dissection. Semin. Vasc. Surg. 2022, 35, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Heo, W.; Song, S.-W.; Lee, S.-Y.; Kim, T.-H.; Lee, J.-S.; Yoo, K.-J.; Cho, B.-K. Locational impact of luminal communication on aortic diameter changes and reintervention in acute type I aortic dissection. Eur. J. Cardio-Thorac. Surg. 2019, 55, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Di Eusanio, M.; Castrovinci, S.; Tian, D.H.; Folesani, G.; Cefarelli, M.; Pantaleo, A.; Murana, G.; Berretta, P.; Yan, T.D.; Bartolomeo, R.D. Antegrade stenting of the descending thoracic aorta during DeBakey type 1 acute aortic dissection repair. Eur. J. Cardio-Thorac. Surg. 2013, 45, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Itoh, S.; Yuri, K.; Adachi, K.; Matsumoto, H.; Yamaguchi, A.; Adachi, H. Reoperation for enlargement of the distal aorta after initial surgery for acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2015, 149 (Suppl. S2), S91–S98.e1. [Google Scholar] [CrossRef]
- Kreibich, M.; Berger, T.; Rylski, B.; Chen, Z.; Beyersdorf, F.; Siepe, M.; Czerny, M. Aortic reinterventions after the frozen elephant trunk procedure. J. Thorac. Cardiovasc. Surg. 2020, 159, 392–399.e1. [Google Scholar] [CrossRef] [PubMed]
- Meisenbacher, K.; Osswald, A.; Bischoff, M.S.; Böckler, D.; Karck, M.; Ruhparwar, A.; Geisbüsch, P. TEVAR Following FET: Current Outcomes of Rendezvous Procedures in Clinical Practice. Thorac. Cardiovasc. Surg. 2021, 70, 314–322. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).